Abstract
Programmed cell death protein 1 (PD-1) negatively regulates T cell effector mechanisms and contributes to tumor cell escape from immune surveillance. To evaluate potential clinical significance of PD-1 in multiple myeloma (MM), we quantified PD-1 expressing T cells in bone marrow (BM) of MM patients using flow cytometry. Our results showed that PD-1 positive T cells in BM from relapsed/refractory patients were significantly higher than those in the newly diagnosed, partial/complete remission MM, and controls, respectively. Additionally, high-risk MM patients had more PD-1 positive T cells in BM than low-risk patients. Moreover, PD-1 positive T cells in relapsed/refractory MM patients were positively associated with myeloma cell counts in BM and clinical stages. PD-1 positive T cells in BM of all MM and relapsed/refractory MM patients were positively correlated with their serum β2-microglobulin concentrations. Our results strongly suggest that PD-1 expressing T cells in BM may be applied as a biomarker to reflect tumor mass and prognosis.
Keywords: Bone marrow, multiple myeloma, programmed cell death protein 1, PD-1 positive T cells, biomarker
Introduction
Multiple myeloma (MM) is a plasma cell malignancy characterized by accumulation of monoclonal plasma cells in the bone marrow (BM) manifested by bone destruction. It is more common among individuals older than 60 years of age [1]. Novel proteasome inhibitors such as bortezomib [2] and immunomodulatory thalidomide and its derivative, lenalidomide [3] have improved MM outcomes. However, MM remains incurable and nearly all patients eventually relapse.
Programmed cell death protein 1 (PD-1, CD279), a member of the CD28 receptor family of co-signaling molecules [4] is expressed on the surface of antigen-activated T and B cells [5]. Engagement of PD-1 with its ligands PD-L1 or PD-L2 inhibit activated T-cells resulting in T cell anergy and functional exhaustion [6]. Several studies indicate that higher PD-1 expression in tumor-infiltrating T cells correlated with poorer patient outcomes for melanomas, renal cell carcinoma, and breast cancer [7-9]. Moreover, increased PD-1-positive lymphocytes in the tumor microenvironment have been considered to be an independent prognostic indicator for poor prognosis in follicular lymphoma [10]. Recent clinical trials demonstrated that blocking the PD-1/PD-L pathway using monoclonal antibodies against PD-1 or PD-L1 markedly induced tumor regression and prolonged disease stabilization in patients with solid tumors [11,12] and several hematologic malignancies [13,14]. PD-1 expression is upregulated in T cells isolated from peripheral blood of MM patients, suggesting a role of the PD-1/PD-L pathway in counteracting T cell-mediated immune response against MM [15]. Ongoing clinical trials of immunomodulatory drugs combined with PD-1 pathway blockade had shown promising preliminary results in relapsed/refractory MM patients [16,17].
To evaluate the potential clinical significance of PD-1 positive T cells in BM or the tumor microenvironment of MM patients, we measured PD-1 expression and associated these data with tumor mass variables. We noted that PD-1 positive T cells were correlated with myeloma cell counts, serum β2-microglobulin (β2-MG) concentrations and clinical stage, indicating that PD-1 expression may have promised as a biomarker to reflect immune status of the myeloma microenvironment, tumor mass and MM prognosis as well as MM subgroup type.
Materials and methods
Patient information
From July 2011 to June 2015, 56 MM patients (22 with newly diagnosed, 14 with relapsed/refractory and 20 with partial/complete remission) were recruited from the Department of Hematology of Jinan Central Hospital, affiliated with Shandong University. Patient information appears in Table 1. Diagnoses of newly diagnosed, relapsed/refractory and partial/complete remission MM were established according to the criteria recommended by the International Myeloma Working Group [18,19]. Of the MM patients, 56 had a diagnostic BM smear, biopsies and flow cytometric analyses, as well as karyotyping and fluorescent in situ hybridization (FI-SH) for 6 patients with newly diagnosed MM. 12 patients with iron deficiency anemia (IDA), a non-immunological and malignant disease, were used as controls, and IDA were diagnosed according to the British Society of G. Guidelines for the Management of Iron Deficiency Anemia were used as controls [20]. All cases were analyzed using 5-color flow cytometry. Clinical and laboratory data were obtained from a review of medical records and the study was approved by the Ethics Committee of Jinan Central Hospital affiliated to Shandong University. Oral consent was obtained from all individual participants included in the study.
Table 1.
Clinical and laboratory feature in MM patients
| Parameters | Newly diagnosed (n=22) | Relapsed/refractory (n=14) | Partial/complete remission (n=20) |
|---|---|---|---|
| Age, years (range) | 67 (50-83) | 60 (45-74) | 59 (43-81) |
| Age >65 years | 12 (55) | 7 (50) | 9 (45) |
| Intact immunoglobin MM | 21 (96) | 14 (100) | 14 (70) |
| IgG | 14 (64) | 11 (79) | 10 (50) |
| IgA | 5 (23) | 3 (21) | 3 (15) |
| IgD | 2 (9) | 0 (0) | 1 (5) |
| Light Chain MM | 1 (4) | 0 (0) | 6 (30) |
| β2-MG >3.5 mg/L | 18 (82) | 10 (71) | 5 (25) |
| Albumin <35 g/L | 16 (73) | 7 (50) | 5 (25) |
| Hemoglobin <10 g/dl | 18 (82) | 7 (50) | 6 (30) |
| Bone lesions | 17 (77) | 11 (79) | 15 (75) |
| BMPC, median (range) | 32 (5-76) | 32 (7-85) | 1 (0-3) |
| Abnormal cytogenetics | 6 (27) |
Qualitative data expressed as n (%). Quantitative data expressed as median (interquartile range). MM, multiple myeloma; IgG, immunoglobulin G; IgA, immunoglobulin A; IgD, immunoglobulin D; β2-MG, beta-2-microglobulin; BMPC, bone marrow plasma cells.
