Abstract
To study clinical characteristics of Mycoplasma pneumoniae pneumonia (MPP) in children of different ages. We investigated the medical records of MPP patients admitted at Children’s Hospital of Soochow University from January 2006 and December 2013. The presence of MP was confirmed by real-time PCR and ELISA. There were overall 3358 children with MPP. 412 (12.3%) were <6 months old. Six months to 1 year, 1-3 years, 3-5 years and ≥5 years old children constituted 423 (12.6%), 1033 (30.8%), 733 (21.8%) and 757 (22.5%) respectively. Fever was less frequent in <6 months old infants, with frequency increasing in older patients. The proportion of patients with wheeze was highest (57.9%) in patients aged 6 months to 1-year-old. Tachypnea, cyanosis and hypoxemia were more prevalent in <6 months old infants (P=0.00). Radiologically, bronchopneumonia was frequent among infants, while segmental/lobar pneumonia was frequent in ≥5 years old patients. White blood cell and platelet counts of infants were significantly higher than other children. Neutrophil count, c-reaction protein levels was significantly different amongst age groups. Our study revealed MPP patients of different ages presented with different clinical findings especially <6 months old and ≥5 years of age, which may indicate different pathological findings in age groups of patients with MPP.
Keywords: Mycoplasma pneumoniae, pneumonia, clinical characteristics, children
Introduction
Mycoplasma pneumoniae (MP) is an important pathogen resulting in severe respiratory disease in children, accounting for up to 40% of community-acquired pneumonia (CAP). As many as 18% of pediatric patients with MP pneumonia (MPP) require hospital admissions [1]. MPP has been reported to be most prevalent in older children, adolescents, and young adults [2-4]. As a result, clinicians tend too often underestimate MP infections in infants. MP infections in children under 5 years of age, along with variable clinical presentations in school-aged children have been recently identified [5-7]. Othman N found that clinical findings such as coryza, tachypnoea, chest wall recession, diarrhea and vomiting, are more common in children under the age of 5 compared to older patients [8]. Furthermore, Ferwerda also reported that MP infection in children aged 5-15 years is associated with lobar pneumonia [9]. Host immune responses play an important role in MP infection, with disease severity, clinical manifestations and laboratory parameters differing according to the age of patients. Hence, studies to elucidate clinical findings in MPP children of different ages is of great importance. We retrospectively reviewed and analyzed the medical data of 3358 MPP patients admitted at Children’s Hospital of Soochow University during Jan 2006 and Dec 2013.
Materials and methods
Patients
This retrospective study was conducted on pediatric patients admitted at the Department of Respiratory Disease of Children’s Hospital of Soochow University during the period between Jan 2006 and Dec 2013. MPP was considered diagnostic according to the following criteria. 1. a pulmonary infiltrate was present on a chest radiograph in combination with a fever, cough or auscultatory findings consistent with pneumonia. 2. MP-DNA was detected in sputum by real-time polymerase chain reaction (PCR) and/or specific IgM antibodies against MP were detected by enzyme-linked immunosorbent assays (ELISA). 3. other infections were excluded. Patients with congenital heart disease, immunodeficiency, bronchial or pulmonary dysplasia and those with incomplete information were excluded from our study. The study was approved by the Medical Ethics Committee of Soochow University. The parents of study participants gave written consent before study enrollment.
Nasopharyngeal secretion specimen collection: Nasopharyngeal secretions were collected from each study participant within 24 h of admission by a laboratory technician. The sample was mixed with 4-8 mL phosphate buffer saline (PBS), and centrifuged for 10 minutes at 300-500 rpm. The supernatant was discarded and the pellet was mixed with 4-8 mL PBS and centrifuged for an additional 10 minutes. The pellet was stored at -80°C for analysis.
Collection of serum sample
Serum (4 mL) was obtained for complete blood counts and estimation of C-reactive protein, alanine transaminase (ALT), myocardium enzymes, IgM/IgG specific antibodies of MP.
