Abstract
FBXW7 is a potential tumor suppressor that regulates ubiquitination and proteolysis of multiple targets such as cyclin E, c-Myc, c-Jun and Notch. However, little knows about the correlation between FBXW7 and prognosis of patients with colorectal cancer (CRC). In this study, we detected FBXW7 expression in CRC tissue microarray which includes 568 cases cancer tissue and their paired adjacent non-cancerous tissues. We found that FBXW7 expression was significantly reduced in CRC tissues versus paired normal colon tissues (P < 0.001). Moreover, low FBXW7 expression was significantly associated with increased lymph node metastasis (P < 0.001) and advanced TNM stage (P < 0.001). Besides, the low expression of FBXW7 indicated the poor prognosis in CRC patients for both overall and disease-free cumulative survival (P < 0.001 and P = 0.003, respectively). Multivariate Cox regression analysis showed that low FBXW7 expression was an independent unfavorable prognostic factor of CRC (hazard ratio = 0.45, P = 0.001). In conclusion, we can indicate that FBXW7 may play essential roles in the progression of CRC and function as an independent prognostic marker for clinical diagnosis and therapy treatment of patients with CRC.
Keywords: FBXW7, colorectal cancer, tissue microarray, metastasis, prognosis
Introduction
Colorectal carcinoma (CRC) has become the third cause of cancer death in humans, worldwide. Besides, this situation was already improving results that got the benefit of the dissemination of early detection tests and the improvement of treatment and the changes in risk factors such as the increased use of aspirin and the reduction of consumption of cigarette and red meat e.g. [1]. It is estimated that there were still additional cases of 134, 490 men and women with a colorectal cancer diagnosis and death cases of 49, 190 in United States, 2016 [2]. Therefore, CRC is still a dangerous major factor for health of human. And, for the treatment of CRC, except for surgery, neoadjuvant chemotherapy and radiotherapy, molecularly targeted therapy of colorectal carcinoma is called upon to become an effective means of treatment. Therefore, it is essential to determine the novel biological markers involved in the progression of CRC that can assist doctors with improving previous diagnosis and target therapy for CRC patients.
Colorectal carcinogenesis involved in a multistep process including the loss of genomic stability that accelerates the improvement to colorectal cancer by facilitating the acquisition of multiple tumor-associated mutations [3]. CRC progression is driven by a series of well-defined genetic alterations, including mutations in APC, BRAF, KRAS, PIK3CA, p53 and F-box and WD repeat domain-containing 7 (FBXW7) [4]. F-box/WD repeat containing protein 7 (FBXW7) was a member of F-box family proteins, which constitute one subunit of Skp1, Cul1, and F-boxprotein (SCF) ubiquitin ligase complex. FBXW7 targeted a set of well-known oncoproteins, including c-Myc, cyclin E, Notch, c-Jun, and Mcl-1, for ubiquitylation and degradation. It provided specificity of the ubiquitylation of these substrate proteins via recognition of a consensus phosphorylated degree [5]. It regulated cell cycle progression and cell growth and differentiation [6]. Due to reduced FBXW7 expression level and loss-of-function mutations are found in a wide range of human cancers, FBXW7 is generally considered as a tumor suppressor [7,9]. Preceding studies disclosed the relationship between ubiquitin ligase FBXW7 and diverse human cancers, such as cholangiocarcinoma, T cell acute lymphoblastic leukemia, pancreatic cancer, and endometrial cancer [10,14].
However, correlation between ubiquitin ligase FBXW7 and prognosis of patients with colorectal cancer remains unclear. Therefore, we speculated that expression of the ubiquitin ligase FBXW7 may play significant roles for prognosis of CRC. In the present study, we applied the tissue microarray (TMA) along with the retrospective CRC patient cohorts to investigate the relationship between FBXW7 protein expression and clinicopathological features in CRC. Meanwhile, exploring whether FBXW7 could regard as a prognosis biomarker to target therapy for patients with CRC.
