Abstract
Objective: miRNAs, altered expression manner in prostate cancer, have involved in cancer development by regulating proliferation, differentiation, invasion, metabolism and apoptosis. Recently, it has been found that miRNA-627 might mediate colorectal cancer cells as a tumor-suppressor. However, its role on prostate cancer cells remains unknown. Methods: Following transfection with miRNA-627, miRNA-627 expression, cell proliferation, cell cycle, cell apoptosis, migration assay, and real-time PCR analysis were performed in prostate cancer cells. Results: It was found that miRNA-627 inhibited cell proliferation, retarded cell cycle and cell migration in prostate cancer cells. Additionally, miRNA-627 promoted cell apoptosis and upregulated the mRNA levels of MAP3K1, PTPRK and SRA1. Conclusion: Collectively, miRNA-627 interrupts prostate cancer development as suppressing cell proliferation, migration and promoting cell apoptosis and tumor suppressor genes expression, which might provide promising therapeutic effect in the treatment of prostate cancer.
Keywords: miRNA-627, proliferation, migration, apoptosis, prostate cancer cells
Introduction
Prostate cancer is one of the most common tumors for elderly male. There is still lack of effective treatment for prostate cancer [1,2]. The pathogenesis of prostate cancer is still unclear. It has been shown that miRNAs are closely related to the occurrence and development of prostate cancer [3,4]. Expression levels of a variety of miRNAs are different in the non-malignant and prostate cancer tissues; there are also different miRNAs expression manners between localized prostate cancer and metastatic prostate cancer [5-7]. Therefore, miRNAs are expected to become important targets for prostate cancer treatment [8]. However, which specific miRNA associated with the occurrence and development of prostate cancer remains unknown.
It is found that miRNAs are participated in the regulation of cell activity and biological processes through mediating the expression of encoded protein [9]. This regulating effect of miRNAs is not a one-to-one relationship; single miRNA can control multiple target genes and then result in different functional changes. Some specific miRNAs are found that involved in the regulation of cancer cell activities including differentiation, proliferation, apoptosis, and metastasis, which can be used as a specific signs and benefit to the clinical diagnosis, treatment and prognosis of tumors [9]. In recent years, numerous studies have found that a variety of miRNAs are abnormally expressed in various kinds of prostate cancer tissue and the prostate cancer cells [10-13]. Sathish K.R. Padi and the colleagues found that miRNA-627 was lowly expressed in colorectal cancer cells, which contributed to the anticancer activities of calcitriol in colon cancer by targeting histone demethylase JMJD1A [14]. Through miRNA-627, calcitriol induced histone methylation and suppressed the expression of growth-promoting genes such as GDF15 [14]. However, the expression level and role of miRNA-627 in prostate cancer are still unknown. Hence, the present study would try to study the impact of miRNA-627 on proliferation, migration and apoptosis of prostate cancer cells, and provide new target for the treatment of prostate cancer.
Materials and methods
Cell culture and transfection
Prostate cancer cell line, DU145, was purchased from the cell bank of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in DMEM/F12 medium (11320, Gibco) supplemented with 10% fetal bovine serum (SV30087.02, Hyclone) in 5% CO2 at 37°C. Cells were passaged every 2 days by using trypsin/EDTA (11668-500, Invitrogen). In the present study, lenti-miRNA-627 was generated to express miRNA-627 (Obio Technology, Shanghai, China); and a similar lentivirus vector encoding the EGFP gene was used as a negative control (NC group) (Obio Technology, Shanghai, China). DU145 cells were transfected with recombinant lentivirus at a multiplicity of infection (MOI) of 0, 10, 20, 40 and 80 particles per cell, with 5 μg/ml polybrene (H9268, Sigma) to choose the proper transfection condition. With the proper MOI, the cells were treated with 2 μg/ml puromycin (P8833, Sigma) to choose stable cell line to do subsequent experiments.