Nasopharyngeal secretion MP-DNA detection and evaluation
DNA lysate (Shanghai Shenyou Biotechnology Company, Shanghai, China) was added to the sputum pellet following washing with PBS. The sample was heated to at 95°C for 10 min, centrifuged for 5 min at 12 000 rpm, and then the supernatant was collected. After extracting DNA from the sputum specimen, MP DNA was detected by fluorescent real-time PCR (BIO-RAD iCycler, USA). The cyclic temperature settings were 93°C, 2 min; 93°C, 45 s→55°C, 60 s→10 cycles; 93°C, 30 s→55°C, 45 s→30 cycles. The fluorescence collection point was set at 55°C, 45 s. Ct value was used to quantify the fluorescence quantitative PCR results. The probe binding sequence was located between the upstream and downstream primer. The fluorescent reporter dye at the 59 end of probe was 6-carboxyfluorescein (FAM), and the quencher at the 39 end of the probe was 6-carboxytetramethylrhodamine (TAMRA). The forward primer was 5’-CCA ACCAAA CAACAA CGTTCA-3’, the reverse primer was 5’-ACCTTGACTGGAGGCCGT TA-3’, and the probe was 5’-FAM-TCA ACT CGA ATA ACG GTG ACTTCT TAC CAC TG-3’-TAMRA. The primers and probe were purchased from Guangzhou Daan Gene Ltd. (Guangzhou, China). A positive MP sample was defined as having an amplification curve, S-shaped and a Ct value <30. The DNA content of the sputum was determined by the following criteria. If the sample C<5.00×102, the DNA content was <2.5×103 gene copies/mL; if 5.00×102≤C≤5.00×108, the DNA content =5.00×103 gene copies/mL; and if C>5.00×108, the DNA content was >5.00×103 gene copies/mL. An MP-negative sample was defined as having an amplification curve that was not S-shaped or a Ct value <30.
Virus detection from nasopharyngeal secretion sample
Direct immunofluorescence was used to detect syncytial virus infection (RSV), influenza virus A (IVA), influenza virus B (IVB), parainfluenza virus (PIV) I, PIV II, PIV III, and adenovirus (ADV). All assay kits were purchased from Chemicon (USA) and all staining procedures were performed according to the manufacturer’s instructions. Immunostained preparations were viewed with a fluorescence microscope (Leica 020-518.500, Germany).
RNA extraction and real-time PCR to detect the human metapneumovirus (hMPV) gene
The forward primer sequences for hMPV was 5’-AACCGTGTACTAAGTGATGCACTC-3’, the reverse primer sequences was 5’-CATTGTTTGACCGGCCCCATAA-3’.
DNA extraction and real-time PCR to detect the human bocavirus (hBoV) gene
The forward primer sequences for hBoV was 5’-TGACATTCAACTACCAACAACCTG-3’, the reverse primer sequences was 5’-CAGATCCTTTTCCTCCTCCAATAC-3’, and the probe was 5’-FAM-AGCACCACAAAACACCTCAGGGG-3’-TAMRA.
Serological analysis for MP
IgM/IgG antibodies against MP were measured using the Serion Elisa Mycoplasma pneumoniae IgM/IgG kit (Institut Virion/Serion GmbH, Würzburg, Germany) with the test cut-off 0.5× mean optical density (OD) value of the kit control serum, as indicated in the insert. A significant rise in IgG titre was considered to be a doubling of the OD value above the cut-off, or a sero-conversion in which the primary serum was antibody negative and the second serum had an OD at least twice the cut-off corresponding to a threefold rise in AU/mL titre. The assay was considered positive if IgM≥1.1 U/mL, according to the manufacturer’s instructions.
Data collection
Demographic characteristics of age and gender, clinical manifestations, physical examination findings, values of laboratory tests and findings on chest X-ray were collected from case records.
Statistical analyses
All data were analyzed using SPSS 18.0 statistical software. The comparisons among groups were performed using the chi square test. For data that did not meet the conditions of the chi square test, Fisher’s exact probability test was used. Data with non-normal distribution were expressed as medians and quartile ranges (M; P25 P75) and differences were evaluated using the Mann-Whitney U test. P<0.05 was considered significant.