Method and materials
Ethics statement
The Institutional Review Boards of Xuzhou Medical University ratified the agreement on this study that conducted in accordance with the approved guidelines. All patients provided written informed consent to their colorectal tissue samples to be used in order to research.
Samples and patients
Colorectal tissue samples that constructed into TMA consisted of 568 cases cancer tissue and their paired adjacent non-cancerous tissues which of all were paraffin-embedded blocks and collected from the Pathology Department of Affiliated Hospital of Xuzhou Medical University. All the patients underwent radical surgery at Affiliated Hospital of Xuzhou Medical University from April, 2010 to March 2015. The patients’ information gained from the Medical Record of the above-named hospital, such as clinicopathological parameters including sex, age, tumor differentiation, tumor diameter, invasion depth, lymph node metastasis, TNM stage and others like marriage, birth place, and surgery date e.g.
In this retrospective CRC cohort of 568 cases, there were male 327 and female 241 and their average age was 61.7 years (range from 21 to 91). For the TNM stage, there were 299 patients at stage I and II, 193 patients at stage III and IV. For the differentiation status, 88 cases known as poorly-differentiated, 391 cases were considered as moderately-differentiated and 82 tumors were well-differentiated. For the pathologic type, almost patients were deemed to be adenocarcinoma (559/568). Every patient gets a complete record of the postoperative follow-up. Survival time was calculated based on the date of surgery to the date of death or to the last follow-up. Besides, date of death was obtained from the records of the postoperative follow-up and verified by the local department of civil affairs.
Tissues microarray construction and immunohistochemistry staining
The CRC TMA was established by contract service at the National Engineering Center for Biochip, Shanghai, China, with duplicate 1.5 mm diameter cores that punched from the paraffin block. Immunohistochemistry (IHC) was implemented following a standard streptavidin-peroxidase (SP) method as previously reported [15] and heat induced epitope retrieval (HIER) was performed with the retrieval buffer, citrate, pH 6.0, prior to commencing with IHC staining protocol. For primary antibody incubation, anti-FBXW7 antibodies were applied at 1:200 dilutions (ab84783, Abcam, USA). The slide without primary antibody incubation served as negative control.
Analysis of immunohistochemistry staining
Two pathologists assessed separately the TMAs under blinded experimental conditions and all differences that arise were resolved by discussion. The staining scores of FBXW7 were evaluated via combining the percentage of cells with the staining intensity and being dependent on the immunoreactivity score (IRS). The intensity of FBXW7 immunostaining was scored as 0-3 (0, negative; 1, weak; 2, moderate; 3, strong); the percentage of immunoreactivity cells was graded as 1 (0-25%), 2 (26-50%), 3 (51-75%) and 4 (76-100%). Relied on the IRS, the level of FBXW7 expression was categorized as low (IRS: 0-4) and high (IRS: 6-12) expression.
Statistical analysis
All the statistical analyses were performed by SPSS 20.0 statistical software package (SPSS Inc., Chicago, IL). The paired Wilcoxon test was used to assess the significance of FBXW7 staining in cancers and their coupled adjacent non-cancerous tissues. The X2 test was implemented to evaluate the relationship between FBXW7 expression and clinicopathological parameters.
Probability of differences in overall survival as a function of time was verified by Kaplan-Meier method and log-rank test. Univariate and multivariate Cox proportional hazards regression analyses were conducted to estimate the crude hazard ratios (HRs), adjusted HRs and 95% confidence intervals (CIs) of HRs. P value < 0.05 was considered as statistically significant.
Results
FBXW7 expression was down-regulated in CRC opposed to adjacent noncancerous tissue
By means of TMAs, we investigated FBXW7 protein expression of 509 (89.6%) of 568 CRC samples and 493 (86.8%) of 568 non-tumor tissues in the TMAs. The rest of samples got lost due to antigen retrieval. We found that FBXW7 was located in the nucleus matrix mostly and less in cytoplasm (Figure 1). The paired Wilcoxon test of 417 cases paired tumor and its adjacent noncancerous tissues revealed that FBXW7 protein expression was dramatically down-regulated in cancers when confronted with adjacent noncancerous tissues (Figure 2) (P < 0.001).
