Abstract
Background: The expression of miRNA-21 was high in cells that were derived from MSCs, but, the role of miRNA-21in the MSCs was unknown. Material/Methods: In this study, flow cytometry, which was used to identify the surface-associated antigens of MSCs. The 10 μmol/l 5-azacytidine was used to induce MSCs to differentiate to cardiomyocyte-like cells. Immunofluorescence, that was for detected the expression of troponin I (cTnI). The samples were assigned to 3 groups: the blank group, the miRNA-21 mimic group, and the negative control (NC) group. The proliferation of MSCs was detected by methyl thiazolylte-trazolium (MTT), the apoptosis of MSCs was analyzed by flow cytometry, Western-blot, which was used to identify the expression of cTNI and myoD in the MSCs. Results: The proliferation of MSCs was increased, because of the over expression of miRNA-21; But, the apoptotic rate of the MSCs were slower in MIRNA-21 group, on account of the expression of miRNA-21 was higher than that of in the NC and CK groups. The expression of cTNI in miRNA-21 group was higher than that of in the NC and CK groups. Conclusions: The results also suggested that, the up-regulation of miRNA-21 enhanced proliferation of MSCs, reducing the apoptosis of MSCs. MiRNA-21 promotes the differentiation of MSCs, which may pave the way for the treatment directed toward restoring miRNA-21 function for myocardial ischemia.
Keywords: MiRNA-21, mesenchymal stem cells, proliferation, apoptosis, differentiation
Introduction
The bone marrow-derived cells, mesenchymal stem cells (MSCs) were confirmed to differentiate into cells, such as: osteoblasts, chondrocytes, adipocytes, cardiomyocyte-like cells and et al. [1-3]. MSCs could secrete vascular endothelial growth factor (VEGF) and other factors [4]. MSCs not only could differentiate into cardiomyocyte-like cells, but also supply large amounts of angiogenic and other factors. So, MSCs have the cardioprotective effects in the treatment of myocardial ischemia [5-7].
MicroRNAs (miRNAs) are a class of non-coding small RNA (21-25 nucleotides) involved in regulation of cell behavior either through inhibition of mRNA translation or promoting mRNA degradation [8]. MiRNAs are important regulators in diverse biological processes not only in cell development, proliferation, differentiation but also in controlling the gene expression [10-12]. Recent reviews reported the role of microRNAs in mesenchymal stem cell differentiation [9,13-16]. It is also known that transient modulation of microRNAs is useful for rapid induction of osteogenesis from mesenchymal stem cells and it is a useful scheme for cell based therapy [17]. So, all the above information suggested that microRNAs are involved in stem cells maintenance and differentiation.
MiRNA-21 has been mapped at 17q23.2, where it overlaps with the gene encoding vacuole membrane protein 1 (VMP1) [11]. Some reports that miRNA-21 has an important role in MSCs Derived Osteoblast Cells, Chondrocytes Cells and Adipocytes Cells. But the number of studies related to the role of miRNA-21 in MSCs is still very limited [8,18,19], especially, in the process of MSCs differentiation into cardiomyocyte-like cells. So, we wanted to survey the up-regulation of miRNA-21 affect to MSCs.
Materials and methods
Animals
The Clean Sprague-Dawley (SD) rats were weighing between 250 to 300 g. The rats came from the Experimental Animal Center of Shanghai Jiaotong University School of Medicine, Shanghai, China (production license No: scxk (hu) 2004-0001; using license No. syxk (hu) 2003-2009). The present study was reviewed and approved by the University Institutional Animal Care and Use Committee, the animals were as the same as in our previous study [42].
Reagents
DMEM (Low-glucose Dulbecco’s modified Eagle’s medium) and FBS (fetal bovine serum) came from Hyclone, MTT (Thiazolyl blue) was obtained from Sigma-Aldrich Chemical Corporation, two dimethyl sulfoxide (DMSO) was obtained from Gibco, The cardiac-specific anti-bodies (cTNI), CD45 was FITC-conjugated goat anti-rat antibodies, CD90 was PE-conjugated rabbit anti-rat antibodies and CD29 was APC-conjugated rabbit anti-rat antibodies, they were purchased from Abcam. PGMLV-MA2 Expression Vector kit with EmGFP and other correlating reagents were purchased from Qiagen. In the study, all of the reagents and instruments for immunofluorescence, western blot analysis, MTT, Construction of the expression lentivirus, flow cytometry and Immunofluorescence were purchased from Gibco, Invitrogen, Fermentas, Thermo Scientific and Sigma-Aldrich Chemical Corporation, respectively.
Cell culture
Bone marrow cells were isolated from the long bones (tibia and femur) of SD rats and cultured in DMEM containing 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin for 24 h to generate BMSCs. BMSCs were harvested using 0.125% trypsin and plated at 1:3. BMSCs were cultured on tissue culture plastic or coverslips in DMEM for 24 h with 10 µmol/l 5-azacytidine. Then, the culture medium was replaced every 2 days.
In order to keep the cell surface molecules better, the cells were detached using accutase for flow cytometry.
Labeling of MSCs
Before the MSCs with DAPI were harvested, in the culture medium of MSCs, the DAPI solution was added, at a final concentration of 50 mg/ml for 30 min [20], then, the excess unbound DAPI must to be removed.
Flow cytometry
Briefly, 3×105 cells/mL were treated with LCB (20, 40 and 80 µM) for 48 h, washed twice with ice-cold PBS, then stained by 5 µL of CD45 monoclonal antibody, CD90 monoclonal antibody and CD29 monoclonal antibody and 5 µL of propidium iodide (PI) for 10 min. Then, the data were analyzed using Summit software (Cytomation, Inc, Fort Collins, CO, USA); the whole process was performed using a flow cytometer (BD, NJ, USA).