Treatment strategies
By following the International Staging System (ISS) [21], 15 and 7 out of 22 newly diagnosed MM patients were classified as stages III and II/I respectively. Based on their general condition, medical insurance status and disease activity [22], 5/22 patients were treated with BCD (bortezomib, cyclophosphamide, and dexamethasone), 7/22 with BTD (bortezomib, thalidomide and dexamethasone) [23,24], 2/22 with VAD (vindesine, adriamycin and dexamethasone) and 1/22 with MP (melphalan, prednisone) regimen, respectively. The remaining 7 out of 22 patients received supportive therapy only. Data of comparing PD-1 expression on T cells in BM before and after 3-4 cycle treatment were available from 6 out of the 22 newly diagnosed MM patients (5/6 with bortezomib based regimens and 1/6 with VAD).
Flow cytometry
BM aspirate samples collected in heparin-anticoagulant were washed 3 times with the phosphate-buffered saline (PBS) and White Blood cells (WBC) concentration of the samples was adjusted to 1×106/ml. Then, 100 μl of washed cells were added to each stain tube. Added titrated amount of antibodies/cocktails to each stain tube (stained panels as below) and incubated for 15 min in the dark at room temperature: Tube 1: CD16-fluorescein isothiocyanate (FITC) (BD Pharmingen, San Jose, CA), CD279-phycoerythrin (PE) (BD Pharmingen), CD3-energy coupled dye (ECD) (Beckman coulter, Miami FL), CD56-peridinin chlorophyll protein (PC5) (Beckman Dickinson, San Jose, CA), CD45-peridinin chlorophyll protein (PC7) (Beckman coulter). Tube 2: CD200-PE (Biolegend, San Diego, CA), CD14-ECD (Beckman coulter), CD38-PC5 (Beckman coulter), CD45-PC7. Tube 3: Cyto-kappa-FITC (Dako, Glostrup Denmark), CD117-PE (Beckman coulter), CD38-PC5, CD45-PC7. Tube 4: Cyto-lambda-FITC, HLA-G-PE (BD Pharmingen), CD38-PC5, CD45-PC7. Tube 5: Cyto-IgG1-FITC, CD138-PE (Beckman coulter), CD19-ECD (Beckman coulter), CD38-PC5, CD45-PC7.
At the end of incubation, 500 μl of BC Optilyse C lysing solution was added into all tubes and incubated for 10-12 min at room temperature in the dark. After centrifuging for 5 min at 1,700 rpm, antibody tubes were washed once with 2 ml PBS containing 0.5% bovine serum albumin (BSA). For tube 1,2, resuspended cells with 0.6 ml of 1% paraformaldehyde in PBS. For tube 3-5, cytoplasmic Lambda and Kappa light chain staining, 0.5 ml of 1× BD FACS permeabilizing solution was added, and incubated for 5 min at room temperature. After washing with PBS containing 0.5% BSA and centrifugation for 5 min, an appropriate volume of fluorescent-conjugated intracellular antibody (Cyto-Kappa/Lambda/IgG1) was added and incubated for 30 min at room temperature in the dark. After washing, cells were resuspended in 1% paraformaldehyde in PBS.
At least 100,000 events were acquired using a FC500 MCL flow cytometer with CXP software (Beckman Coulter, Miami FL). All analyses included an isotype-matched control tube to establish a positive staining threshold for plasma cells and lymphocytes [25].
Data analysis
All data were analyzed using FCS Express software. Distinct cell populations (clusters) were characterized using antibodies and gating with CD45 fluorescence versus forward and side scatter. Neoplastic plasma and T cells were identified based on patterns of aberrant antigen expression and/or light chain restriction. A population was considered antigen positive when at least 20% of the cluster of interest exceeded a 2% isotype control cutoff [26]. Due to potential shifts in relative fluorescence over time that were related to instrument or reagent changes, when possible, antigen expression was compared with additional internal normal cell populations.
Cytogenetic analysis
Interphase FISH analysis was performed to measure cytogenetic aberrations in purified plasma myeloma cells according to the manufacturer’s specifications. Del (13q) abnormality was analyzed with a probe specific for the 13q14 locus (Cytocell RB-1 kit); Del (17p13) was assessed using a specific probe for the 17p13.1 locus (VYSIS TP53 kit); 1q21 dual-color probe (Cytocell CKS1B/CDKN2C kit) was used to measure amplification of 1q21. An IGH probe (VYSIS IGH kit) was used to assess translocation and deletion of 14q32. A VYSIS IGH/FGFR3, VYSIS IGH/CCND, and VYSIS IGH/MAF dual-color dual fusion probe were used to assay t(4;14), t(11;14), and t(14;16), respectively.
