Abstract
Vav1 play key roles in the progression of human cancer. However, the role of Vav1 in gastric cancer (GC) remains to be elucidated. The present study aimed to investigate the potential relevance of the clinicopathological characteristics and prognostic significance of Vav1 in GC patients. Methods: The protein expression of Vav1 was analyzed by immunohistochemistry in formalin-fixed samples obtained from 105 GC patients. Results: Positive Vav1 expression was correlated with larger tumor size and more lymph node metastasis (P<0.05) in GC tissues. Serosal invasion (hazard ratio [HR] = 2.764, P = 0.007), lymph node metastasis (HR = 1.298, P = 0.002) and Vav1 expression (HR = 0.436, P = 0.006) were identified as independent factors of the overall survival (OS) of GC patients. For advanced GC patients, lymph node metastasis (HR = 1.310, P = 0.003) and Vav1 expression (HR = 0.443, P = 0.010) were the independent prognostic factors of OS. Conclusion: Vav1 performs important functions in the aggressiveness of GC, and Vav1 may serve as a novel prognostic factor in GC.
Keywords: Stomach, cancer, Vav1, prognosis
Introduction
Gastric cancer (GC) is one of the most common malignancies worldwide. Despite its declining incidence, GC remains as the second leading cause of cancer-related deaths [1,2]. Although the diagnosis and treatment for GC have made great progress, and basic and clinical studies have been conducted in recent years, its long-term outcome remains poor [3]. The etiology of GC is a complex process that involves the activation of oncogenes and the inactivation of tumor suppressor genes at different stages. However, its exact pathogenesis remains unclear [4]. Hence, there is still an urgent need to explore novel and special promising predictive factors to improve the prognosis of GC.
The Vav family of proteins is a family of intracellular signal transduction proteins that function as guanine nucleotide exchange factors (GEF) [5]. As one of its family members, Vav1 was first identified in the Barbacid laboratory in 1989 [6]. Based on its structure and function, Vav1 has also been identified as a member of the Dbl-homology domain superfamily [7]. Vav1 has been considered to be able to control a diverse array of signaling pathways. Furthermore, Vav1 plays critical roles in the development and activation of T-cells [8]. Fernandez et al. [9] found that Vav1 contributed to the tumorigenic properties of pancreatic cancer cells by regulating both cellular proliferation and cell survival pathways through the regulation of the EGF-Src-Vav1-Rac1-Pak1-NF-B-CyclinD1 signaling axis. It has been demonstrated that Vav1 plays an important role in the actin cytoskeletal reorganization following T-cell activation through the T-cell receptor [10]. Furthermore, Vav1 was been shown to promote matrix degrading processes via its GEF activity in tumor cell migration. In lung cancer, Lazer et al. [11] was able to prove that the depletion of the Vav1 gene could reduce Rac-GTP activation and decrease the expression of TGF-α. Furthermore, they found that the knockdown of Vav1 expression by siRNA reduced the potential to generate tumors in nude mice.
Vav1 was shown to be involved in diverse human cancers, including breast cancer, ovarian cancer and lung cancer [12]. However, the expression, clinical values and prognostic implications of Vav1 in GC have not been studied to date. In the present study, we identified the relationships between the expression and clinicopathological characteristics of Vav1, as well as its correlation with the overall survival (OS) of patients.
Materials and methods
Tissue samples
Patients with histologically confirmed GC in Tianjin Nankan Hospital and Tianjin Cancer Hospital from 2005 to 2010 were reviewed. The eligibility criteria for the present study were as follows: (1) patients with no history of gastrectomy or other malignancies, (2) patients with no distant metastasis or peritoneal dissemination, and (3) the number of dissected lymph nodes was not less than 15. The exclusion criteria were as follows: (1) patients who underwent palliative surgery; (2) patients who received treatment such as chemotherapy, or radiation therapy, prior to radical surgery.
The data obtained from the registry were listed as follows: gender (male or female), age (≤60 or >60), tumor size (≤5 cm or >5 cm), tumor location (lower 1/3, middle 1/3, upper 1/3, or greater than 1/3), degree of differentiation (well/moderate or poor), serosal invasion (T1, T2, T3, or T4), lymph node metastasis (N0, N1, N2, or N3), and Vav1 expression (positive or negative).