Results
Demographic characteristics
A total of 3358 patients aged from 1 month to 14 years were identified in the study; 412 (12.3%) patients were in <6 months old age group (the mean age was 2.81±1.46 months). 423 (12.6%) patients were in 6 months to 1 year age group (the mean age was 8.92±1.88 months). 1033 (30.8%) patients were between 1 and 3 years age group (the mean age was 22.4±7.3 months). 733 (21.8%) patients were in 3-5 years age group (the mean age was 47.54±7.13 months). 757 (22.5%) patients were ≥5 years (the mean age was 90.5±22.2 months).
Comparison of clinical symptoms and signs in children with different ages
Children in <6 months age group had a lower incidence of fever than the other groups while children in 3-5 years and ≥5 years group had a higher incidence of fever (both P<0.05). Children in <6 months, 6 months-1 year and 1-3 years group had higher incidences of rales and presented more frequently with a productive cough, whereas children in ≥5 years group presented more frequently with a dry cough, the difference was statistically significant. Children in 6 months-1 year age group had the highest incidence of wheezing (57.9%), and children in ≥5 years age group had the lowest incidence of wheezing (both P<0.05). The incidences of rhinorrhea, tachypnea, cyanosis and hypoxemia in <6 months age group were significantly higher than those in other groups (all P<0.05). There was no difference in the incidence of dyspnea and length of hospital stay among different groups (Table 1).
Table 1.
Comparison of clinical symptoms and signs of MPP in children with different age groups
| Clinical characteristics | <6 months n=412, n (%) | 6~12 months n=423, n (%) | 1~3 years n=1033, n (%) | 3~5 years n=733, n (%) | ≥5 years n=757, n (%) | Χ2 or Z value | P |
|---|---|---|---|---|---|---|---|
| Male | 253 (61.4) | 287 (67.8) | 567 (54.9) | 347 (47.3) | 379 (50) | 59.55 | 0.000 |
| Fever | 86 (20.9) | 211 (49.9) | 684 (66.2) | 597 (81.4) | 633 (83.6) | 604.15 | 0.000 |
| <38°C | 46 (53.5) | 194 (45.9) | 340 (49.7) | 66 (11.1) | 32 (4.2) | 474.25 | 0.000 |
| 38-39°C | 25 (29.1) | 148 (34.9) | 218 (31.9) | 197 (33) | 105 (13.9) | 145.31 | 0.000 |
| ≥39°C | 15 (17.4) | 81 (19.2) | 126 (18.4) | 334 (55.9) | 620 (81.9) | 1228.51 | 0.000 |
| Cough | 409 (99.3) | 415 (98.1) | 1025 (99.2) | 733 (100) | 757 (100) | 22.68 | 0.000 |
| Dry cough | 58 (14.1) | 77 (18.2) | 301 (29.1) | 410 (55.9) | 622 (82.2) | 123.20 | 0.000 |
| Wet cough | 354 (85.9) | 346 (81.8) | 732 (70.9) | 323 (44.1) | 135 (17.8) | 154.50 | 0.000 |
| Wheezing | 173 (41.9) | 245 (57.9) | 426 (41.2) | 155 (21.1) | 59 (7.8) | 436.78 | 0.000 |
| Rhinorhea | 264 (64) | 141 (33.3) | 179 (17.3) | 194 (26.5) | 135 (17.8) | 375.67 | 0.000 |
| Tachypnea | 69 (16.7) | 49 (11.6) | 87 (8.4) | 45 (6.1) | 25 (3.3) | 74.83 | 0.000 |
| Dyspnea | 22 (5.3) | 21 (4.9) | 37 (3.6) | 25 (3.4) | 22 (2.9) | 6.36 | 0.174 |
| Cyanosis | 20 (4.9) | 8 (1.9) | 16 (1.5) | 7 (0.1) | 6 (1) | 30.98 | 0.000 |
| Rales | 372 (90.3) | 396 (93.6) | 909 (88) | 490 (66.9) | 165 (21.8) | 1180.72 | 0.000 |
| SaO2<92% | 64 (15.5) | 46 (10.9) | 82 (7.9) | 38 (7.4) | 21 (2.8) | 75.83 | 0.000 |
| Length of hospital stay (d) | 8.2±3.3 | 8.1±3.1 | 7.8±2.7 | 7.8±3.4 | 8.0±3.2 | 2.28 | 0.059 |
Comparison of radiographic findings in children with different ages
Children in <5 years age group more commonly displayed evidence of bronchopneumonia on chest radiographs compared with that of children in the ≥5 years age group (P<0.05) (Figure 1). Approximately 58.1% of the children in ≥5 year age group had segmental/lobar pneumonia (Figure 2), and frequently presented with pleural effusion and atelectasis. The incidence of interstitial pneumonia in children aged 6 months and 1-year age group was higher (6.3%) than that in other age groups (P<0.05) (Table 2).