Figure 1.
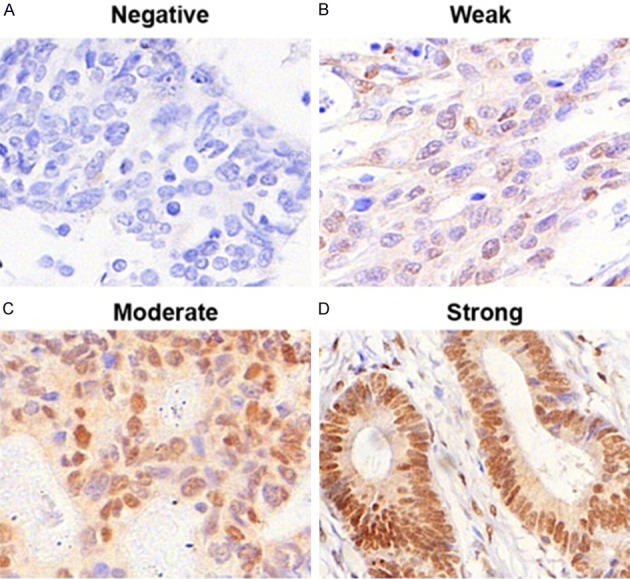
The intensity of FBXW7 immunostaining in colorectal carcinoma tissues. A: Negative staining; B: Weak staining; C: Moderate staining; D: Strong staining. Note: magnification × 200.
Figure 2.
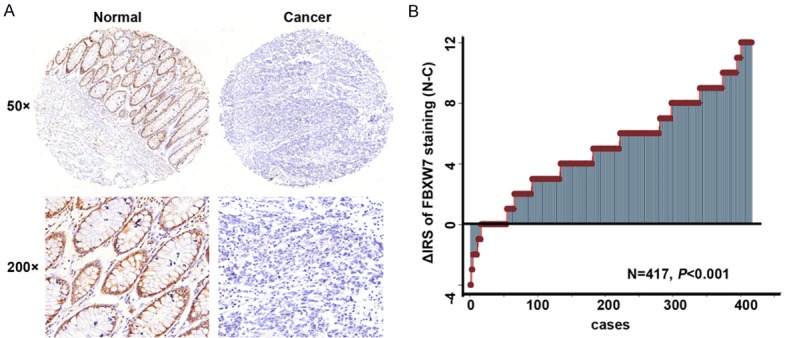
Expression of FBXW7 in paired colorectal carcinoma and adjacent noncancerous tissues. A: FBXW7 immunostaining in TMAs are shown. Note: Top panel, magnification × 50; Bottom panel, magnification × 200. B: FBXW7 expression levels were significantly lower in colorectal carcinoma compared with corresponding adjacent noncancerous tissues (P < 0.001, the paired Wilcoxon test). Note: C, colorectal carcinoma tissues; N, paired adjacent noncancerous tissues.
The relationship between FBXW7 expressions and clinicopathological parameters in CRC patients
The correlation between FBXW7 expression and clinicopathological parameters in CRC was displayed in Table 1. While FBXW7 expression was deemed to be low (IRS: 0-4) and high (IRS: 6-12) expression, we found that low FBXW7 protein expression was 70.5% (359/509), high FBXW7 protein expression was 29.5% (150/509) in CRC tissues. Fisher’s exact test was used to examine the correlation of FBXW7 expression in cancer with clinicopathological characteristics. The data revealed that FBXW7 expression was significantly negatively associated with the lymph node metastasis and TNM stages (Table 1, P < 0.001). Moreover, our results showed that there was marginal correlations of FBXW7 expression with the depth of invasion (P = 0.072) and distant metastasis (P = 0.074). But, FBXW7 expression was not associated with age, sex, tumor diameter and the differentiation.
Table 1.