Immunohistochemistry analysis
Formalin-fixed tissues were embedded in paraffin, cut into 4-μm sections, and mounted on silane-coated slides for immunohistochemistry analysis. The sections were deparaffinized with dimethylbenzene and rehydrated through 100%, 95%, 90%, 80% and 75% of ethanol. Antigen retrieval treatment was conducted at 95°C for 20 minutes in 1×10-5 mol/mL of sodium citrate buffer (pH 6.0), and endogenous peroxidases were blocked using 3% hydrogen peroxide for 15 minutes at room temperature. Next, the sections were washed in PBS, blocked with 10% goat serum (Zhong Shan Biotechnology) for 30 minutes, and incubated with rabbit anti-human Vav1 polyclonal antibody (ab97574, 1:50 dilution; Abcam) in a humidified chamber at 4°C overnight. Following three additional washes using PBS, the sections were incubated with HRP-conjugated secondary antibody for one hour at room temperature. Finally, the visualization signal was developed with 3,3’-diaminobenzidine solution, and all slides were counterstained with 20% hematoxylin. Then, the slides were dehydrated and mounted on cover slips. For negative controls, PBS was used in place of the primary antibody.
Evaluation of immunohistochemical variables
All sections were blindly assessed by two independent observers. In case of disagreements in the assessment, a third independent assessment was performed. The staining score for each slide was assessed according to staining intensity and the percentage of positive cells. Under a microscope at ×400 magnification, five fields of vision were randomly selected. The staining intensity was graded as follows: 0 (no staining), 1 (light yellow), 2 (yellowish brown), and 3 (brown). The positive cells were graded according to the percentage of positive cells as follows: 0 (no positive tumor cells), 1 (<10% positive tumor cells), 2 (11-50% positive tumor cells), 3 (51-80% positive tumor cells), and 4 (>80% positive tumor cells). Then, the percentage of positive cells and the staining intensity were multiplied to generate the immunoreactivity score. Based on this score, the immunoreactivity was divided into two groups: negative expression (a total score of 0-3), and positive expression (a total score of >3) [13].
Statistical analysis
Associations between the expression and clinicopathological characteristics of Vav1 were analyzed by chi-square test. The 5-YSR was determined using Kaplan-Meier method, and log-rank test was used to determine significance. Factors with potential importance on univariate analyses were included in multivariate analyses. Multivariate analysis of the OS was performed using Cox proportional hazards model with forward step procedures. Hazard ratios (HR) were then generated. All statistical analyses were carried out using SPSS software 17.0 (SPSS, Chicago, IL, US). A P-value <0.05 was considered statistically significant.
Results
Patient characteristics
Based on these inclusion and exclusion criteria, a total of 105 GC patients were eligible for the present study. The 5-year survival rate (5-YSR) for all GC patients was 44.8%. All surgical treatments were completed based on the R0 standard. The mean number of dissected lymph nodes was 22.43 ± 6.72 (range: 15-62). The other clinicopathological characteristics of GC patients were shown in Table 1.
Table 1.
Clinicopathological characteristics of GC patients
| Variables | Number of each group (%) |
|---|---|
| Gender | |
| Male | 60 (57.1) |
| Female | 45 (42.9) |
| Age (year) | |
| ≤60 | 64 (61.0) |
| >60 | 41 (39.0) |
| Size of tumor (cm) | |
| ≤5 | 75 (71.4) |
| >5 | 30 (28.6) |
| Location of tumor | |
| Lower 1/3 | 40 (38.1) |
| Middle 1/3 | 20 (19.0) |
| Upper 1/3 | 38 (36.2) |
| More than 1/3 | 7 (6.7) |
| Degree of differentiation | |
| Well/Moderate | 11 (10.5) |
| Poor | 94 (89.5) |
| Lauren’s classification | |
| Intestinal type | 73 (69.5) |
| Diffuse type | 32 (69.5) |
| Serosal invasion* | |
| T1 | 8 (7.6) |
| T2 | 4 (3.8) |
| T3 | 10 (9.5) |
| T4 | 83 (79.0) |
| Lymph node metastasis* | |
| N0 | 39 (37.1) |
| N1 | 26 (24.8) |
| N2 | 19 (18.1) |
| N3 | 21 (20.0) |
| Vav1 expression | |
| Negative | 62 (59.0) |
| Positive | 43 (41.0) |
7th TNM classification.