Immunofluorescence microscopy
The MSCs were fixed in 4% formal-dehyde for a 20 min incubation, permeabilised for 10 min in 0.3% Triton X-100, blocked in 5% donkey serum at room temperature for 30 min, and probed with the primary antibodies diluted in 5% donkey serum in PBS for 2 h at room temperature (RT) or overnight at 4°C. Then, the cells were incubated with the secondary antibody (the anti-bodies for cTNI) for 30 min. Finally, coverslips were sealed with nail polish. Immunofluorescent stains were analyzed at high resolution with a Zeiss laser scanning confocal microscope, LSM-780. Z-stacks of images were processed and 3D-reconstructed with Imaris software (version 7.00, Bitplane). Imaris, Photoshop and Illustrator (Adobe) software was used for image processing in compliance with Nature’s guide for digital images. All quantifications were done with ImageJ, Imaris and Volocity software on high-resolution confocal images.
Western blot analysis
Proteins were separated on a polyacrylamide gel before transfer to a PVDF membrane. The blotting membrane was blocked with bovine serum albumin and incubated with primary antibodies followed by incubation with HRP-coupled goat anti-rabbit IgG H&L (1:5000, Abcam, ab6721) or HRP-coupled rabbit anti-goat IgG H&L (1:5000, Abcam, ab6741), respectively. The proteins were detected using SuperSignal® West Dura Extended Duration Substrate (Thermo Scientific, Prod # 34075).
Construction of the expression lentivirus vector of miRNA-21
The pre-miRNA-21sequence was according to the miRBase accession no. MI0000850. The corresponding shRNA primer sequence was as follows: 4145hsa-miR-21-F (XhoI): CCGCTCGAG TTGTTTTGCTTGGGAGGAAAATAAACA; and 4145hsa-miR-21-R (BamHI): CCG GGATCC CAATGCAGCTTAGTTTTCCTTTATTTATTTGTG. The corresponding restriction enzymes digest the XhoI and BamHI. The Vector Cloning kit was used for restructuring; the shRNA oligonucleotide was inserted into the shRNA expression vector (Invitrogen), and the cells were transfected with plasmids infected with E. coli DH5. The oligonucleotides were annealed and cloned into the PGMLV-MA2 vector (LV-miRNA-21).
Transfection
The protocol was the same as in our previous study [42]. As a ratio of 1:5, a plasmid expressing GFP together with a plasmid expressing shRNA specific for GFP co-transfected the 293T cells, with FuGENE® transfection reagent.
Lentiviral vector production
The protocol was the same as in our previous study [42].
The 293T cells were transfected by using the calcium-phosphate method [21-23]. Then recombinant lentiviruses were produced. After 48 and 72 h, we got the infectious lentiviruses [21-23]. Through ultracentrifugation (2 h at 50,000×g) and subsequently purified on a sucrose 20% gradient (2 h at 46,000×g), recombinant lentiviruses were concentrated [16]. Vector concentrations were analyzed as previously described [24]; the activity of the viral titer was measured (5×108 TU/ml).
Transfection of MSCs with LV-miRNA-21
The MSCs were successfully transfected with LV-miRNA-21 or LV-GFP.
MTT cell proliferation assay
The protocols for MTT assays were as previously described [25]. Each well was added with medium containing 0.25 mg/ml MTT. Cells were incubated at 37°C for 20 min; following, 0.2 ml DMSO/well taken the place of the medium. Then, the MTT dye conversion was determined, and using DMSO as the blank control.
Detection of MSCs apoptosis by flow cytometry
Apoptosis analysis was conducted by flow cytometry using the Annexin V-FITC double staining. Briefly, 3×105 cells/mL were treated with LCB (20, 40 and 80 µM) for 48 h. Afterwards, cells were washed twice with ice-cold PBS, and then stained by 5 µL of Annexin V-FITC and 5 µL of propidium iodide (PI) for 10 min. The stained cells were analyzed using a flow cytometer (BD, NJ, USA).
Statistical analysis
Images were analyzed by image programmer software. Statistical analyses were performed using paired t-tests and the means ± standard deviation (SD), using SPSS and GraphPad Prism 5 Demo software.
Results
Characterization of MSCs
The MSCs present a small population of single cells, were spindle-shaped with a single nucleus after 3 days in primary culture (Figure 1A). The cells resembled long spindle-shaped fibroblast cells and began to form colonies after 7 to 10 days (Figure 1B and 1C).
Figure 1.