Relationship between FBXW7 expression and clinicopathological features of CRC patients
| Variables | FBXW7 expression (n = 509 cases) | ||
|---|---|---|---|
|
| |||
| Low (%) | High (%) | P a | |
| All patients | 359 (100) | 150 (100) | |
| Age (years) | 1.000 | ||
| ≤ 60 | 147 (41) | 62 (41) | |
| > 60 | 212 (59) | 88 (59) | |
| Gender | 0.280 | ||
| Males | 211 (59) | 80 (53) | |
| Females | 148 (41) | 70 (47) | |
| Depth of invasion* | 0.072 | ||
| T1/T2 | 14 (4) | 12 (8) | |
| T3/T4 | 340 (96) | 132 (92) | |
| Lymph node metastasis | < 0.001 | ||
| N0 | 207 (58) | 115 (77) | |
| N1/N2/N3 | 152 (42) | 35 (23) | |
| Distant metastasis# | 0.074 | ||
| M0 | 340 (95) | 148 (99) | |
| M1 | 17 (5) | 2 (1) | |
| TNM stage | < 0.001 | ||
| I | 41 (11) | 28 (19) | |
| II | 150 (42) | 92 (61) | |
| III | 150 (42) | 27 (18) | |
| IV | 18 (5) | 3 (2) | |
| Tumor diameter | 0.592 | ||
| ≤ 5 cm | 258 (72) | 104 (69) | |
| > 5 cm | 101 (28) | 46 (31) | |
| Differentiation$ | 0.692 | ||
| Poor | 57 (16) | 26 (18) | |
| Moderate/high | 299 (84) | 120 (82) | |
Two-sided Fisher’s exact tests.
The depth of invasion of cancer in 11 patients cannot be assessed.
The distant metastasis of cancer in 2 patients cannot be assessed.
We lost the data of 7 CRC patients.
Low FBXW7 protein expression relates to poor overall survival in CRC patients
We implemented the Kaplan-Meier survival curve and the log-rank test to examine that whether the expression of FBXW7 was relevant to overall and disease-free survival for CRC patients. Our data suggested that a low level of FBXW7 protein expression trend to be correlative with the worse overall and disease-free survival for CRC patients, while compared with ones with high FBXW7 expression (P < 0.001 and P = 0.003, respectively, Figure 3). Meanwhile, for the overall survival rate, the cumulative survival rate was 84% in the high level group and 63% inthe low level group of FBXW7. For the disease-free survival rate, the cumulative survival rate fell down from 82% to 61% along with low group to high group of FBXW7 expression.
Figure 3.
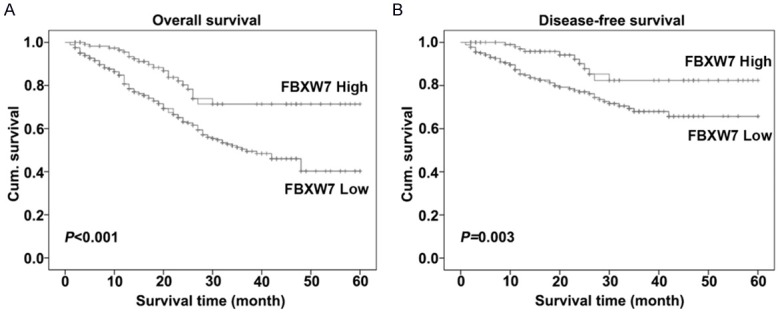
Kaplan-Meier curves showed overall and disease-free survival of colorectal carcinoma patients depending on expression levels of FBXW7. Low FBXW7 expression relevant to a worse overall (A) and disease-free (B) cumulative survival for colorectal carcinoma patients (P < 0.001 and P = 0.003, respectively).
FBXW7 serves as an independent molecular prognostic indicator for CRC
To further evaluate the prognostic value of FBXW7 expression, the univariate and multivariate Cox regression analyses were performed. The univariate Cox regression analysis showed that gender, lymph node metastasis, depth of invasion, distant metastasis, TNM stage and FBXW7 expression, except for age, histological type and tumor diameter, were the prognostic factors for the CRC patients (Table 2). The multivariate Cox regression analysis went on declaring that FBXW7 expression was an independent prognostic biomarker for the CRC patients after adjusting with age, gender, histological type, tumor diameter and TNM stage (HR = 0.45, 95% CI = 0.29-0.71, P = 0.001, Table 3).