Relationships between the expression and various clinicopathological characteristics of Vav1
In order to elucidate the biological significance of Vav1 in GC, the immunohistochemical expression of Vav1 was examined in GC tissues. Vav1 protein was mainly localized in the cytoplasm of GC cells (Figure 1). The present analysis identified tumor size (P<0.001) and lymph node metastasis (P = 0.001) as relative factors of Vav1 expression in GC tissues. In contrast, Vav1 expression was not associated with gender, age, tumor location, degree of differentiation, Lauren’s classification and serosal invasion (P>0.05) (Table 2).
Figure 1.
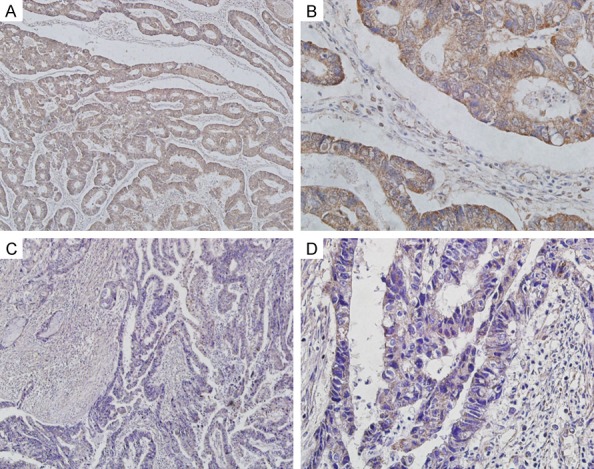
(A, B) Vav1 was positively expressed in GC tissues. (C, D) Vav1 was negatively expressed in GC tissues. Magnification is ×100 (A and C) or ×400 (B and D).
Table 2.
Correlations between Vav1 expression and clinicopathological characteristics in GC patients
| Variables | Vav1 expression | Chi-square value | P value | |
|---|---|---|---|---|
|
| ||||
| Positive | Negative | |||
| Gender | 0.030 | 0.864 | ||
| Male | 25 | 35 | ||
| Female | 18 | 27 | ||
| Age | 1.289 | 0.256 | ||
| ≤60 | 29 | 35 | ||
| >60 | 14 | 27 | ||
| Size of tumor | 14.655 | <0.001 | ||
| ≤5 | 22 | 53 | ||
| >5 | 21 | 9 | ||
| Location of tumor | 5.327 | 0.149 | ||
| Lower 1/3 | 12 | 28 | ||
| Middle 1/3 | 8 | 12 | ||
| Upper 1/3 | 18 | 20 | ||
| More than 1/3 | 5 | 2 | ||
| Degree of differentiation | 0.107 | 0.744 | ||
| Well/Moderate | 4 | 7 | ||
| Poor | 39 | 55 | ||
| Lauren’s classification | 0.149 | 0.700 | ||
| Intestinal type | 29 | 44 | ||
| Diffuse type | 14 | 18 | ||
| Serosal invasion* | 3.554 | 0.314 | ||
| T1 | 1 | 7 | ||
| T2 | 1 | 3 | ||
| T3 | 4 | 6 | ||
| T4 | 37 | 46 | ||
| Lymph node metastasis* | 15.638 | 0.001 | ||
| N0 | 10 | 29 | ||
| N1 | 7 | 19 | ||
| N2 | 13 | 6 | ||
| N3 | 13 | 8 | ||
7th TNM classification.
Survival analysis for GC patients
The univariate analysis revealed significant relationships between OS and tumor size, degree of differentiation, serosal invasion, lymph node metastasis and Vav1 expression; but not with gender, age, tumor location and Lauren’s classification. Serosal invasion (hazard ratio [HR] = 2.764, P = 0.007), lymph node metastasis (HR = 1.298, P = 0.002) and Vav1 expression (HR = 0.436, P = 0.006) were identified as independent factors of OS for all enrolled GC patients following the multivariate analysis (Cox proportional hazards model) (Table 3). In clinic, GC patients with negative Vav1 expression presented a significantly better OS than patients with positive Vav1 expression (Figure 2).