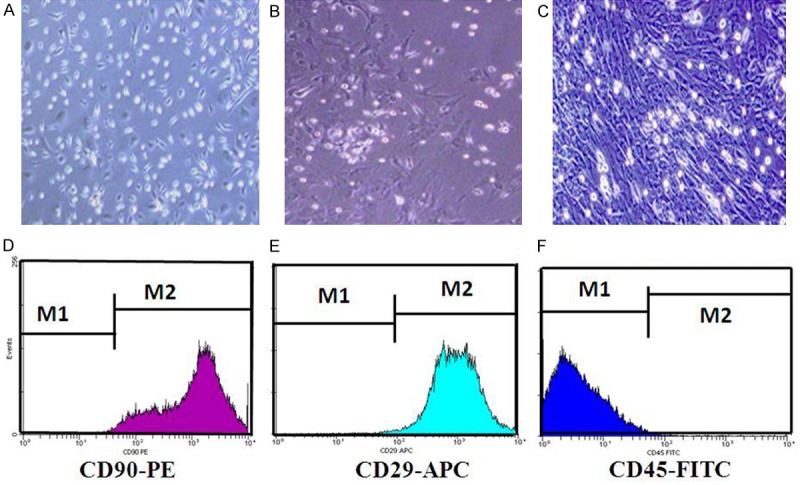
Characterization of Mesenchymal stem cells (MSCs) culturing in vitro. (A) Morphological observation of rat MSCs after culturation for 3 d 100×. The cells were spindle shaped with one nucleus. (B) Morphological observation of rat MSCs after culturation for 7 d 100×. (C) Morphological observation of rat MSCs after culturation for 10 d 100×. The cells looked like long spindle-shaped fibroblastic cells and began to form colonies. Respectively, (D-F) were behalf of surface-associated antigens (CD90, CD29 and CD45) of MSCs were dedected by flow cytometry. Surface-associated antigens of MSCs were positive for CD90 (D), CD29 (E), and negative for CD45 (F). The percentage of CD90 and CD29 is about 99%. But, the percentage of CD45 is only about 1%.
The surface antigen [31], such as CD90 (Figure 1D) and CD29 (Figure 1E) were positive in MSCs and CD45 was negative (Figure 1F).
We saw that the morphology of MSCs were changed after induced by 5-aza, the cells were be enlarged in volume, and increased in quantity; showing a long spindle shape. After 14 days, the cells connect to each other, arrayed trend to uniformity. This Characterization of MSCs in MIRNA-21 group was more significantly than its in NC (control) group and CK (normal) group (Figure 2).
Figure 2.
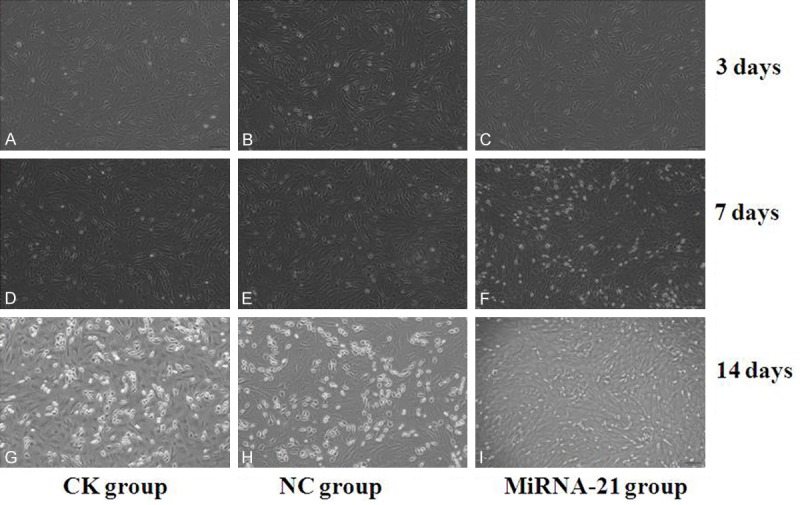
Characterization of Mesenchymal stem cells (MSCs) induced by 5-azacytidine in vitro. MSCs of (A-C) were culturing for 3 days after treatment with 5-azacytidine; MSCs of (D-F) were culturing for 7 days after treatment with 5-azacytidine; MSCs of (G-I) were culturing for 14 days after treatment with 5-azacytidine. (A, D, G) were in CK (normal) group; (B, E, H) were in NC (control) group; (C, F, I) were in miRNA-21 group. (D-F) Morphological observation of rat MSCs after been treated by 5-azacytidine for 7 d 100×. The cells had enlarged and had assumed ball-like or stick-like morphologies. (G-I) Morphological observation of rat MSCs after treated by 5-azacytidine for 14 d 100×. 2 weeks later, the cells connected with adjoining cells. The cells in miRNA-21 group were enlarger in volume, and more much in quantity than the cells in NC (control) group and CK (normal) group. This characterization of MSCs in MIRNA-21group was more significantly than its in NC group and CK group.
MiRNA-21 accelerated the proliferation of MSCs
The 3 groups of cells (MIRNA-21, CK and NC group) were cultured for 1, 2, and 3 weeks. MTT assay, which used to detected the proliferation of the cells at the end of 1, 2, and 3 weeks. As shown in Figure 3, the proliferation of MSCs was increasing because of the over expression of miRNA-21; however, the proliferation of the cells in the NC group and the normal group were not altered and similar.
Figure 3.
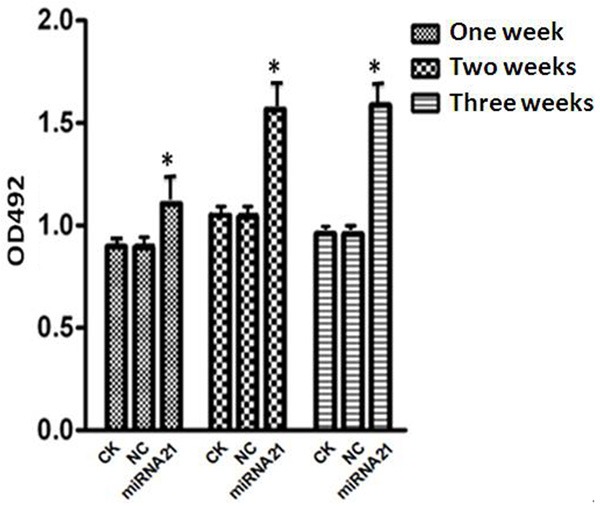
Cell proliferation was detected by MTT assay: Cell proliferation was detected by MTT assay at the end of 1, 2 and 3 week. When the 3 groups of cells (NC, CK and MIRNA-21 group) were cultured for 1, 2 and 3 weeks under the same conditions, the proliferation of the cells in the MIRNA-21 group was increased compared with the other 2 groups; this difference was statistically significant (*P=0.025; P<0.05).