Cell proliferation assay
CCK8 (CK04, Dojindo, Japan) assay was used for detecting cell proliferation. In the present study, 100 μl cells (2,000 cells/per well) were added into a 96-well plate. After cultured in an incubator chamber (311, Thermo) at 37°C and 5% CO2, cells were transfected with lenti-miRNA-627 and control vector. At the time points of 0, 24, 48, 72 and 96 h, 10 μl CCK8 solution was dropped into each well. The cells were incubated for another four hours. A microplate reader (Infinite M1000, TECAN) was performed to measure the absorbance of each plate at 450 nm (OD 450 nm).
Cell cycle assay
Collected about 1×106 prostate cells of each group to perform cell cycle analysis. Fixed by pre-cooling 75% ethyl alcohol, the cells were incubated overnight at 4°C. Next day, removed the alcohol. The cells were washed with PBS again; and then suspended in a mixture combined by 500 μl PBS, 500 μl RNase A (R9009, Sigma) and 100 μl propidium iodide (PI) (P-4170, Sigma). One hour later, cells were filtered by using 40 μm cell strainers (352340, BD Falcon) and then performed on flow cytometry (FACSCalibur, BD). The data was analyzed by ModFit software (Verity Software House), and the figures were performed by GraphPad Prism (Ver5, GraphPad Software).
Cell migration assay
Scratch test was used to detect the migration ability of the prostate cancer cells. A 200 μl pipette was used to vertically scratch the surface of cells. Cells were washed with PBS for three times and then cultured with a serum-free culture medium. Cells were cultured in a 5% CO2 incubator at 37°C, this moment was marked as 0 h. At 12, 24 and 48 h time points, the scratches were observed and the migration ability of the prostate cancer cells was determined through measuring the wound increased area.
Cell apoptosis assay
Annexin-PE was purchased from Beyotime Biotechnology (C1062, Wuhan, China) to evaluate cell apoptosis. The prostate cancer cells (1×106) were collected and then centrifuged at 500 g for 5 min. The cells were suspended and then went to centrifugation again. Next, the cells were suspended with 200 μl Annexin-V-PE to label the cells. After 10 minutes cultured in the dark at room temperature, the cells were detected by flow cytometry (FACSCalibur, BD, USA).
Real-time PCR assay
Total RNA was extracted from prostate cancer cells by using TRIzol reagent (15596018, Invitrogen) according to the manufacturer’s instructions. Synthesis of cDNA was performed by using the Transcriptor First Strand cDNA Synthesis Kit (04896866001, Roche). Gene differences were detected by using SYBR green (04913850001, Roche). In the present study, actin gene expression was used as an internal control. The reaction conditions were as follows: 95°C for 30 sec; 95°C for 5 sec and 60°C for 34 sec (40 cycles); 95°C for 1 min; 55°C for 1 min; 55°C to 95°C for 4 sec (81 cycles). The expression levels of each gene were quantified through the analysis of the Cq. The primer of miRNA-627 was purchased from GeneCopoeiaTM (HmiRQP0735). The sequences of other primers pairs (Sangon Biotech, Shanghai, China) in the present study were displayed as follows: MAP3K1: 5’-CAGAGATGTCAAAGGTGCC-3’ and 5’-TGTTGACCTCTTAGTACCTCAG-3’; PTPRK: 5’-TCGACTTGTCCCAGGGCT-3’ and 5’-CAGATAACCTTCCTGTGGTCTTG-3’; SRA1: 5’-GTGGCCACACAAGGAAGCA-3’ and 5’-CGGTGGCTTGAAAGCTCTTG-3’; actin: 5’-TTCTACAATGAGCTGCGTG-3’ and 5’-CTCAAACATGATCTGGGTC-3’.
Statistical analysis
Data was presented as mean ± SD. Student’s t test was used for statistical analyses between two groups. Significant difference was obtained when a p value <0.05. SPSS 17.0 was used for all data analysis.