Figure 1.
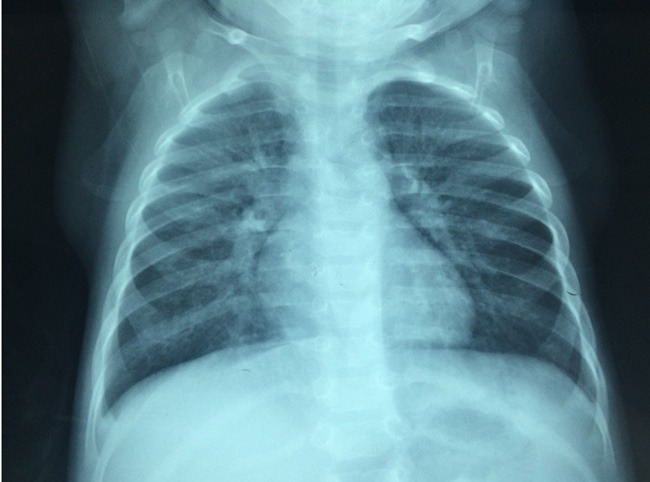
Radiographic characteristics of a patient with Mycoplasma pneumoniae pneumonia. Thickened lung markings accompanied by fuzzy, messy, reticular high-density shadows are seen on this chest X-ray.
Figure 2.
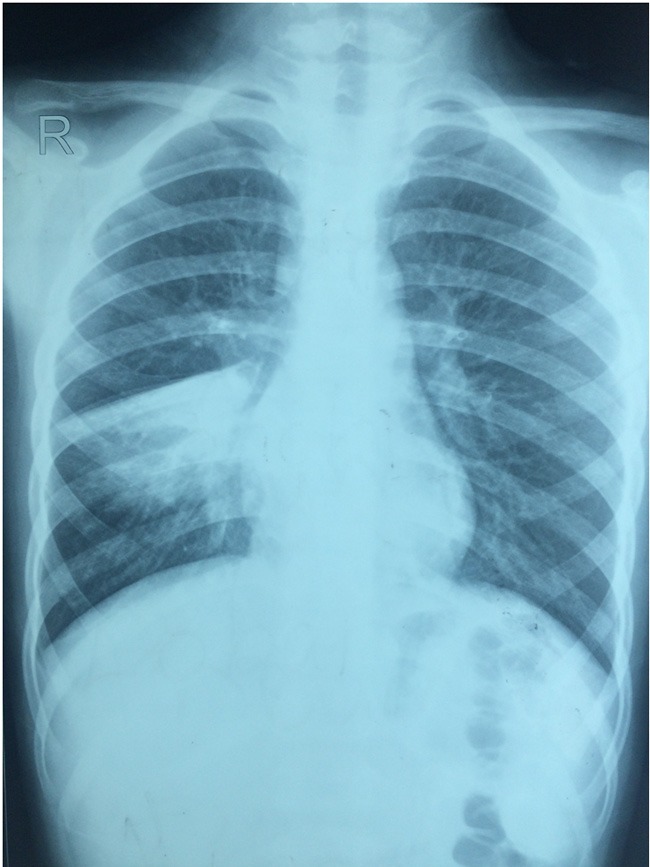
Radiographic characteristics of a patient with Mycoplasma pneumoniae pneumonia. The chest X-ray shows high density shadow in right middle lungs.