MiRNA-21 slowed the apoptosis of MSCs
The 3 groups of cells (MIRNA-21, CK and NC group) were cultured for 1, 2, and 3 weeks. The apoptosis of MSCs was detected by flow cytometry. The apoptotic rate of the cells in the MIRNA-21 group was slower than that of the cells in the NC and CK groups (Figure 4).
Figure 4.
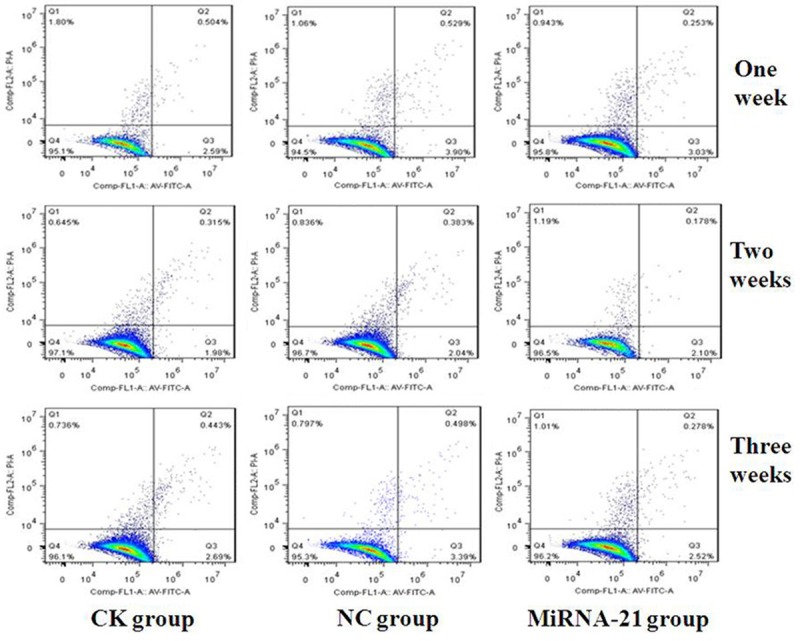
Cell apoptosis of mesenchymal stem cells (MSCs) in the 3 groups (NC, CK and MIRNA-21 group) detected by flow cytometry. The 3 groups of cells (NC, CK and MIRNA-21 group) were cultured for 1, 2 and 3 weeks under the same conditions. The cell cycle was analyzed by flow cytometry. The apoptotic rate of the cells in the MIRNA-21 group was slower than that of cells in NC and CK group; the percentage of apoptotic cells in the MIRNA-21 group was approximately 3.094±0.050% at 1 week, 2.278±0.045% at 2 weeks, 2.125±0.035% at 3 weeks. The percentage of apoptotic cells in the NC was approximately 4.429±0.027% at 1 week, 2.425±0.045% at 2 weeks, 3.888±0.019% at 3 weeks; and the percentage of apoptotic cells CK group was approximately 3.383±0.025% at 1 week, 2.395±0.023% at 2 weeks, 3.133±0.013% at 3 weeks, respectively. The difference between the MIRNA-21 group and the NC and CK groups was statistically significant (P=0.031, P=0.020, respectively; P<0.05).
MiRNA-21 improved the MSCs differentiation into cardiomyocyte-like cells
Immunofluorescence assays was used to detected cTnI and CD90 after 1, 2 and 3 weeks following MSC exposure to 5-azacytidine in vitro. The cTnI was indicated after MSCs were induced to differentiation into cardiomyocyte-like cells by 10 µmol/l 5-azacytidine. But the expression of CD90, the special surface antigen of stem cells, was null along with the expression of cTNI that was more and more. The expression of cTNI in miRNA-21 group was higher than that of in the NC and CK groups (Figures 5, 6 and 7). This to say that MiRNA-21 improved the MSCs differentiation into cardiomyocyte-like cells. And after MSCs differentiating into cardiomyocyte-like cells, MSCs lost its Characterization of stem cell.
Figure 5.
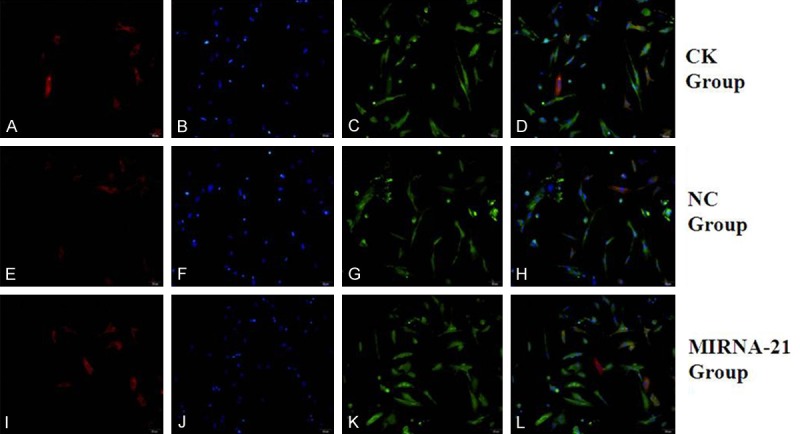
Expression of cardiac structural proteins (aTNI) and CD90 by MSCs after treatment with 5-azacytidine for 1 week in vitro, determined by immunofluorescence. (A, E, I) Positive staining for cTnI protein of MSCs (red fluorescence; magnification, ×200). (C, G, K) Positive staining for CD90 of MSCs (green fluorescence; magnification, ×200). (B, F, J) DAPI-labeled nuclei of MSCs (blue fluorescence; magnification, ×200). (D) Merged image of (A, C); (H) Merged image of (E, G); (L) Merged image of (I, K) (magnification, ×200). (A-D) in CK group; (E-H) in NC group; (I-L) in MIRNA-21 group. MSCs, mesenchymal stem cells; cTnI, cardiac troponin I.