Results
The miRNA-627 expression level was significantly increased in the prostate cancer cells with lenti-miRNA-627 transfection
To further ascertain the function of miRNA-627 on prostate cancer, we used lentivirus vector to overexpress miRNA-627 named lenti-miRNA-627 in DU145 cell line (a prostate cancer cell line). Firstly, to choose the appropriate multiplicity of infection (MOI), we checked the effects of 0, 10, 20, 40 and 80 particles per cell. As revealed in Figure 1A, it was found that the transfection efficacy was significantly increased in MOI20, MOI40 and MOI80. According to the principle of high transfection efficacy, low MOI and cytotoxicity, MOI20 was selected in subsequent experiments (Figure 1B and 1C). Next, expression level of miRNA-627 in the group with lenti-miRNA-627 transfection was detected; it was found that miRNA-627 expression level was significantly increased in the prostate cancer cells with lenti-miRNA-627 transfection (Figure 1D). These results indicated that lenti-miRNA-627 transfection in the prostate cancer cells was successful and the transfection efficacy of MOI20 was appropriate in the present study.
Figure 1.
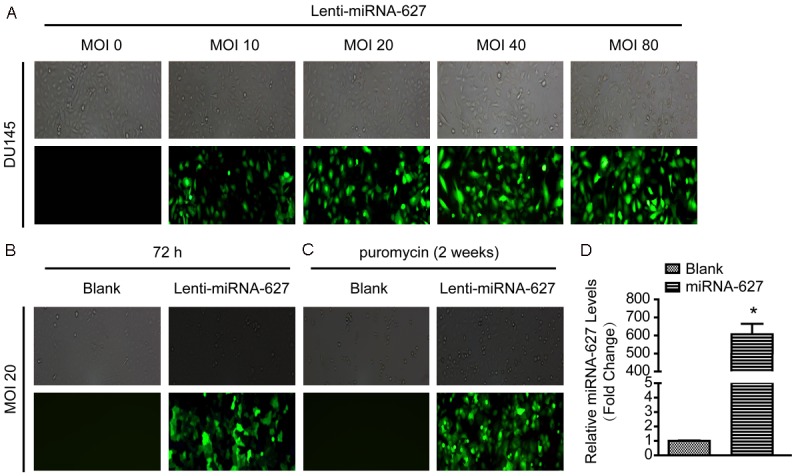
miRNA-627 expression level was significantly increased in DU145 cells with lenti-miRNA-627 transfection. A: The transfection efficacy of multiplicity of infection (MOI) 0, 10, 20, 40 and 80 particles per cell were detected with GFP. B: The expression of GFP was increased in the MOI 20 after 72 h transfection. C: After 72 h transfection, non-transfected DU145 cells were screened with puromycin for 2 weeks. D: The level of miRNA-627 was significantly increased in DU145 cells with lenti-miRNA-627 transfection (n=6 samples per group). *P<0.05 vs. Blank.
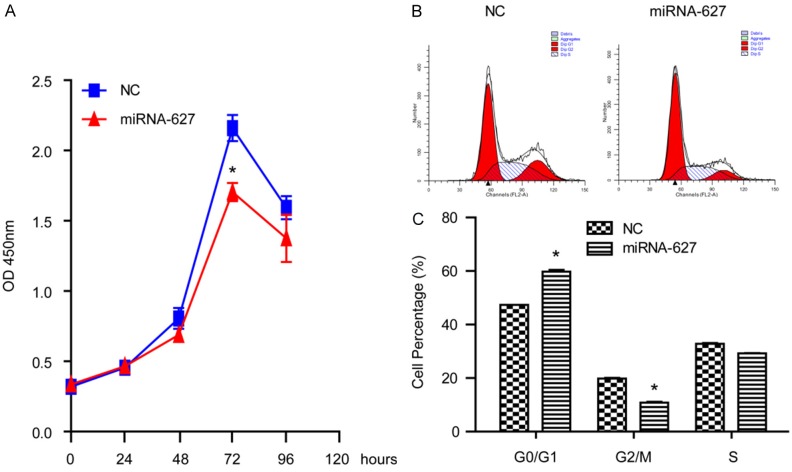
miRNA-627 inhibited the proliferation of the prostate cancer cells and retarded the cells in the phase of G0/G1
To investigate the influence of miRNA-627 on cell proliferation, we performed a CCK8 assay. As shown in Figure 2A, upregulation of miRNA-627 significantly suppressed cell proliferation. It was revealed that after 72 h, the inhibition effect of miRNA-627 reached highest in the prostate cancer cells. The result indicated that miRNA-627 might play a suppressive role on prostate cancer cells proliferation in vitro. In order to elucidate the mechanism of cell proliferation regulated by miRNA-627, the effect of miRNA-627 on cell cycle progression was further evaluated. It was revealed that the G2/M-phase population was significantly decreased whereas the G0/G1-phase was significantly increased in lenti-miRNA-627 group, when compared to the negative control (NC) group (Figure 2B and 2C). These results indicated that miRNA-627 induced cell cycle arrest in the phase of G0/G1 and then inhibited the proliferation of the prostate cancer cells.