Table 2.
Univariate Cox regression analysis of FBXW7 expression and clinicopathological variables predicting the survival of CRC patients
| Variables | (n = 509 cases) | |
|---|---|---|
|
| ||
| HR (95% CI) | P | |
| Age (≤ 65 vs. > 65) | 1.35 (0.98-1.85) | 0.066 |
| Gender (Males vs. Females) | 1.39 (1.03-1.89) | 0.033 |
| Lymph node metastasis (N0 vs. N1/N2/N3) | 1.42 (1.05-1.94) | 0.024 |
| Depth of invasion (T1/T2 vs. T3/T4) | 7.00 (1.73-28.3) | 0.006 |
| Distant metastasis (M0 vs. M1) | 2.31 (1.18-4.53) | 0.015 |
| TNM stage (I-II vs. III/IV) | 1.48 (1.09-2.01) | 0.011 |
| Histological type (Moderate/high vs. Poor) | 1.36 (0.94-1.99) | 0.101 |
| Tumor diameter (≤ 5 cm vs. > 5 cm) | 1.21 (0.87-1.70) | 0.246 |
| FBXW7 expression (Low vs. High) | 0.46 (0.30-0.70) | < 0.001 |
Abbreviations: HR, hazard ratio; CI, confidence interval.
Table 3.
Multivariate Cox regression analysis models assessing the effects of covariates on overall survival in CRC patients
| Variables | (n = 509 cases) | |
|---|---|---|
|
| ||
| HR (95% CI) | P | |
| Age (≤ 65 vs. > 65) | 1.29 (0.93-1.80) | 0.120 |
| Gender (Males vs. Females) | 1.50 (1.10-2.06) | 0.011 |
| Histological type (Moderate/high vs. Poor) | 1.35 (0.92-1.98) | 0.123 |
| Tumor diameter (≤ 5 cm vs. > 5 cm) | 1.24 (0.88-1.74) | 0.209 |
| TNM stage (I-II vs. III/IV) | 1.40 (1.01-1.92) | 0.041 |
| FBXW7 expression (Low vs. High) | 0.45 (0.29-0.71) | 0.001 |
Abbreviations: HR, hazard ratio; CI, confidence interval.
Discussion
Tumor suppressors are defined as those in which loss of function leads to tumor formation. FBXW7 is a tumor suppressor gene that is responsible for the degradation of several proto-oncogenes and its functional inactivation can dysregulate the cell division process, and potentially lead to tumorigenesis [5]. Since then, an explosion of studies explicitly addressed the role of FBXW7 in human tumors. FBXW7 has been implicated in astrocytoma, and in cancers of the lung, breast, gastric, liver, pancreatic, cervix, and esophagus [13]. Several mechanisms have been reported for the inactivation of FBXW7 in the progress of human cancer including mutation, deletion, and hypermethylation, of which the FBXW7 mutation is most common [13]. A lot of effort has been concentrated on finding FBXW7 mutation in various types of human cancers, which has shown that the overall point mutation frequency is 6% to 35% in human cancers with tissue specificity [13]. However, little is known about the roles of FBXW7 in CRC, in consideration of its function, we hypothesized that FBXW7 may play a part in the progression of CRC.
In our study, we first observed that FBXW7 protein expression was frequently at low expression in tumor tissue when compared with its paired non-tumor tissues, which agreed with previous studies in gastric cancer [16], hepatocellular carcinoma [17], breast cancer [18], lung cancer [19] and glioma malignancy [20]. Hence, our result indicated that FBXW7 expression may play a cardinal role in tumorigenesis of CRC. Depend upon our result of low expression of FBXW7 in CRC tissues, we suspected FBXW7 gene may act as important roles in the development of CRC. Unfortunately, little was known about the roles of FBXW7 in the development of CRC, hence future studies are required to illuminate the precise mechanism of FBXW7 involved in the development of CRC.