Figure 6.
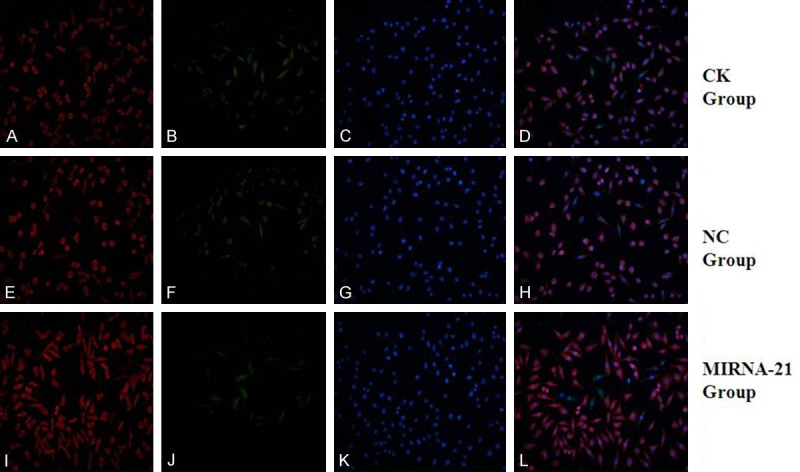
Expression of cardiac structural proteins (aTNI) and CD90 by MSCs after treatment with 5-azacytidine for 2 weeks in vitro, determined by immunofluorescence. (A, E, I) Positive staining for cTnI protein of MSCs (red fluorescence; magnification, ×200). (C, G, K) Positive staining for CD90 of MSCs (green fluorescence; magnification, ×200). (B, F, J) DAPI-labeled nuclei of MSCs (blue fluorescence; magnification, ×200). (D) Merged image of (A, C); (H) Merged image of (E, G); (L) Merged image of (I, K) (magnification, ×200). (A-D) in CK group; (E-H) in NC group; (I-L) in MIRNA-21 group. MSCs, mesenchymal stem cells; cTnI, cardiac troponin I.
Figure 7.
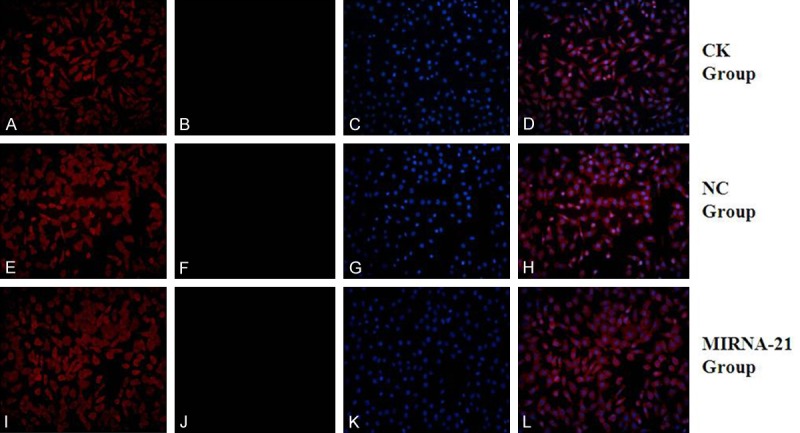
Expression of cardiac structural proteins (aTNI) and CD90 by MSCs after treatment with 5-azacytidine for 3 weeks in vitro, determined by immunofluorescence. (A, E, I) Positive staining for cTnI protein of MSCs (red fluorescence; magnification, ×200). (C, G, K) Positive staining for CD90 of MSCs (green fluorescence; magnification, ×200). (B, F, J) DAPI-labeled nuclei of MSCs (blue fluorescence; magnification, ×200). (D) Merged image of (A, C); (H) Merged image of (E, G); (L) Merged image of (I, J) (magnification, ×200). (A-D) in CK group; (E-H) in NC group; (I-L) in MIRNA-21 group. MSCs, mesenchymal stem cells; cTnI, cardiac troponin I.
Western blot analysis for indenting the expression of cTNI and myOD in the MSCs. The expression of cTNI in miRNA-21 group was higher than that of in the NC and CK groups (Figure 8). As the cells were cultured in vitro for 1, 2 and 3 weeks, the expression of cTNI in miRNA-21 group was more and more higher than that of in the NC and CK groups (Figure 8); But, the expression of myOD in each group was slower and slower, especially, the expression of myOD in miRNA-21 group was notably slower than that of in the NC and CK groups. So, we can say that MSCs differentiate into cardiomyocyte-like cells and muscle cells, but, most of them differentiate into cardiomyocyte-like cells. After two weeks later, under effects of miRNA-21, MSCs differentiate into cardiomyocyte-like cells, there were majority of expression of cTNI. After three weeks later, under effects of miRNA-21, the only expression of cTNI in the process of MSCs differentiation. In that, miRNA-21 stimulated the MSCs differentiation into cardiomyocyte-like cells.
Figure 8.