Figure 2.
miRNA-627 inhibited the proliferation of DU145 cells and retarded the cells in the phase of G0/G1. A: The CCK8 assays revealed that the proliferation of DU145 cells was inhibited by miRNA-627 overexpression (n=6 samples per group). B and C: Representative and statistical results of cell cycle in DU145 cells of the indicated groups (n=3 independent experiments). *P<0.05 vs. NC (negative control).
miRNA-627 suppressed the migration of the prostate cancer cells
To measure the effect of miRNA-627 on cell migration, scratch test was used. As demonstrated in Figure 3A and 3B, miRNA-627 resulted in a higher wound increased area than that of the NC group at the time points of 12 h, 24 h and 48 h. These results indicated that miRNA-627 inhibited migration in the prostate cancer cells.
Figure 3.
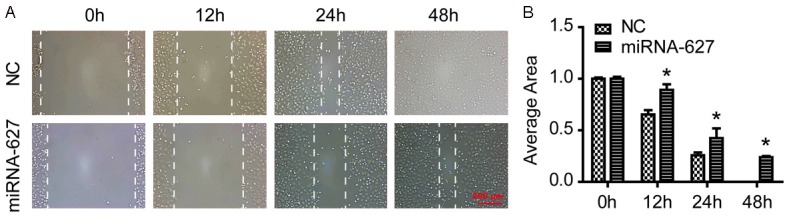
miRNA-627 suppressed the migration of the prostate cancer cells. A and B: Representative and statistical results of wound average area detected by scratch test in DU145 cells (n=3 independent experiments). *P<0.05 vs. NC (negative control).
miRNA-627 promoted apoptosis of the prostate cancer cells
To determine the effect of miRNA-627 on cell apoptosis, flow cytometry was applied to measure the apoptosis percentage. As demonstrated in Figure 4A and 4B, miRNA-627 resulted in a higher apoptosis percentage than that of the NC group. The result indicated that miRNA-627 promoted cell apoptosis in the prostate cancer cells.
Figure 4.
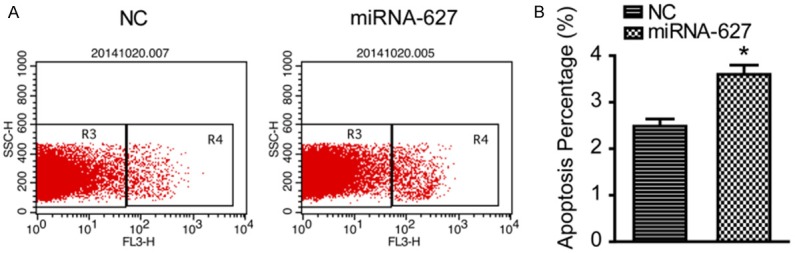
miRNA-627 promoted apoptosis of the prostate cancer cells. A and B: Representative and statistical results of cell apoptosis detected by flow cytometry in DU145 cells (n=3 independent experiments). *P<0.05 vs. NC (negative control).