A total of 200 interphase nuclei per DNA probe were analyzed. Cut-off values recommended by the European Myeloma Network (EMN) were used. For deletion and numerical aberrations, cut-off level was set at 20%; for translocation at the IgH locus and other translocations, the cut-off level was set at 10% [27].
Statistical analysis
Statistical analyses were carried out in Graphpad Prism, version 6.0b (Graphpad Software, Inc. CA 92037 USA). The data of PD-1 positive cells in newly diagnosed, relapsed/refractory, partial/complete remission MM patients, and controls was evaluated using ordinary one-way ANOVA and an unpaired t-test was applied to compare differences respectively, PD-1 positive T cells between the high-risk and low-risk MM patients was analyzed using an unpaired t-test. The correlation between PD-1 positive T cells in all and relapsed/refractory MM patients and serum β2-microglobulin concentration was assessed using the Pearson’s correlation coefficient test. Pearson’s correlation coefficient was used to assess significant linear relationships between PD-1 positive T cells in relapsed/refractory MM and myeloma cell counts and with clinical stages. Results were expressed as means ± standard error of the mean (SEM). A p-value less than 0.05 was considered statistically significant.
Results
PD-1 expression in BM T cells from MM patients
PD-1 expression in BM of MM patients (Figure 1A) and controls (Figure 1B) was quantified by flow cytometry and PD-1 positive T cells in of all MM patients exceed those of controls, but this difference was not statistically significant (P=0.3384). PD-1 positive cells in relapsed/refractory MM patients were greater than those of the newly diagnosed and partial/complete remission patients, and controls (P=0.0058, P=0.0049, P=0.0136, Figure 1C). According to the ISS criteria, stage III was defined as high-risk and stage I-II was low-risk. Data show that PD-1 positive T cells in BM of high-risk MM patients were significantly greater than those of low-risk MM patients (P=0.0325, Figure 1D).
Figure 1.
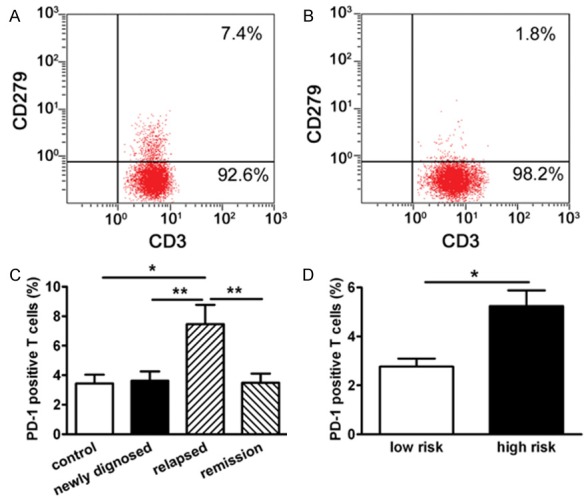
PD-1 expression in T cells in BM of MM patients. PD-1 expression was quantified with flow cytometry as described in Materials and methods. Representative dot plots illustrating PD-1 (CD279+) expression on T cells (CD3+) in BM of relapsed/refractory MM patient (A) and control (B). (C) Percentage of PD-1 positive T cells (CD3+CD279+) in BM of relapsed/refractory (n=14) patients were significantly higher than that in newly diagnosed (n=22), remission (partial + complete remission; n=20) patients and Controls (n=12), respectively (P<0.05 for three comparisons). (D) PD-1 positive T cells in BM were significantly higher in high-risk (n=42) than low-risk MM patients (n=14). *P<0.05, **P<0.01.
PD-1 positive T cells were associated with prognostic markers and tumor mass
Serum β2-MG concentrations in peripheral blood is prognostic marker for MM [28]. Our data show that PD-1 positive T cells in BM of all MM and relapsed/refractory MM patients were positively correlated with serum β2-MG (Figure 2A, 2B). No correlations between PD-1 positive T cells and serum β2-MG in newly diagnosed MM patients were found (P=0.9544, r=-0.01294).
Figure 2.
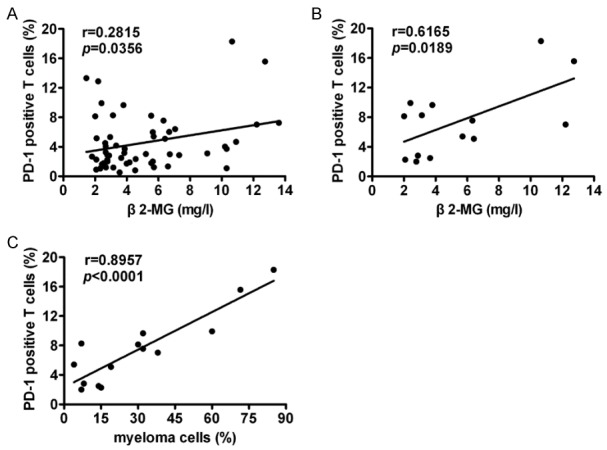
PD-1 positive T cells in BM of MM patients were associated with tumor burden and prognostic indicators. A. PD-1 positive T cells in BM of all MM patients positively correlated with serum β2-MG concentrations in peripheral blood (n=56). B. PD-1 positive T cells in BM of relapsed/refractory MM patients positively correlated with serum β2-MG concentrations in peripheral blood (n=14). C. PD-1 positive T cells in relapsed/refractory MM patients positively correlated with myeloma cell numbers in BM (n=14).