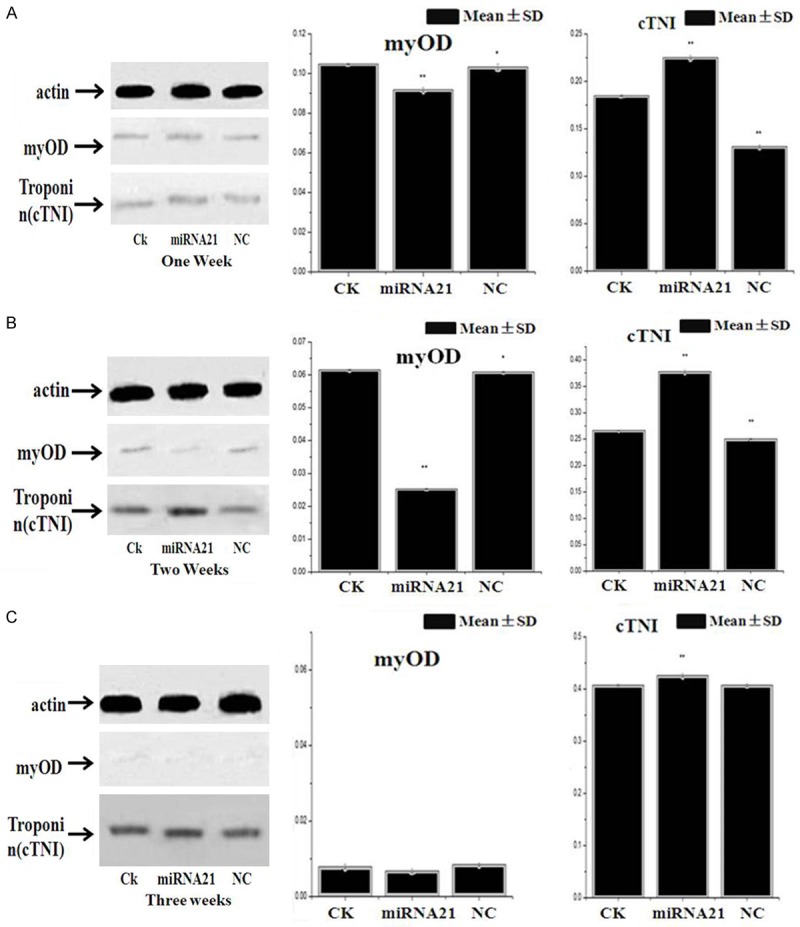
Expression levels of cTNI and myOD protein detected by western blot analysis. A. The expression level of cTNI protein in MIRNA-21 group was higher than that of in NC and CK group after 1 week later; But, the expression level of myOD protein was lower than that of in NC and CK group (*P<0.05). B. The expression level of cTNI protein in MIRNA-21 group was higher than that of in NC and CK group after 2 weeks later; But, the expression level of myOD protein was lower than that of in NC and CK group (*P<0.05). C. The expression level of cTNI protein in MIRNA-21 group was higher than that of in NC and CK group after 3 weeks later; But, the expression level of myOD protein was lower than that of in NC and CK group (*P<0.05). From one week to three weeks, we could see that the expression level of myOD protein was lower and lower, then to null; But, the epression levels of cTNI protein was higher and higher.
Discussion
The first, Friedenstein described the MSCs in 1968 [26]. MSCs were reported that be able to proliferate and differentiate in vitro [27-29]. However, the ratio of MSCs in bone-marrow is only 0.001-0.01%. Ficoll density gradient separation, the first time used by Wakitani et al. [30] to isolate MSCs from rat bone marrow. MSCs could differentiate into cardiomyocyte lineages in vivo and in vitro [32-34]. These MSCs could be developed into cardiomyocyte-like cells [35-37]. In the present study, we use immunofluorescent staining and western blot detected the expression of cTnI in cardiomyocyte-like cells.
The effect of miRNA-21 following MSCs differentiation was examined, because that the mechanisms of cell-based therapies have remained to be elucidated, and the ratio of MSCs in bone-marrow is very low.
MiRNAs are a class of small non-coding RNAs whose mature products are 22 nucleotides long miRNAs; They negatively regulate gene expression by inducing translational inhibition or transcript degradation. MiRNAs are important regulators in all sorts of biological processes including cell development, proliferation, differentiation and controlling the gene expression [10-12]. Recently, many reports also have explored the role of microRNAs in mesenchymal stem cell differentiation [9,13-16]. It is known that transient modulation of microRNAs is useful for induction of osteogenesis from mesenchymal stem cells and it is a useful scheme for cell based therapy [17]. So, recent, the information suggested that microRNAs are involved in stem cells maintenance and differentiation.
MiRNA-21 has been mapped to chromosome 17q23.2, where it overlaps with the protein-coding gene VMP1 (or TMEM49). Recent studies demonstrated that the expression of genes encoding several tumour suppressor proteins such as phosphatase and tensin homolog on chromosome ten (PTEN), tumour suppressor gene tropomyosin 1 (TPM1), maspin, programmed cell death 4 (PDCD4), matrix metalloproteinases inhibitors RECK and TIMP3 are targeted by miRNA-21. It has been known that miRNA-21 is involved in differentiation of many types of MSCs derived cells, but the number of studies related to the role of miRNA-21 in MSCs is still very limited [8,18,19]. In this study, we discovered the effect of miRNA-21 in the process of MSCs differentiating into the cardiomyocyte-like cells.