Table 3.
Univariate and multivariate analyses of the overall survival in GC patients
| Variables | 5-YSR (%) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
|
|
|
||||
| Chi-square value | P value | HR value | P value | ||
| Gender | 0.188 | 0.665 | |||
| Male | 43.3 | ||||
| Female | 46.7 | ||||
| Age | 0.025 | 0.874 | |||
| ≤60 | 42.2 | ||||
| >60 | 48.8 | ||||
| Size of tumor | 8.552 | 0.003 | |||
| ≤5 | 52.0 | ||||
| >5 | 26.7 | ||||
| Location of tumor | 4.261 | 0.235 | |||
| Lower 1/3 | 52.5 | ||||
| Middle 1/3 | 40.0 | ||||
| Upper 1/3 | 42.1 | ||||
| More than 1/3 | 28.6 | ||||
| Degree of differentiation | 4.774 | 0.029 | |||
| Well/Moderate | 72.7 | ||||
| Poor | 41.5 | ||||
| Lauren’s classification | 0.119 | 0.730 | |||
| Intestinal type | 46.6 | ||||
| Diffuse type | 40.6 | ||||
| Serosal invasion* | 14.947 | 0.002 | 2.764 | 0.007 | |
| T1 | 100.0 | ||||
| T2 | 75.0 | ||||
| T3 | 70.0 | ||||
| T4 | 34.9 | ||||
| Lymph node metastasis* | 32.604 | <0.001 | 1.298 | 0.002 | |
| N0 | 69.2 | ||||
| N1 | 42.3 | ||||
| N2 | 26.3 | ||||
| N3 | 19.0 | ||||
| Vav1 expression | 28.317 | <0.001 | 0.436 | 0.006 | |
| Negative | 64.5 | ||||
| Positive | 16.3 | ||||
7th TNM classification.
Figure 2.
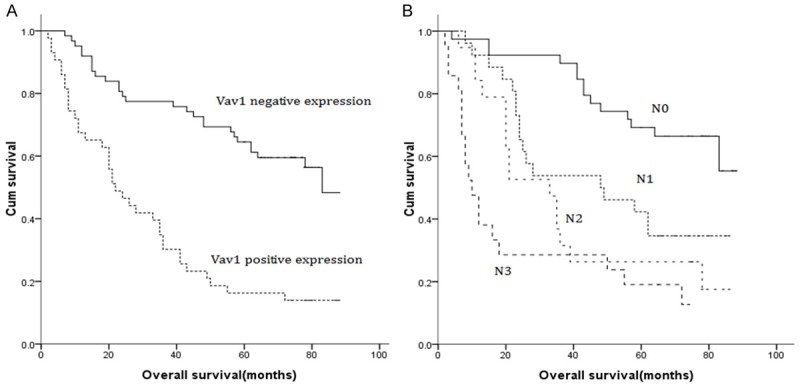
A. Survival curve for GC patients according to Vav1 expression (negative or positive). B. Survival curve for GC patients according to N stage (N0, N1, N2 and N3).
Furthermore, the prognostic value of Vav1 expression in T4N0-3M0 GC patients were examined. The univariate analysis revealed significant relationships between OS and tumor size, degree of differentiation, lymph node metastasis and Vav1 expression. The multivariate Cox regression analysis revealed that lymph node metastasis (HR = 1.310, P = 0.003) and Vav1 expression (HR = 0.443, P = 0.010) were the independent prognostic factors for OS (Figure 3; Table 4).
Figure 3.
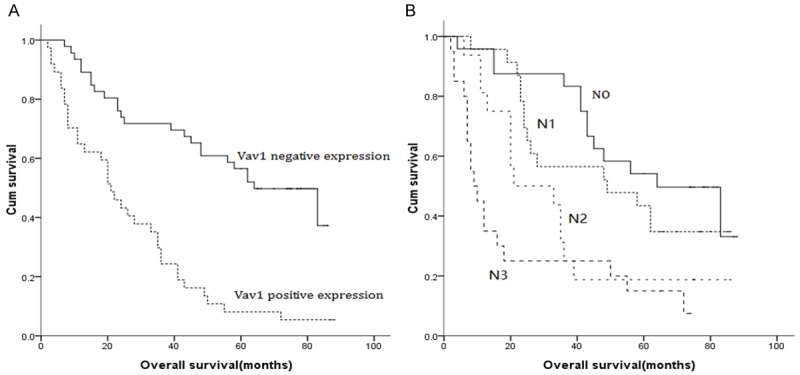
A. Survival curve for T4N0-3M0 GC patients according to Vav1 expression (negative or positive). B. Survival curve for T4N0-3M0 GC patients according to N stage (N0, N1, N2 and N3).