Table 2.
Comparison of radiographic findings of MPP in children with different ages
| <6 months n=412, n (%) | 6~12 months, n=423, n (%) | 1~3 years n=1033, n (%) | 3~5 years n=733, n (%) | ≥5 years n=757, n (%) | Χ2 or Z value | P | |
|---|---|---|---|---|---|---|---|
| Bronchopneumonia | 393 (95.4) | 407 (6.2) | 940 (90.9) | 620 (84.6) | 289 (38.2) | 1002.11 | 0.000 |
| Interstitial pneumonia | 17 (4.1) | 11 (2.6) | 65 (6.3) | 27 (3.6) | 28 (3.7) | 13.88 | 0.008 |
| Segmental/lobar pneumonia | 2 (0.5) | 5 (3.2) | 28 (2.7) | 86 (11.7) | 440 (58.1) | 1242.81 | 0.000 |
| Atelectasis | 3 (0.7) | 2 (0.5) | 5 (0.5) | 17 (2.3) | 98 (12.9) | 154.70 | 0.000 |
| Pleural effusion | 0 (0) | 1 (1.2) | 12 (1.2) | 24 (3.3) | 77 (10.2) | 149.09 | 0.000 |
Comparison of laboratory values in children with different ages
Significant differences were present in white blood cells (WBC) counts, proportion of neutrophils (N%), platelet (PLT) count and C-reaction protein (CRP) levels among different age groups. The percentage of ALT and creatine kinase isoenzyme (CKMB) elevation differed significantly among different age groups (Table 3).
Table 3.
Comparison of laboratory values associated with MPP in children with different ages
| <6 months n=412 | 6~12 months n=423 | 1~3 years n=1033 | 3~5 years n=733 | ≥5 years n=757 | Χ2 or Z value | P | |
|---|---|---|---|---|---|---|---|
| WBC, mean ± SD, ×109 | 10.45±4.90 | 10.59±4.95 | 9.97±5.22 | 8.99±4.76 | 8.40±3.72 | 26.21 | 0.000 |
| N%, mean ± SD | 37.10±19.36 | 39.54±17.89 | 48.62±18.0 | 57.08±16.66 | 61.79±16.10 | 229.27 | 0.000 |
| PLT, mean ± SD, ×109 | 365±114 | 354±113 | 320±101 | 297±98 | 297±100 | 53.63 | 0.000 |
| CRP, median (quartiles), mg/L | 0.985 (0.12-5.00) | 0.87 (0.17-6.63) | 1.93 (0.21-9.27) | 6.12 (0.81-13.71) | 10.26 (1.84-22.78) | 34.94 | 0.000 |
| ALT elevation, n (%) | 85 (20.6) | 90 (21.3) | 298 (28.8) | 230 (31.3) | 246 (32.5) | 32.39 | 0.000 |
| CK-MB, elevation, n (%) | 140 (33.9) | 130 (30.7) | 320 (30.9) | 145 (19.8) | 95 (12.5) | 118.81 | 0.000 |
Note: ALT elevation: ALT>35 U/L, CK-MB elevation: CK-MB>26 U/L.
Discussion
MP is one of the most common pathogens responsible for CAP in children, accounting for 10-30% CAP in China and 7-37% CAP in other countries [10,11]. Korppi, in a prospective study involving 210 CAP patients found MP infection in 30% of patients, and reported that MP was the most common pathogen in patients older than 5 years of age [2]. Juve’n T found MP infections in 26% of 254 CAP patients [12] and Chiang WC found the presence of MP in 20.3% of 1702 CAP patients [13]. In our presented study, we detected a prevalence of MP infection of 30.8%, similar to Korppi’s study, but lower than Chen CJ’s study [11]. MP was the most common single pathogen, indicating that it is an important pathogen in the causation of pneumonia. Keping Chen, showed that in a study of 1204 CAP patients in Nanjing from 2011 to 2013, 40.78% of CAP pneumonia patients were admitted due to MP infection, with pre-school age children most frequently infected with MP [14].