By targeting the mRNA encoding sprouty homolog 1 (Spry1) which negatively regulates the osteogenic differentiation of MSCs. The differentiation of MSCs to osteoblast was promoted by miRNA-21 [38]. Thus the above report suggested that miRNA-21 can be used for osteoporosis and other inflammatory bone diseases as novel therapeutic strategies [38]. Besides, during the first 4 days of adipogenesis and osteogenesis, up-regulation of miRNA-21 resulted in the activity of ERK-MAPK signaling pathway increased and its downregulation of miRNANA-21 decreased the pathway. The report showing that miRNA-21 is involved in various pathological and physiological processes especially on determining the fate of stem cell [19]. The growth differentiation factor 5 (GDF-5) was identified as the direct target for miRNA-21 during the regulation of chondrogenesis [39]. MiRNA-21 plays a vital role in human adipose-derived mesenchymal stem cells (hADSCs), which can be used as therapeutic tools in regenerative medicine and tumour biology [40]. Since miRNA-21 is up-regulated in many types of MSCs derived stem cells, their unique molecular signatures can be used as prognosis and therapeutic targets. Consistent evidence suggested that miRNA-21 is an important oncogene, which acts as a significant part in the regulation of stem cell behavior.
In our study, we also discovered that miRNA-21 accelerated the proliferation of MSCs, slowed down the apoptosis of MSCs and enhanced the MSCs differentiating into the cardiomyocyte-like cells. It has been revealed that overexpression of miRNA-21 in hADSCs inhibits the tumour growth and contrastingly, the inhibition of miRNA-21 increases the tumour growth in the same cells [40]. The finding suggests that miRNA-21 plays a novel and important role in mesenchymal stem cells [41]. So, we can conclude that miRNA-21 considered to be as a powerful biomarker which is directly used as a therapeutic target. But, more investigation is still required.
Acknowledgements
This study was supported by a grant from the Fund of the School Medicine, Shanghai Jiao Tong University, Shanghai, China (no. 13XJ10014).
Disclosure of conflict of interest
None.
References
- 1.Beyer Nardi N, da Silva Meirelles L. Mesenchymal stem cells: isolation, in vitro expansion and characterization. Handb Exp Pharmacol. 2006;174:249–282. [PubMed] [Google Scholar]
- 2.Brighton CT, Hunt RM. Early histological and ultrastructural changes in medullary fracture callus. J Bone Joint Surg Am. 1991;73:832–847. [PubMed] [Google Scholar]
- 3.Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med (Maywood) 2001;226:507–520. doi: 10.1177/153537020122600603. [DOI] [PubMed] [Google Scholar]
- 4.Sensebé L, Krampera M, Schrezenmeier H, Bourin P, Giordano R. Mesenchymal stem cells for clinical application. Vox Sanguinis. 2010;98:93–107. doi: 10.1111/j.1423-0410.2009.01227.x. [DOI] [PubMed] [Google Scholar]
- 5.Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M, Yamagishi M, Mori H, Kangawa K, Kitamura S. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogen-esis. Am J Physiol Heart Circ Physiol. 2004;287:H2670–H2676. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- 6.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, Kitamura S, Mori H. Monolayered mesen-chymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 7.Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, Yamagishi M, Tokudome T, Mori H, Miyatake K, Kitamura S. Transplantation of mesen-chymal stem cells improves cardiac function in a rat model ofdilated cardiomyopathy. Circulation. 2005;112:1128–1135. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 8.Eguchi T, Watanabe K, Hara ES, Ono M, Kuboki T, Calderwood SK. OstemiR: a novel panel of microRNA biomarkers in osteoblastic and osteocytic differentiation from mesencymal stem cells. PLoS One. 2013;8:e58796. doi: 10.1371/journal.pone.0058796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fakhry M, Hamade E, Badran B, Buchet R, Magne D. Molecular mechanisms of mesenchymal stem cell differentiation towards osteoblasts. World J Stem Cells. 2013;5:136–148. doi: 10.4252/wjsc.v5.i4.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lakshmipathy U, Hart RP. Concise review: microRNA expression in multipotent mesenchymal stromal cells. Stem Cells. 2008;26:356–363. doi: 10.1634/stemcells.2007-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sekar D, Hairul Islam VI, Thirugnanasambantham K, Saravanan S. Relevance of miR-21 in HIV and non-HIV-related lymphomas. Tumour Biol. 2014;35:8387–8393. doi: 10.1007/s13277-014-2068-9. [DOI] [PubMed] [Google Scholar]
- 12.Mathieu J, Ruohola-Baker H. Regulation of stem cell populations by microRNAs. Adv Exp Med Biol. 2013;786:329–351. doi: 10.1007/978-94-007-6621-1_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou DD, Tang XB. Epithelial-mesenchymal transition and cancer stem cells. Zhonghua Bing Li Xue Za Zhi. 2013;42:62–65. doi: 10.3760/cma.j.issn.0529-5807.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 14.Zhang R, Wang D, Xia Z, Chen C, Cheng P, Xie H, Luo X. The role of microRNAs in adipocyte differentiation. Front Med. 2013;7:223–230. doi: 10.1007/s11684-013-0252-8. [DOI] [PubMed] [Google Scholar]
- 15.Ceppi P, Peter ME. MicroRNAs regulate both epithelial-to mesenchymal transition and cancer stem cells. Oncogene. 2014;33:269–278. doi: 10.1038/onc.2013.55. [DOI] [PubMed] [Google Scholar]