The percentage of myeloma cells were calculated from 200 nucleated cell counts in a bone marrow smear, which was defined as tumor mass. PD-1 positive T cells in BM of relapsed/refractory MM patients were positively correlated with myeloma cell counts in BM (Figure 2C). Moreover, Myeloma cell counts in BM of patients with relapsed/refractory MM were positively correlated with serum β2-MG in peripheral blood (P=0.0174, r=0.6228). No correlations between PD-1 positive T cells in BM and myeloma cell counts in newly diagnosed MM patients were found (P=0.7475, r=0.07278).
In high-risk MM patient PD-1 positive T cells in BM were positively correlated with myeloma cell counts (P=0.0445, r=0.3898) and serum β2-MG in peripheral blood (P=0.0459, r=0.6408). However, no such correlation was found in low-risk and newly diagnosed MM patients.
PD-1 positive T cells correlated with tumor mass and prognostic indicators in IgG MM patients
All MM patients were divided into IgG and non-IgG types, including IgA, IgD and light chain types. Our results show that PD-1 positive T cells in BM of newly diagnosed and relapsed/refractory IgG type MM patients were positively correlated with myeloma cell counts in BM (Figure 3A) and serum β2-MG in peripheral blood (Figure 3B). Moreover, PD-1 positive T cells of relapsed/refractory IgG type MM patients were positively correlated with myeloma cell counts (Figure 3C) and serum β2-MG (Figure 3D). However, PD-1 positive T cells of newly diagnosed and relapsed/refractory non-IgG type MM patients did not corelate with myeloma cell counts (P=0.7112, r=-0.1264) or β2-MG (P=0.4823, r=-0.2373).
Figure 3.
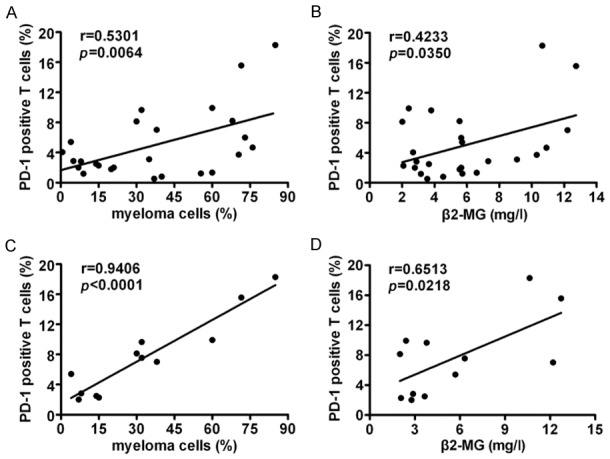
PD-1 expressing T cells in IgG type MM patients correlated with tumor burden and prognostic indictors. PD-1 expressing T cells in newly diagnosed, relapsed/refractory IgG type MM patients positively correlated with myeloma cells in BM (n=25) (A) and serum β2-MG concentrations in peripheral blood (n=25) (B). (C) PD-1 expressing T cells in relapsed/refractory IgG type MM patients positively correlated with myeloma cells in BM (n=12). (D) PD-1 expressing T cells in relapsed/refractory IgG type MM patients positively correlated with serum β2-MG concentrations in peripheral blood (n=12).
Chemotherapy reduced PD-1 expression in BM T cells from MM patients
In this study, 6 newly diagnosed MM patients were studied to compare PD-1 positive T cells in BM before and after chemotherapy or immunochemotherapy. Five patients were treated with the BCD (bortezomib, cyclophosphamide, and dexamethasone), and BTD (bortezomib, thalidomide and dexamethasone) regimens and one was treated with a conventional regimen without bortezomib including VAD (vindesine, adriamycin and dexamethasone). After 3-4 cycles of treatments, 3/6 achieved a very good complete remission and 3/6 complete remission. As expected, percentages of PD-1 positive T cells in BM of MM patients after treatment were significantly lower than that of before treatment (P=0.0434 Figure 4), indicating that chemotherapy may break tumor tolerant BM microenvironment through reducing PD-1 positive T cells or PD-1 expression. Clearly, the underlying mechanisms of such effects need to be clarified.
Figure 4.