Table 4.
Univariate and multivariate analyses of the overall survival in T4N0-3M0 GC patients
| Variables | 5-YSR (%) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
|
|
|
||||
| Chi-square value | P value | HR value | P value | ||
| Gender | 0.294 | 0.588 | |||
| Male | 37.3 | ||||
| Female | 31.3 | ||||
| Age | 0.023 | 0.879 | |||
| ≤60 | 32.7 | ||||
| >60 | 38.2 | ||||
| Size of tumor | 9.434 | 0.002 | |||
| ≤5 | 42.4 | ||||
| >5 | 16.7 | ||||
| Location of tumor | 4.254 | 0.235 | |||
| Lower 1/3 | 45.2 | ||||
| Middle 1/3 | 38.9 | ||||
| Upper 1/3 | 21.4 | ||||
| More than 1/3 | 33.3 | ||||
| Degree of differentiation | 5.405 | 0.020 | |||
| Well/Moderate | 71.4 | ||||
| Poor | 31.6 | ||||
| Lauren’s classification | 0.007 | 0.935 | |||
| Intestinal type | 35.7 | ||||
| Diffuse type | 33.3 | ||||
| Lymph node metastasis* | 21.349 | <0.001 | 1.310 | 0.003 | |
| N0 | 54.2 | ||||
| N1 | 43.5 | ||||
| N2 | 18.8 | ||||
| N3 | 15.0 | ||||
| Vav1 expression | 25.680 | <0.001 | 0.443 | 0.010 | |
| Negative | 56.5 | ||||
| Positive | 8.1 | ||||
7th TNM classification.
Discussion
Vav1 is a hematopoietic-specific signal transducer that plays important roles in gene transcription, actin cytoskeleton reorganization, and activation and development of immune cells [14]. The expression of Vav1 was first found in hematopoietic system, and has been proven to be involved in normal hematopoietic system development and homeostasis [15]. In recent years, Vav1 was found to be overexpressed in many solid tumors, such as neuroblastomas [16], pancreatic ductal adenocarcinomas [17], primary lung cancer [18], melanomas [19] and ovarian cancers [20]. Hornstein et al. [16] demonstrated there were no rearrangements or mutations in the Vav1 gene in human neuroblastomas cells, and they found the expression level of Vav1 was elevated in the majority of neuroblastoma patients. Qi et al. [13] reported that positive Vav1 expression was correlated with more lymph node metastasis, advanced T stage and poor histological differentiation. Furthermore, they also found that Vav1 expression was an independent prognostic factor for OS and disease-free survival in non-small cell lung cancer. Fernandez et al. [21] found that due to the demethylation of the gene promoter, Vav1 was observed to be highly expressed in most pancreatic cancer cell lines. Furthermore, they were able to prove that patients with positive Vav1 expression had the worse OS, compared with pancreatic cancer patients with negative VAV1 expression. Grassilli et al. [22] reported that in breast cancer, higher levels of Vav1 were positively correlated with the low incidence of relapse, while the Kaplan-Meier curves revealed an elevated risk of distant metastasis in patients with low Vav1 expression levels, compared with patients with high Vav1 expression levels. In early-stage ovarian cancer patients, Wakahashi et al. [20] demonstrated that Vav1 overexpression was associated with an unfavorable prognosis. These findings suggest that Vav1 plays important roles in human cancers. In the present study, we investigated Vav1 expression in GC, and its correlation with the clinicopathological characteristics of patients, including OS.
We first investigated the Vav1 expression data obtained by immunohistochemistry, and analyzed these for correlations with clinicopathological characteristics. In the present study, we found that positive Vav1 expression was associated with the larger size of the primary tumor and greater lymph node metastasis. These results indicate that Vav1 may affect the invasion, metastasis and progression of GC.