Expression levels of MAP3K1, PTPRK and SRA1 were increased in the prostate cancer cells after infected with lenti-miRNA-627
To further investigate the role of miRNA-627 on the prostate cancer cells, the mRNA levels of MAP3K1, PTPRK and SRA1 were measured by RT-PCR. As shown in Figure 5A-C, miRNA-627 could increase the mRNA levels of MAP3K1, PTPRK and SRA1. The result indicated that miRNA-627 might promote the expression of tumor suppressor genes in the prostate cancer cells.
Figure 5.
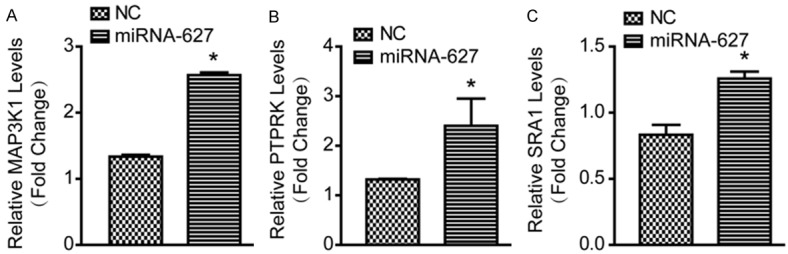
Expression levels of MAP3K1, PTPRK and SRA1 were increased in the prostate cancer cells after transfected with lenti-miRNA-627. The relative mRNA levels of MAP3K1 (A), PTPRK (B) and SRA1 (C) in DU145 cells of the indicated groups (n=6 samples per group). *P<0.05 vs. NC (negative control).
Discussion
miRNAs have been indicated to play important roles in the regulation of cancer cell functions including differentiation, proliferation, apoptosis, and metastasis [9,15]. In the present study, it was demonstrated that the overexpression level of miRNA-627 was contributed to the antitumor activities including inhibiting cell proliferation and cell migration, promoting cell apoptosis and the expression of MAP3K1, PTPRK and SRA1 in the prostate cancer cells.
The miRNA-627 is a newly found member of miRNA family [16]. Previously, it was found that miRNA-627 level in human colon cancer was significantly lower than that in the normal tissues [14]. However, there was no correlation between miRNA-627 expression and tumor grade of differentiation or tumor sites (left or right colon). It might indicate that miRNA-627 expression was decreased during the early stage of carcinogenesis in the colon [14]. It has been shown that miRNA-627 could slow down colon cancer growth in vivo, and this antitumor effect could be related to JMJD1A, which is an iron- and 2-oxoglutarate-dependent dioxygenase which catalyzes the demethylation of mono- and dimethylated H3K9 and involves in promoting colon cancer growth [14]. It was found that cytochrome P450 enzyme CYP3A4-mediated metabolism of irinotecan could potentially serve as a mechanism of drug resistance in cancer chemotherapy [17]. More importantly, another study demonstrated that miRNA-627-mediated downregulation of CYP3A4 could provide a mechanism to overcome such resistance to irinotecan [18]. These indicated that induction of miRNA-627 expression might serve as an important mechanism of vitamin D in modulating the intratumoral CYP3A4 activity in colon cancer [18]. Strategies to selectively deliver miRNA-627 may prove to be effective to reduce the CYP3A4 level in cancer cells and improve tumor response to irinotecan. Through the above studies, it indicates that miRNA-627 serves as an antitumor effector in colon cancer [14,18]. And it was firstly found that miRNA-627 might also contribute to the antitumor activities by up-regulating tumor suppressors in prostate cancer in the present study. Firstly, miRNA-627 could inhibit the proliferation of the prostate cancer cells by inducing cell cycle arrest in the phase of G0/G1. Secondly, miRNA-627 could suppress the migration of the prostate cancer cells, which is the important process of neoplasm metastasis [19]. Thirdly, miRNA-627 could promote apoptosis of the prostate cancer cells, which is related to cell migration and neoplasm metastasis [20]. Lastly, miRNA-627 could upregulate the levels of MAP3K1, PTPRK and SRA1 [21-23]. Thus, our data have established a novel action of miRNA-627 in prostate cancer.