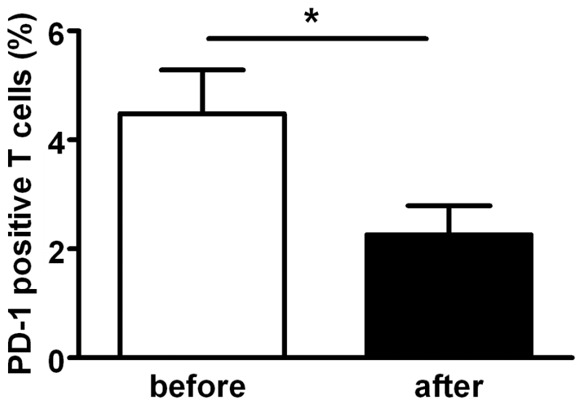
Treatment reduced PD-1 positive T cells in BM of MM patients. 6 of 22 newly diagnosed MM patients were studied to compare PD-1 expression on T cell in BM before and after chemotherapy or immuno-chemotherapy. Five patients were treated with BCD (bortezomib, cyclophosphamide and dexamethasone), BTD (bortezomib, thalidomide and dexamethasone) regimens and one was treated with VAD (vindesine, adriamycin and dexamethasone) without bortezomib for 3-4 cycles. As expected, the percentages of PD-1 positive T cells in BM of MM patients after treatment were significantly lower than that of before treatment. Data are means ± SEm (*P<0.05).
Discussion
MM development/growth strongly depended on the MM microenvironment due to suppression of anti-MM immune effector responses [29]. Recent studies suggest that immune checkpoint PD-1/PD-L1 signaling is a key pathway regulating the critical balance between immune activation and tolerance that allows for immune surveillance escape [6]. Blockade of PD-1/PD-L1 signaling with anti-PD1 monoclonal antibodies reduced solid tumors and offers a novel immunotherapeutic strategy [11,12]. Several recent studies of hematologic malignancies confirmed increased expression of PD-1 and PD-L1 in lymphomas, chronic lymphocytic leukemia, and MM [13,30,31]. However, PD-1 expression in T cells in BM of MM and MM subgroups and their relationship with tumor mass, prognostic factors, clinical stage, type and treatment response is unclear.
Reports suggest that PD-1 is found on a proportion of T cells of MM patients [32] and PD-L1 expression is greater in a myeloma cell line and in primary myeloma tumor cells from MM patients [33,34]. PD-1 expression is induced in activated T cells in the BM microenvironment and activates phosphatases [35]. PD-1 engagement upregulates expression of basic leucine ATF-like transcription factor (BATF), which impairs T-cell proliferation and cytokine secretion [36]. Therefore, upregulation of PD-1 and engagement with its ligands can severely inhibit T cell function. Direct interaction between PD-1 in T cells and PD-L1 in myeloma cells delivers an inhibitory signal and induces resistance to anti-myeloma chemotherapy and disease progression [15,37].
We investigated PD-1 expression in T cells in BM of patients with newly diagnosed, relapsed/refractory, and partial/complete remission MM and as well as controls and identified correlations with variables of tumor mass. PD-1 positive T cells of relapsed/refractory MM patients were significantly higher than those of newly diagnosed, partial/complete remission, and control patients. PD-1 positive T cells of high-risk patients with all types of MM were significantly higher compared to that of low-risk MM patients. PD-1 positive T cells in advanced MM and MM subgroups were positively correlated with myeloma cell counts, serum β2-MG and clinical MM stage. This study indicates that the PD-1 positive T cells in advanced MM patients are positively associated with tumor mass and PD-1 plays an important role in the regulation of tumor escape from immune surveillance due to suppression of anti-MM immune effector responses. Enhancing anti-MM immune response by targeting checkpoint molecules may therefore improve outcome in advanced and subgroup MM patients. Application of anti-PD-1 antibody together with pomalidomide has shown treatment effects for refractory/relapsed MM [16,17].
We observed that the percentages of PD-1 positive T cells in BM of newly diagnosed, relapsed/refractory IgG and relapsed/refractory IgG MM patients were positively correlated with myeloma cell counts and serum β2-MG, but PD-1 positive T cells of those with newly diagnosed, relapsed/refractory non-IgG type MM, and relapsed/refractory non-IgG MM did not correlate with myeloma cell counts or serum β2-MG. Thus, advanced and subgroup IgG type MM had more PD-1 positive T cells correlated with tumor mass, and the PD-1/PD-L1 pathway promoted tumor progression indirectly in IgG type but not non-IgG MM patients, so other mechanisms may explain MM development/growth. In addition to PD-1, other T-cell co-stimulatory or co-inhibitory molecules, such as CD40, OX40 (CD134), T cell immunoglobulin mucin-3 (TIM-3), and cytotoxic T lymphocyte associated antigen-4 (CTLA-4) are being explored as potential targets for an antibody-mediated tumor immunotherapy and blockade of PD-1. Also, CTLA-4 is associated with increased proliferation of antigen-specific effector CD8+, CD4+ T cells, antigen-specific cytokine release, and upregulation of key signaling molecules for T cell function [36,37]. Furthermore, anti-PD-1 antibody, Nivolumab did not produce a response in MM [40]; PD-1 expression is downregulated in clonal BM cytotoxic T cells compared with non-clonal T cells in MM patients which may explain T cells activity as PD-1/PD-L1 do not interact in MM cells [41].