Previous studies have shown that MP infections mostly affect school-aged children and adolescents, and less frequently infants [2-4]. Domi’nguez A reported that 18% of CAP due to MP were younger than 5 years old [5]. Indeed, another Australian study found that 39% MP patients were younger than 5 years old [8]. Recently, MP infection has been increasingly reported in infants. Using throat swabs positive for MP-DNA-PCR, Defilippi found patients <2 years accounted for 56.4% of MP infections [15]. Sørensen CM demonstrated MP was more common in infants, and patients <1 year accounted for 18%, of whom the youngest was a 57-day-old infant [16]. In our study, we found that children in all age groups were predisposed to MPP. Further, patients <3 years accounted for 55.6% of all MPP, a higher number compared to reports from other countries. The result helped clinicians in our hospital to consider MP as a diagnosis in younger patients presenting with such clinical characteristics.
Although children in all age groups can be infected with MP, the clinical characteristics are not identical and often significantly differ. A study by Defilippi found cough and fever were the two most frequent symptoms in MP infection [15]. However, dyspnea, upper respiratory tract involvement, diarrhea and vomiting were reported as more common manifestations in patients <2 years old children [15]. Xia Y reported expectoration and wheezing, accompanied by low-grade fever as common presentations among infants with MP infection. They observed gastrointestinal symptoms as the most common extra-pulmonary manifestation among infants who had evident pulmonary signs. In contrast, the majority of the school-age children presented with high fever and severe dry cough. Studies have reported rash as the most common extra-pulmonary manifestation in these patients [17]. Our findings were similar to these reports. Furthermore, Youn YS reported that older children infected with MP had prolonged fevers and severe pulmonary lesions. Patients with segmental/lobar pneumonia were older in age, had a longer duration of fever and lower WBC and lymphocyte counts, compared with those with bronchopneumonia [18]. We found in the 3-5 years age group and ≥5 years age group, neutrophil and CRP level were frequently elevated. On the contrary, in the 1-3 years age group, lymphocytes were elevated and CRP was often normal, which is inconsistent with Yun-Ju Ma’s findings [19]. We speculate that wheezing was related to elevated PLT count to some extent for the finding that patients in the <6 months and 6-12 months age groups had higher PLT counts and a higher incidence of wheezing. A previous study found plasma levels of TGF-β and P-selectin were significantly higher in wheezing children, activating inflammation and therefore wheezing [20]. In another study, Defilippi found thrombocytosis was more frequent in the children <2 years [15]. Thrombocytosis was observed in 8% of patients at admission, however, thrombocytosis was reported in 33% of patients at discharge in a study by Youn YS. Thus, we hypothesize that the degree of platelet counts in MP infections may correlate with the degree of inflammation.
In Korppi’s study, MPP patients <4 years had a higher rate of hospitalization than patients >5 years old and hospitalized MPP patients were predisposed to dyspnea and wheezing [2]. Othman N reported that tachypnea and chest wall recession were significantly more common in children less than 5 years of age, than in children 5-15 years old [8]. Further, Yun-Ju Ma reported children younger than 5 years had significantly longer hospitalization rates, higher intensive care unit (ICU) admission rates, higher complication rates and were more likely to receive oxygen supplementation and even surgical intervention [19]. We found MPP in patients <6 months old was more severe, consistent with reports of other studies, and this may relate to the immaturity of the airway in such patients. No significant difference was found in length of hospitalization between different age groups, consistent with Yun-Ju Ma’ findings [19]. It still has not been clearly elucidated the mechanism of the differences in MPP individuals, however host immunity may play an important role [21].