In the aspect of the prognostic value of Vav1 expression, the Kaplan-Meier curves prove that patients with positive Vav1 expression presented poorer OS, compared to patients with negative Vav1 expression. Multivariate Cox regression analysis confirmed that Vav1 expression was an independent prognostic factor of GC prognosis. These findings suggest that GC patients with Vav1 overexpression may be a high-risk group with poor survival, and may require systemic therapy after surgery. As it is known, 70% of GC patients are diagnosed as advanced GC and more than 60% of GC patients were accompanied by tumor invaded serosa (visceral peritoneum) or adjacent structures on diagnosis in China [23,24]. We assessed the prognostic value of Vav1 expression in T4N0-3M0 GC, and found that Vav1 expression was the independent prognostic factor of OS for advanced GC patients, based on the multivariate Cox regression analysis. We deduced that Vav1 expression may be a promising prognostic factor of OS in patients with advanced GC.
The present study provides evidences that the expression of Vav1 is correlated to tumor invasion and metastasis. The expression of this protein may serve as a potential prognostic biomarker for GC.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Yang L. Incidence and mortality of gastric cancer in China. World J Gastroenterol. 2006;12:17–20. doi: 10.3748/wjg.v12.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park SR, Park YS, Ryu MH, Ryoo BY, Woo CG, Jung HY, Lee JH, Lee GH, Kang YK. Extra-gain of HER2-positive cases through HER2 reassessment in primary and metastatic sites in advanced gastric cancer with initially HER2-negative primary tumours: results of GASTric cancer HER2 reassessment study 1 (GASTHER1) Eur J Cancer. 2015;53:42–50. doi: 10.1016/j.ejca.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Liu H, Liu Y, Kong F, Xin W, Li X, Liang H, Jia Y. Elevated levels of SET and MYND domain-containing protein 3 are correlated with overexpression of transforming growth factor-β1 in NSCLC. J Am Coll Surg. 2015;221:579–590. doi: 10.1016/j.jamcollsurg.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 5.Helou YA, Petrashen AP, Salomon AR. Vav1 regulates T-cell activation through a feedback mechanism and crosstalk between the T-cell receptor and CD28. J Proteome Res. 2015;14:2963–2975. doi: 10.1021/acs.jproteome.5b00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katzav S, Martin-Zanca D, Barbacid M. Vav, a novel human oncogene derived from a locus ubiquitously expressed in hematopoietic cells. EMBO J. 1989;8:2283–2290. doi: 10.1002/j.1460-2075.1989.tb08354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazer G, Katzav S. Guanine nucleotide exchange factors for RhoGTPases: good therapeutic targets for cancer therapy? Cell Signal. 2011;23:969–979. doi: 10.1016/j.cellsig.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Rapley J, Tybulewicz VL, Rittinger K. Crucial structural role for the PH and C1 domains of the Vav1 exchange factor. EMBO Rep. 2008;9:655–661. doi: 10.1038/embor.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Zapico ME, Gonzalez-Paz NC, Weiss E, Savoy DN, Molina JR, Fonseca R, Smyrk TC, Chari ST, Urrutia R, Billadeau DD. Ectopic expression of VAV1 reveals an unexpected role in pancreatic cancertumorigenesis. Cancer Cell. 2005;7:39–49. doi: 10.1016/j.ccr.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 10.Fischer KD, Kong YY, Nishina H, Tedford K, Marengère LE, Kozieradzki I, Sasaki T, Starr M, Chan G, Gardener S, Nghiem MP, Bouchard D, Barbacid M, Bernstein A, Penninger JM. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr Biol. 1998;8:554–562. doi: 10.1016/s0960-9822(98)70224-6. [DOI] [PubMed] [Google Scholar]