PD-1 positive T cells in relapsed/refractory and high-risk MM patients were significantly higher than that of other subgroups and low-risk MM patients and PD-1 positive T cells in BM of MM patients were associated with tumor mass, prognostic indicators, and clinical stage. Likely, PD-1 weakened T-cell mediated antitumor responses, upregulated Janus kinase (JAK)/signal transducer and activated transcription-3 (STAT3) and phosphatidylinositol 3-kinase (PI3K/AKT) pathways during refractory and relapsed MM [42] and overexpressed PD-L1 on MM cells [15]. PD-1/PD-L1 interactions have been shown to mediate tumor escape from immune control in several animal models [32,43]. CT-011, an anti PD-1 antibody, enhances NK-cell activity against autologous primary MM cells, and lenalidomide downregulates PD-L1 in MM cells and augments CT-011-mediated enhancement of NK cell activity against MM [31]. PD-1 in T cells and PD-L1 in MM cells surrounding tumors contribute to relapsed/refractory and drug resistance mechanisms so immune-based therapeutic strategies that target checkpoint signaling with PD-1 or PD-L1 blocking antibodies may inhibit tumor cell growth and restore host immune function in MM [44].
PD-1 expression is reported to be upregulated in T cells isolated from patients with MM, and after autologous transplantation, expression of PD-1 on T cells returned to normal controls [17]. We found that 6/22 newly diagnosed MM patients accepted conventional therapy and bortezomib based regimens, and after 3-4 cycles of treatment, myeloma cell counts and serum β2-MG were significantly decreased. Meanwhile, PD-1 positive T cells were reduced. Thus, proteasome inhibitors and conventional treatment not only reduced tumor mass of MM patients but also reduced PD-1 positive T cells, indicating an important role of the PD-1 pathway in mediating the immunosuppressive state of MM patients and treatment response. MM strategies aimed to increase immune functions may have important therapeutic implications for MM. Several clinical trials with anti-PD-1 antibodies as a single therapy or combined with novel agents in patients with refractory/ relapsed plasma cell myeloma showed responses [16,17].
Although our findings show that PD-1 positive T cells in BM of advanced MM patients are positively associated with tumor mass and prognostic indicators, additional studies could provide stronger evidence. Our conclusions are limited due to the insufficient numbers of newly diagnosed patients before and after treatment in this study and the lack of cytogenetic and molecular abnormality analyses for most patients. Future studies should be conducted in a larger sample of MM patients to verify expression of PD-1 on T cells or T cell subsets and expression of PD-L1 in myeloma cells [45].
In conclusion, our novel findings show that PD-1 positive T cells in BM from relapsed/refractory patients were significantly higher than those in the newly diagnosed MM, and controls, and were positively associated with parameters of tumor mass and prognosis which highlight that PD-1 expressing T cells in BM may be applied as an independent biomarker to reflect prognosis. This notion needs to be further confirmed in a large-scale cohort study before a solid conclusion can be made.
Acknowledgements
This work was supported in part by research funding from the National Natural Science Foundation of China (81372545), Youth fund of Second Hospital of Shandong University (Y2013010029), Business Plan Foundation of Jinan for the Scholar to Study Abroad (20100206), Fundamental Research Funds of Shandong University (2014QY004-16) and Primary Research & Developement Plan of Shandong Province (2017GSF18136). We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Disclosure of conflict of interest
None.
References
- 1.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 2.Moreau P, Richardson PG, Cavo M, Orlowski RZ, San Miguel JF, Palumbo A, Hraousseau JL. Proteasome inhibitors in multiple myeloma: 10 years later. Blood. 2012;120:947–959. doi: 10.1182/blood-2012-04-403733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quach H, Kalff A, Spencer A. Lenalidomide in multiple myeloma: current status and future potential. Am J Hematol. 2012;87:1089–1095. doi: 10.1002/ajh.23234. [DOI] [PubMed] [Google Scholar]
- 4.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 6.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapon M, Randriamampita C, Maubec E, Badoual C, Fouquet S, Wang SF, Marinho E, Farhi D, Garcette M, Jacobelli S, Rouquette A, Carlotti A, Girod A, Prevost-Blondel A, Trautmann A, Avril MF, Bercovici N. Progressive upregulation of PD-1 in primary and metastatic melanomas associated with blunted TCR signaling in infiltrating T lymphocytes. J Invest Dermatol. 2011;131:1300–1307. doi: 10.1038/jid.2011.30. [DOI] [PubMed] [Google Scholar]