- 16.Kang HY. MicroRNA-21 regulates stemness in cancer cells. Stem Cell Res Ther. 2013;4:110. doi: 10.1186/scrt321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakhshandeh B, Hafizi M, Ghaemi N, Soleimani M. Downregulation of miRNA-221 triggers osteogenic differentiation in human stem cells. Biotechnol Lett. 2012;34:1579–1587. doi: 10.1007/s10529-012-0934-3. [DOI] [PubMed] [Google Scholar]
- 18.Trohatou O, Zagoura D, Bitsika V, Pappa KI, Antsaklis A, Anagnou NP, Roubelakis MG. Sox2 suppression by miR-21governs human mesenchymal stem cell properties. Stem Cells Transl Med. 2014;3:54–68. doi: 10.5966/sctm.2013-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mei Y, Bian C, Li J, Du Z, Zhou H, Yang Z, Zhao RC. miR-21 modulates the ERK-MAPK signaling pathway by regulating SPRY2 expression during human mesenchymal stem cell differentiation. J Cell Biochem. 2013;114:1374–1384. doi: 10.1002/jcb.24479. [DOI] [PubMed] [Google Scholar]
- 20.Chedrawy EG, Wang JS, Nguyen DM, Shum-Tim D, Chiu RC. Incorporation and integration of implanted myogenic and stem cells into native myocardial fibers: anatomic basis for functional mprovements. J Thorac Cardiovasc Surg. 2002;124:584–590. doi: 10.1067/mtc.2002.122544. [DOI] [PubMed] [Google Scholar]
- 21.Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 22.Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guenechea G, Gan OI, Inamitsu T, Dorrell C, Pereira DS, Kelly M, Naldini L, Dick JE. Transduction of human CD34+ CD38- bone marrow and cord blood-derived SCID-repopulating cells with third-generation lentiviral vectors. Mol Ther. 2000;1:566–573. doi: 10.1006/mthe.2000.0077. [DOI] [PubMed] [Google Scholar]
- 24.Naldini L, Blömer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci U S A. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park C, Moon DO, Choi IW, Choi BT, Nam TJ, Rhu CH, Kwon TK, Lee WH, Kim GY, Choi YH. Curcumin induces apoptosis and inhibits prostaglandin E2 production in synovial fibroblasts of patients with rheumatoid arthritis. Int J Mol Med. 2007;20:365–372. [PubMed] [Google Scholar]
- 26.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic transplants of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. [PubMed] [Google Scholar]
- 27.Dennis JE, Charbord P. Origin and differentiation of human and murine stroma. Stem Cells. 2002;20:205–214. doi: 10.1634/stemcells.20-3-205. [DOI] [PubMed] [Google Scholar]
- 28.Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 29.Martin DR, Cox NR, Hathcock TL, Niemeyer GP, Baker HJ. Isolation and characterization of multipotential mesenchymal stem cells from feline bone marrow. Exp Hematol. 2002;30:879–886. doi: 10.1016/s0301-472x(02)00864-0. [DOI] [PubMed] [Google Scholar]
- 30.Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 31.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. TheInternational Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 32.Xu W, Zhang X, Qian H, Zhu W, Sun X, Hu J, Zhou H, Chen Y. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp Biol Med (Maywood) 2004;229:623–631. doi: 10.1177/153537020422900706. [DOI] [PubMed] [Google Scholar]
- 33.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 35.Yoon J, Min BG, Kim YH, Shim WJ, Ro YM, Lim DS. Differentiation, engraftment and functional effects of pre-treated mesenchymal stem cells in a rat myocardial infarct model. Acta Cardiol. 2005;60:277–284. doi: 10.2143/AC.60.3.2005005. [DOI] [PubMed] [Google Scholar]
- 36.Xie XJ, Wang JA, Cao J, Zhong X. Differentiation of bone marrow mesenchymal stem cells induced by myocardial medium under hypoxic conditions. Acta Pharmacol Sin. 2006;27:1153–1158. doi: 10.1111/j.1745-7254.2006.00436.x. [DOI] [PubMed] [Google Scholar]
- 37.Forte G, Minieri M, Cossa P, Antenucci D, Sala M, Gnocchi V, Fiaccavento R, Carotenuto F, De Vito P, Baldini PM, Prat M, Di Nardo P. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells. 2006;24:23–33. doi: 10.1634/stemcells.2004-0176. [DOI] [PubMed] [Google Scholar]
- 38.Yang N, Wang G, Hu C, Shi Y, Liao L, Shi S, Cai Y, Cheng S, Wang X, Liu Y, Tang L, Ding Y, Jin Y. Tumor necrosis factor alpha suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiency-induced osteoporosis. J Bone Miner Res. 2013;28:559–573. doi: 10.1002/jbmr.1798. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Jia J, Yang S, Liu X, Ye S, Tian H. MicroRNA-21 controls the development of osteoarthritis by targeting GDF-5 in chondrocytes. Exp Mol Med. 2014;46:e79. doi: 10.1038/emm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin KK, Lee AL, Kim JY, Lee SY, Bae YC, Jung JS. miR-21 modulates tumor outgrowth induced by human adipose tissue-derived mesenchymal stem cells in vivo. Biochem Biophys Res Commun. 2012;422:633–638. doi: 10.1016/j.bbrc.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 41.Sekar D, Saravanan S, Karikalan K, Thirugnanasambantham K, Lalitha P, Islam VI. Role of MicroRNA 21 in mesenchymal stem cell (MSC) differentiation: a powerful biomarker in mscs derived cells. Curr Pharm Biotechnol. 2015;16:43–48. doi: 10.2174/138920101601150105100851. [DOI] [PubMed] [Google Scholar]
- 42.Yang W, Zheng H, Wang Y, Lian F, Hu Z, Xue S. Nesprin-1 plays an important role in the proliferation and apoptosis of mesenchymal stem cells. Int J Mol Med. 2013;32:805–812. doi: 10.3892/ijmm.2013.1445. [DOI] [PubMed] [Google Scholar]