- 11.Lazer G, Idelchuk Y, Schapira V, Pikarsky E, Katzav S. The haematopoietic specific signal transducer Vav1 is aberrantly expressed in lung cancer and plays a role intumourigenesis. J Pathol. 2009;219:25–34. doi: 10.1002/path.2579. [DOI] [PubMed] [Google Scholar]
- 12.Oberley MJ, Wang DS, Yang DT. Vav1 in hematologic neoplasms, a mini review. Am J Blood Res. 2012;2:1–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Qi Y, Kong FM, Deng Q, Li JY, Cui R, Pu YD, Zhai QL, Jia YJ, Li YM. Clinical significance and prognostic value of Vav1 expression in non-small cell lung cancer. Am J Cancer Res. 2015;5:2491–2497. [PMC free article] [PubMed] [Google Scholar]
- 14.Wan YJ, Yang Y, Leng QL, Lan B, Jia HY, Liu YH, Zhang CZ, Cao Y. Vav1 increases Bcl-2 expression by selective activation of Rac2-Akt in leukemia T cells. Cell Signal. 2014;26:2202–2209. doi: 10.1016/j.cellsig.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Horrillo A, Fontela T, Arias-Salgado EG, Llobat D, Porras G, Ayuso MS, González-Manchón C. Generation of mice with conditional ablation of the Cd40lg gene: new insights on the role of CD40L. Transgenic Res. 2014;23:53–66. doi: 10.1007/s11248-013-9743-2. [DOI] [PubMed] [Google Scholar]
- 16.Hornstein I, Pikarsky E, Groysman M, Amir G, Peylan-Ramu N, Katzav S. The haematopoietic specific signal transducer Vav1 is expressed in a subset of human neuroblastomas. J Pathol. 2003;199:526–533. doi: 10.1002/path.1314. [DOI] [PubMed] [Google Scholar]
- 17.Denicola G, Tuveson DA. VAV1: a new target in pancreatic cancer? Cancer Biol Ther. 2005;4:509–511. doi: 10.4161/cbt.4.5.1781. [DOI] [PubMed] [Google Scholar]
- 18.Sebban S, Farago M, Rabinovich S, Lazer G, Idelchuck Y, Ilan L, Pikarsky E, Katzav S. Vav1 promotes lung cancer growth by instigating tumor-microenvironment cross-talk via growth factor secretion. Oncotarget. 2014;5:9214–9226. doi: 10.18632/oncotarget.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartolomé RA, Molina-Ortiz I, Samaniego R, Sánchez-Mateos P, Bustelo XR, Teixidó J. Activation of Vav/Rho GTPase signaling by CXCL12 controls membrane-type matrix metalloproteinase-dependent melanoma cell invasion. Cancer Res. 2006;66:248–258. doi: 10.1158/0008-5472.CAN-05-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wakahashi S, Sudo T, Oka N, Ueno S, Ueno S, Yamaguchi S, Fujiwara K, Ohbayashi C, Nishimura R. VAV1 represses E-cadherin expression through the transactivation of Snail and Slug: a potential mechanism for aberrant epithelial to mesenchymal transition in human epithelial ovarian cancer. Transl Res. 2013;162:181–190. doi: 10.1016/j.trsl.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Zapico ME, Gonzalez-Paz NC, Weiss E, Savoy DN, Molina JR, Fonseca R, Smyrk TC, Chari ST, Urrutia R, Billadeau DD. Ectopic expression of VAV1 reveals an unexpected role in pancreatic cancer tumorigenesis. Cancer Cell. 2005;7:39–49. doi: 10.1016/j.ccr.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 22.Grassilli S, Brugnoli F, Lattanzio R, Rossi C, Perracchio L, Mottolese M, Marchisio M, Palomba M, Nika E, Natali PG, Piantelli M, Capitani S, Bertagnolo V. High nuclear level of Vav1 is a positive prognostic factor in early invasive breast tumors: a role in modulating genes related to the efficiency of metastatic process. Oncotarget. 2014;5:4320–436. doi: 10.18632/oncotarget.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abe N, Watanabe T, Suzuki K, Machida H, Toda H, Nakaya Y, Masaki T, Mori T, Sugiyama M, Atomi Y. Risk factors predictive of lymph node metastasis in depressed early gastric carcinoma. Am J Surg. 2002;183:168–172. doi: 10.1016/s0002-9610(01)00860-1. [DOI] [PubMed] [Google Scholar]
- 24.Deng J, Liang H, Sun D, Pan Y. The prognostic analysis of lymph node-positive gastric carcinoma patients following curative resection. J Surg Res. 2010;161:47–53. doi: 10.1016/j.jss.2008.12.019. [DOI] [PubMed] [Google Scholar]