Almost immediately, we evaluated the association of clinicopathological features with FBXW7 protein expression in CRC patients. Our data showed that FBXW7 protein expression was correlated to clinicopathological parameters such as lymph node metastasis and advanced TNM stage. Obviously, these results indicated that the decreasing expression of FBXW7 may hold a special function in promoting metastasis of CRC and the ability of migration and invasion of CRC cells. However, there are no validation references to illustrate the precise mechanism of metastasis that FBXW7 involved. Our data provided a clue that FBXW7 takes part in metastasis of CRC, yet supplementary studies including in vivo and in vitro assay are further integral to disclose the concrete mechanisms.
In the end of study, we assessed the prognostic value of FBXW7 expression for CRC patients’ prognosis. Our results suggested low expression levels of FBXW7 were tightly correlated to the worse overall and disease-free survival of CRC patients. Likewise, FBXW7 was an independent CRC prognostic factor according to multivariate Cox regression analysis. These results suggested that FBXW7 may serve as a novel molecular prognostic indicator for CRC.
Conclusion, our study found that FBXW7 is a novel biomarker in colorectal carcinoma. The low expression of FBXW7 is associated with lymph node metastasis and advanced TNM stage and acts as an independent prognostic indicator for poor outcome in patient with colorectal carcinoma. Meanwhile, our study provided a novel thought on the diagnosis and treat for patients of colorectal carcinoma.
Disclosure of conflict of interest
None.
References
- 1.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 5.Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8:83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- 6.Song Y, Zhou X, Bai W, Ma X. FBW7 increases drug sensitivity to cisplatin in human nasopharyngeal carcinoma by downregulating the expression of multidrug resistance-associated protein. Tumour Biol. 2015;36:4197–4202. doi: 10.1007/s13277-015-3056-4. [DOI] [PubMed] [Google Scholar]
- 7.Mao JH, Perez-Losada J, Wu D, Delrosario R, Tsunematsu R, Nakayama KI, Brown K, Bryson S, Balmain A. Fbxw7/Cdc4 is a p53-dependent, haploinsufficient tumour suppressor gene. Nature. 2004;432:775–779. doi: 10.1038/nature03155. [DOI] [PubMed] [Google Scholar]
- 8.Maser RS, Choudhury B, Campbell PJ, Feng B, Wong KK, Protopopov A, O’Neil J, Gutierrez A, Ivanova E, Perna I, Lin E, Mani V, Jiang S, McNamara K, Zaghlul S, Edkins S, Stevens C, Brennan C, Martin ES, Wiedemeyer R, Kabbarah O, Nogueira C, Histen G, Aster J, Mansour M, Duke V, Foroni L, Fielding AK, Goldstone AH, Rowe JM, Wang YA, Look AT, Stratton MR, Chin L, Futreal PA, DePinho RA. Chromosomally unstable mouse tumours have genomic alterations similar to diverse human cancers. Nature. 2007;447:966–971. doi: 10.1038/nature05886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuoka S, Oike Y, Onoyama I, Iwama A, Arai F, Takubo K, Mashimo Y, Oguro H, Nitta E, Ito K, Miyamoto K, Yoshiwara H, Hosokawa K, Nakamura Y, Gomei Y, Iwasaki H, Hayashi Y, Matsuzaki Y, Nakayama K, Ikeda Y, Hata A, Chiba S, Nakayama KI, Suda T. Fbxw7 acts as a critical fail-safe against premature loss of hematopoietic stem cells and development of TALL. Genes Dev. 2008;22:986–991. doi: 10.1101/gad.1621808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moberg KH, Bell DW, Wahrer DC, Haber DA, Hariharan IK. Archipelago regulates cyclin E levels in drosophila and is mutated in human cancer cell lines. Nature. 2001;413:311–316. doi: 10.1038/35095068. [DOI] [PubMed] [Google Scholar]