- 8.Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757–1761. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 9.Muenst S, Soysal SD, Gao F, Obermann EC, Oertli D, Gillanders WE. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013;139:667–676. doi: 10.1007/s10549-013-2581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi H, Tomita N, Sakata S, Tsuyama N, Hashimoto C, Ohshima R, Matsuura S, Ogawa K, Yamamoto W, Kameda Y, Enaka M, Inayama Y, Kasahara M, Takekawa Y, Onada N, Motomura S, Ishigatsubo Y, Takeuchi K. Prognostic significance of programmed cell death-1-positive cells in follicular lymphoma patients may alter in the rituximab era. Eur J Haematol. 2013;90:286–290. doi: 10.1111/ejh.12075. [DOI] [PubMed] [Google Scholar]
- 11.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armand P, Nagler A, Weller EA, Devine SM, Avigan DE, Chen YB, Kaminski MS, Holland HK, Winter JN, Mason JR, Fay JW, Rizzieri DA, Hosing CM, Ball ED, Uberti JP, Lazarus HM, Mapara MY, Gregory SA, Timmerman JM, Andorsky D, Or R, Waller EK, Rotem-Yehudar R, Gordon LI. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J. Clin. Oncol. 2013;31:4199–4206. doi: 10.1200/JCO.2012.48.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenblatt J, Glotzbecker B, Mills H, Vasir B, Tzachanis D, Levine JD, Joyce RM, Wellenstein K, Keefe W, Schickler M, Rotem-Yehudar R, Kufe D, Avigan D. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. J Immunother. 2011;34:409–418. doi: 10.1097/CJI.0b013e31821ca6ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zagouri F, Terpos E, Kastritis E, Dimopoulos MA. Emerging antibodies for treatment of multiple myeloma. Expert Opin Emerg Drugs. 2016;21:225–237. doi: 10.1080/14728214.2016.1186644. [DOI] [PubMed] [Google Scholar]
- 17.Badros AZ, Hyjek E, Ma N, Lesokhin A, Dogan A, Rapoport AP, Kocoglu M, Lederer E, Philip S, Milliron T, Dell C, Goloubeva O, Singh Z. Pembrolizumab, pomalidomide and low-dose dexamethasone for relapsed/refractory multiple myeloma. Blood. 2017;130:1189–1197. doi: 10.1182/blood-2017-03-775122. [DOI] [PubMed] [Google Scholar]
- 18.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J, Sonneveld P, Ludwig H, Gahrton G, Beksac M, Crowley J, Belch A, Boccadaro M, Cavo M, Turesson I, Joshua D, Vesole D, Kyle R, Alexanian R, Tricot G, Attal M, Merlini G, Powles R, Richardson P, Shimizu K, Tosi P, Morgan G, Rajkumar SV International Myeloma Working Group. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 19.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E, Richardson P, Landgren O, Paiva B, Dispenzieri A, Weiss B, LeLeu X, Zweegman S, Lonial S, Rosinol L, Zamagni E, Jagannath S, Sezer O, Kristinsson SY, Caers J, Usmani SZ, Lahuerta JJ, Johnsen HE, Beksac M, Cavo M, Goldschmidt H, Terpos E, Kyle RA, Anderson KC, Durie BG, Miguel JF. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:538–548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 20.Goddard AF, James MW, McIntyre AS, Scott BB British Society of Gastroenterology. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60:1309–1316. doi: 10.1136/gut.2010.228874. [DOI] [PubMed] [Google Scholar]
- 21.Greipp PR, San Miguel J, Durie BG, Growley JJ, Barlogie B, Blade J, Boccadoro M, Child JA, Avet-Loiseau H, Kyle RA, Lahuerta JJ, Ludwig H, Morgan G, Powles R, Shimizu K, Shustik C, Sonneveld P, Tosi P, Turesson I, Westin J. International staging system for multiple myeloma. J. Clin. Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 22.The International Myeloma working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disordors: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–757. [PubMed] [Google Scholar]
- 23.Reeder CB, Reece DE, Kukreti V, Chen C, Trudel S, Hentz J, Noble B, Pirooz NA, Spong JE, Piza JG, Zepeda VH, Mikhael JR, Leis JF, Bergsagel PL, Fonseca R, Stewart AK. Cyclophosphamide, bortezomib and dexamethasome induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia. 2009;23:1337–1341. doi: 10.1038/leu.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufman JL, Nooka A, Vrana M, Gleason C, Heffner LT, Lonial S. Bortezomib, thalidomide, and dexamethasone as induction therapy for patients with symptomatic multiple myeloma: a retrospective study. Cancer. 2010;116:3143–3151. doi: 10.1002/cncr.25143. [DOI] [PubMed] [Google Scholar]
- 25.Rawstron AC, Davies FE, DasGupta R, Ashcroft AJ, Patmore R, Drayson MT, Owen RG, Jack AS, Child JA, Morgan GJ. Flow cytometric disease monitoring in multiple myeloma: the relationship between normal and neoplastic plasma cells predicts outcome after transplantation. Blood. 2002;100:3095–3100. doi: 10.1182/blood-2001-12-0297. [DOI] [PubMed] [Google Scholar]
- 26.Spears MD, Olteanu H, Kroft SH, Harrington AM. The immunophenotypic stability of plasma cell myeloma by flow cytometry. Int J Lab Hematol. 2011;33:483–491. doi: 10.1111/j.1751-553X.2011.01317.x. [DOI] [PubMed] [Google Scholar]