Taken together, our findings firstly indicated that miRNA-627 exerted carcinostatic properties in prostate cancer development as suppressing cell proliferation, migration and promoting cell apoptosis and tumor suppressor genes expression, which might provide promising therapeutic effect in the treatment of prostate cancer. However, animal experiments should be applied to confirm the effect of miRNA-627 in prostate cancer in vivo, and the mechanism of miRNA-627 in prostate cancer should be further investigated in the future.
Acknowledgements
This work was supported by the Special fund of Fujian provincial finance department (Fujian finance (2011) 724), and Special fund of Union Hospital of Fujian Medical University (2015TC-1-069).
Disclosure of conflict of interest
None.
References
- 1.Lee DJ, Mallin K, Graves AJ, Chang SS, Penson DF, Resnick MJ, Barocas DA. Recent changes in prostate cancer screening practices and prostate cancer epidemiology. J Urol. 2017;198:1230–1240. doi: 10.1016/j.juro.2017.05.074. [DOI] [PubMed] [Google Scholar]
- 2.Garisto JD, Klotz L. Active surveillance for prostate cancer: how to do it right. Oncology (Williston Park) 2017;31:333–340. 345. [PubMed] [Google Scholar]
- 3.Vanacore D, Boccellino M, Rossetti S, Cavaliere C, D’Aniello C, Di Franco R, Romano FJ, Montanari M, La Mantia E, Piscitelli R, Nocerino F, Cappuccio F, Grimaldi G, Izzo A, Castaldo L, Pepe MF, Malzone MG, Iovane G, Ametrano G, Stiuso P, Quagliuolo L, Barberio D, Perdona S, Muto P, Montella M, Maiolino P, Veneziani BM, Botti G, Caraglia M, Facchini G. Micrornas in prostate cancer: an overview. Oncotarget. 2017;8:50240–50251. doi: 10.18632/oncotarget.16933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luu HN, Lin HY, Sorensen KD, Ogunwobi OO, Kumar N, Chornokur G, Phelan C, Jones D, Kidd L, Batra J, Yamoah K, Berglund A, Rounbehler RJ, Yang M, Lee SH, Kang N, Kim SJ, Park JY, Di Pietro G. miRNAs associated with prostate cancer risk and progression. BMC Urol. 2017;17:18. doi: 10.1186/s12894-017-0206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szczyrba J, Loprich E, Wach S, Jung V, Unteregger G, Barth S, Grobholz R, Wieland W, Stohr R, Hartmann A, Wullich B, Grasser F. The microRNA profile of prostate carcinoma obtained by deep sequencing. Mol Cancer Res. 2010;8:529–538. doi: 10.1158/1541-7786.MCR-09-0443. [DOI] [PubMed] [Google Scholar]
- 6.Ambs S, Prueitt RL, Yi M, Hudson RS, Howe TM, Petrocca F, Wallace TA, Liu CG, Volinia S, Calin GA, Yfantis HG, Stephens RM, Croce CM. Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res. 2008;68:6162–6170. doi: 10.1158/0008-5472.CAN-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porkka KP, Pfeiffer MJ, Waltering KK, Vessella RL, Tammela TL, Visakorpi T. MicroRNA expression profiling in prostate cancer. Cancer Res. 2007;67:6130–6135. doi: 10.1158/0008-5472.CAN-07-0533. [DOI] [PubMed] [Google Scholar]
- 8.Tong AW, Fulgham P, Jay C, Chen P, Khalil I, Liu S, Senzer N, Eklund AC, Han J, Nemunaitis J. MicroRNA profile analysis of human prostate cancers. Cancer Gene Ther. 2009;16:206–216. doi: 10.1038/cgt.2008.77. [DOI] [PubMed] [Google Scholar]
- 9.Felekkis K, Touvana E, Stefanou C, Deltas C. microRNAs: a newly described class of encoded molecules that play a role in health and disease. Hippokratia. 2010;14:236–240. [PMC free article] [PubMed] [Google Scholar]
- 10.Deng JH, Deng Q, Kuo CH, Delaney SW, Ying SY. MiRNA targets of prostate cancer. Methods Mol Biol. 2013;936:357–369. doi: 10.1007/978-1-62703-083-0_27. [DOI] [PubMed] [Google Scholar]