- 11.Kemp Z, Rowan A, Chambers W, Wortham N, Halford S, Sieber O, Mortensen N, von Herbay A, Gunther T, Ilyas M, Tomlinson I. CDC4 mutations occur in a subset of colorectal cancers but are not predicted to cause loss of function and are not associated with chromosomal instability. Cancer Res. 2005;65:11361–11366. doi: 10.1158/0008-5472.CAN-05-2565. [DOI] [PubMed] [Google Scholar]
- 12.Calhoun ES, Jones JB, Ashfaq R, Adsay V, Baker SJ, Valentine V, Hempen PM, Hilgers W, Yeo CJ, Hruban RH, Kern SE. BRAF and FBXW7 (CDC4, FBW7, AGO, SEL10) mutations in distinct subsets of pancreatic cancer: potential therapeutic targets. Am J Pathol. 2003;163:1255–1260. doi: 10.1016/S0002-9440(10)63485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akhoondi S, Sun D, von der Lehr N, Apostolidou S, Klotz K, Maljukova A, Cepeda D, Fiegl H, Dafou D, Marth C, Mueller-Holzner E, Corcoran M, Dagnell M, Nejad SZ, Nayer BN, Zali MR, Hansson J, Egyhazi S, Petersson F, Sangfelt P, Nordgren H, Grander D, Reed SI, Widschwendter M, Sangfelt O, Spruck C. FBXW7/hCDC4 is a general tumor suppressor in human cancer. Cancer Res. 2007;67:9006–9012. doi: 10.1158/0008-5472.CAN-07-1320. [DOI] [PubMed] [Google Scholar]
- 14.Chou A, Toon CW, Clarkson A, Sioson L, Houang M, Watson N, DeSilva K, Gill AJ. Loss of ARID1A expression in colorectal carcinoma is strongly associated with mismatch repair deficiency. Hum Pathol. 2014;45:1697–1703. doi: 10.1016/j.humpath.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Xu W, Liu H, Song J, Fu HX, Qiu L, Zhang BF, Li HZ, Bai J, Zheng JN. The appearance of Tregs in cancer nest is a promising independent risk factor in colon cancer. J Cancer Res Clin Oncol. 2013;139:1845–1852. doi: 10.1007/s00432-013-1500-7. [DOI] [PubMed] [Google Scholar]
- 16.Lee JW, Soung YH, Kim HJ, Park WS, Nam SW, Kim SH, Lee JY, Yoo NJ, Lee SH. Mutational analysis of the hCDC4 gene in gastric carcinomas. Eur J Cancer. 2006;42:2369–2373. doi: 10.1016/j.ejca.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 17.Tu K, Yang W, Li C, Zheng X, Lu Z, Guo C, Yao Y, Liu Q. Fbxw7 is an independent prognostic marker and induces apoptosis and growth arrest by regulating YAP abundance in hepatocellular carcinoma. Mol Cancer. 2014;13:110. doi: 10.1186/1476-4598-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chin K, DeVries S, Fridlyand J, Spellman PT, Roydasgupta R, Kuo WL, Lapuk A, Neve RM, Qian Z, Ryder T, Chen F, Feiler H, Tokuyasu T, Kingsley C, Dairkee S, Meng Z, Chew K, Pinkel D, Jain A, Ljung BM, Esserman L, Albertson DG, Waldman FM, Gray JW. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell. 2006;10:529–541. doi: 10.1016/j.ccr.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Villaruz LC, Socinski MA. Temsirolimus therapy in a patient with lung adenocarcinoma harboring an FBXW7 mutation. Lung Cancer. 2014;83:300–301. doi: 10.1016/j.lungcan.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu Z, Inomata K, Mitsui H, Horii A. Promoter hypermethylation is not the major mechanism for inactivation of the FBXW7 beta-form in human gliomas. Genes Genet Syst. 2008;83:347–352. doi: 10.1266/ggs.83.347. [DOI] [PubMed] [Google Scholar]