- 27.Ross FM, Avet-Loiseau H, Ameye G, Gutierrez NC, Liebisch P, O’Connor S, Dalva K, Fabris S, Testi AM, Jarosova M, Hodkinson C, Collin A, Kerndrup G, Kuglik P, Ladon D, Bernasconi P, Maes B, Zemanova Z, Michalova K, Michau L, Neben K, Hermansen NE, Rack K, Rocci A, Protheroe R, Chiecchio L, Poirel HA, Sonneveld P, Nyegaard M, Johnsen HE European Myeloma Network. Report from the European Myeloma Network on interphase FISH in multiple myeloma and related disorders. Haematologica. 2012;97:1272–1277. doi: 10.3324/haematol.2011.056176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao ZF, Fu WJ, Yuan ZG, Wang DX, Chen YB, Hou J. Prognostic factors and staging systems of multiple myeloma. Chin Med J. 2007;120:1655–1658. [PubMed] [Google Scholar]
- 29.Hallek M, Bergsagel PL, Anderson KC. Multiple myeloma: increasing evidence for a multistep transformation process. Blood. 1998;91:3–21. [PMC free article] [PubMed] [Google Scholar]
- 30.Gassner FJ, Zaborsky N, Catakovic K, Rebhandl S, Huemer M, Egle A, Hartmann TN, Greil R, Geisberger R. Chronic lymphocytic leukaemia induces an exhausted T cell phenotype in the TCL1 transgenic mouse model. Br J Haematol. 2015;170:515–522. doi: 10.1111/bjh.13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kearl TJ, Jing W, Gershan JA, Johnson BD. Programmed death receptor-1/programmed death receptor ligand-1 blockade after transient lymphodepletion to treat myeloma. J Immunol. 2013;190:5620–5628. doi: 10.4049/jimmunol.1202005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, Saudemont A, Quesnel B. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEKdependent pathway. Blood. 2007;110:296–304. doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 33.Benson DM Jr, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, Baiocchi RA, Zhang J, Yu J, Smith MK, Greenfield CN, Porcu P, Devine SM, Rotem-Yehudar R, Lozanski G, Byrd JC, Caligiuri MA. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116:2286–2294. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray A, Das DS, Song Y, Richardson P, Munshi NC, Chauhan D, Anderson KC. Targeting PD1-PDL1 immune checkpoint in plasmacytoid dendritic cell interactions with T cells, natural killer cells and multiple myeloma cells. Leukemia. 2015;29:1441–1444. doi: 10.1038/leu.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229:114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quigley M, Pereyra F, Nilsson B, Porichis F, Fonseca C, Eichbaum Q, Julg B, Jesneck JL, Brosnahan K, Imam S, Russell K, Toth I, Piechocka-Trocha A, Dolfi D, Angelosanto J, Crawford A, Shin H, Kwon DS, Zupkosky J, Francisco L, Freeman GJ, Wherry EJ, Kaufmann DE, Walker BD, Ebert B, Haining WN. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat Med. 2010;16:1147–1151. doi: 10.1038/nm.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar A, Hozo I, Wheatley K, Djulbegovic B. Thalidomide versus bortezomib based regimens as first-line therapy for patients with multiple myeloma: a systematic review. Am J Hematol. 2011;86:18–24. doi: 10.1002/ajh.21904. [DOI] [PubMed] [Google Scholar]
- 38.Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013;73:3591–603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melero I, Grimaldi AM, Perez-Gracia JL, Ascierto PA. Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination. Clin Cancer Res. 2013;19:997–1008. doi: 10.1158/1078-0432.CCR-12-2214. [DOI] [PubMed] [Google Scholar]
- 40.Lesokhin AM, Ansell SM, Armand P, Scott EC, Halwani A, Gutierrez M, Millenson MM, Cohen AD, Schuster SJ, Lebovic D, Dhodapkar M, Avigan D, Chapuy B, Ligon AH, Freeman GJ, Rodig SJ, Cattry D, Zhu L, Grosso JF, Bradley Garelik MB, Shipp MA, Borrello I, Timmerman J. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase IB study. J. Clin. Oncol. 2016;34:2698–2704. doi: 10.1200/JCO.2015.65.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suen H, Brown R, Yang S, Ho PJ, Gibson J, Joshua D. The failure of immune checkpoint blockade in multiple myeloma with PD-1 inhibitors in a phase 1 study. Leukemia. 2015;29:1621–1622. doi: 10.1038/leu.2015.104. [DOI] [PubMed] [Google Scholar]
- 42.Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001;20:5991–6000. doi: 10.1038/sj.onc.1204833. [DOI] [PubMed] [Google Scholar]
- 43.Hallett WH, Jing W, Drobyski WR, Johnson BD. Immunosuppressive effects of multiple myeloma are overcome by PD-L1 blockade. Biol Blood Marrow Transplant. 2011;17:1133–1145. doi: 10.1016/j.bbmt.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 44.Atanackovic D, Luetkens T, Kroger N. Coinhibitory molecule PD-1 as a potential target for the immunotherapy of multiple myeloma. Leukemia. 2014;28:993–1000. doi: 10.1038/leu.2013.310. [DOI] [PubMed] [Google Scholar]
- 45.Görgün G, Samur MK, Cowens KB, Paula S, Bianchi G, Anderson JE, White RE, Singh A, Ohguchi H, Suzuki R, Kikuchi S, Harada T, Hideshima T, Tai YT, Laubach JP, Raje N, Magrangeas F, Minvielle S, Avet-Loiseau H, Munshi NC, Dorfman DM, Richardson PG, Anderson KC. Lenalidomide enhances immune checkpoint blockade-induced immune response in multiple myeloma. Clin Cancer Res. 2015;21:4607–4618. doi: 10.1158/1078-0432.CCR-15-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]