- 11.Sun T, Wang Q, Balk S, Brown M, Lee GS, Kantoff P. The role of microRNA-221 and microRNA-222 in androgen-independent prostate cancer cell lines. Cancer Res. 2009;69:3356–3363. doi: 10.1158/0008-5472.CAN-08-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leite KR, Sousa-Canavez JM, Reis ST, Tomiyama AH, Camara-Lopes LH, Sanudo A, Antunes AA, Srougi M. Change in expression of miRlet7c, miR-100, and miR-218 from high grade localized prostate cancer to metastasis. Urol Oncol. 2011;29:265–269. doi: 10.1016/j.urolonc.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Suh SO, Chen Y, Zaman MS, Hirata H, Yamamura S, Shahryari V, Liu J, Tabatabai ZL, Kakar S, Deng G, Tanaka Y, Dahiya R. MicroRNA-145 is regulated by DNA methylation and p53 gene mutation in prostate cancer. Carcinogenesis. 2011;32:772–778. doi: 10.1093/carcin/bgr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padi SK, Zhang Q, Rustum YM, Morrison C, Guo B. MicroRNA-627 mediates the epigenetic mechanisms of vitamin D to suppress proliferation of human colorectal cancer cells and growth of xenograft tumors in mice. Gastroenterology. 2013;145:437–446. doi: 10.1053/j.gastro.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He SJ, Xiang CQ, Zhang Y, Lu XT, Chen HW, Xiong LX. Recent progress on the effects of microRNAs and natural products on tumor epithelial-mesenchymal transition. Onco Targets Ther. 2017;10:3435–3451. doi: 10.2147/OTT.S139546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummins JM, He Y, Leary RJ, Pagliarini R, Diaz LA Jr, Sjoblom T, Barad O, Bentwich Z, Szafranska AE, Labourier E, Raymond CK, Roberts BS, Juhl H, Kinzler KW, Vogelstein B, Velculescu VE. The colorectal microRNAome. Proc Natl Acad Sci U S A. 2006;103:3687–3692. doi: 10.1073/pnas.0511155103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Sun M, Zhang Q, Yang X, Qian SY, Guo B. Vitamin D enhances the efficacy of irinotecan through miR-627-mediated inhibition of intratumoral drug metabolism. Mol Cancer Ther. 2016;15:2086–2095. doi: 10.1158/1535-7163.MCT-16-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erdogan B, Webb DJ. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem Soc Trans. 2017;45:229–236. doi: 10.1042/BST20160387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Li Y, Ding M, Zhang H, Xu X, Tang J. Molecular mechanisms and clinical applications of miR-22 in regulating malignant progression in human cancer (review) Int J Oncol. 2017;50:345–355. doi: 10.3892/ijo.2016.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu C, Wang S, Zhu S, Wang H, Gu J, Gui Z, Jing J, Hou X, Shao Y. MAP3K1-targeting therapeutic artificial miRNA suppresses the growth and invasion of breast cancer in vivo and in vitro. Springerplus. 2016;5:11. doi: 10.1186/s40064-015-1597-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun PH, Ye L, Mason MD, Jiang WG. Protein tyrosine phosphatase kappa (PTPRK) is a negative regulator of adhesion and invasion of breast cancer cells, and associates with poor prognosis of breast cancer. J Cancer Res Clin Oncol. 2013;139:1129–1139. doi: 10.1007/s00432-013-1421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo P, Jing W, Zhu M, Li ND, Zhou H, Yu MX, Liang CZ, Tu JC. Decreased expression of LncRNA SRA1 in hepatocellular carcinoma and its clinical significance. Cancer Biomark. 2017;18:285–290. doi: 10.3233/CBM-160305. [DOI] [PubMed] [Google Scholar]