Chest X-ray findings were quite different in MPP patients with different ages. Younger patients had mostly bronchopneumonia while older patients had segmental/lobar pneumonia. These findings are consistent with previous reports [15,18,22]. Indeed, MP-related lobar pneumonia and associated pleural effusion in older patients may be caused by overactive self-immune response.
MP infection has been reported to be closely related to wheezing [15,22,23]. The occurrence of wheezing in MP-related lower respiratory tract infections was 21% and 33%, respectively in studies by Choi and Defilippi. We found a higher rate of wheezing in infants with MP infection and the incidence was higher than that reported in the aforementioned reports. Furthermore, MP infection was the second common pathogen resulting in bronchiolitis, which leads to a longer wheezing duration than RSV [24]. Thus, MP should not be neglected in infants with prolonged wheezing when studies for other pathogens are negative.
There were some limitations in the present study. One major limitation is that we did not include outpatients with MPP which may influence our findings. Secondly, younger children with CAP had a greater tendency for hospitalization, compared to school-aged children.
In conclusion, the clinical characteristics of MPP patients in varying age groups are often different. In our study, the differences were more obvious between <6 months and ≥5 years age group. MPP patients of <6 months old presented mainly with a productive cough, wheezing accompanied by low to moderate grade fever, and had evident pulmonary signs. Gastrointestinal symptoms were the most common extra-pulmonary manifestation and their chest x-ray films showed bronchopneumonia. Lymphocyte proportion and PLT counts were elevated in patients and CRP was often normal. Conversely, MPP patients ≥5 years old presented with a high fever, a severe dry cough and often had less pulmonary signs. These patients often had a rash and abnormal LFTs as the most common extra-pulmonary manifestations. Chest X-ray findings showed segmental/lobar pneumonia with associated pleural effusion and atelectasis. Neutrophil counts and CRP were elevated in these patients. Furthermore, the clinical characteristics in 1-3 years and 3-5 years old patient groups were in between these manifestations. The differences observed between ages may be related to the mechanisms involved in lung injury. Further studies are needed to elucidate the exact molecular signaling events involved.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 81573167), Science and Technology Project of Suzhou (SYS201435), and Science and Technology Project of Suzhou Health Bureau (lczx201409).
Disclosure of conflict of interest
None.
References
- 1.Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17:697–728. doi: 10.1128/CMR.17.4.697-728.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korppi M, Heiskanen-Kosma T, Jalonen E, Saikku P, Leinonen M, Halonen P, Mäkela PH. Aetiology of community-acquired pneumonia in children treated in hospital. Eur J Pediatr. 1993;152:24–30. doi: 10.1007/BF02072512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammerschlag MR. Mycoplasma pneumoniae infections. Curr Opin Infect Dis. 2001;14:181–186. doi: 10.1097/00001432-200104000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Principi N, Esposito S, Blasi F, Allegra L Mowgli Study Group. Role of mycoplasma pneumoniae and chlamydia pneumoniae in children with community-acquired lower respiratory tract infections. Clin Infect Dis. 2001;32:1281–1289. doi: 10.1086/319981. [DOI] [PubMed] [Google Scholar]
- 5.Dominguez A, Minguell S, Torres J, Serrano A, Vidal J, Salleras L. Community outbreak of acute respiratory infection by mycoplasma pneumoniae. Eur J Epidemiol. 1996;12:131–134. doi: 10.1007/BF00145497. [DOI] [PubMed] [Google Scholar]
- 6.Bosnak M, Dikici B, Bosnak V, Dogru O, Ozkan I, Ceylan A, Haspolat K. Prevalence of mycoplasma pneumoniae in children in Diyarbakir, the south-east of Turkey. Pediatr Int. 2002;44:510–512. doi: 10.1046/j.1442-200x.2002.01606.x. [DOI] [PubMed] [Google Scholar]
- 7.Sun H, Chen Z, Yan Y, Huang L, Wang M, Ji W. Epidemiology and clinical profiles of mycoplasma pneumoniae infection in hospitalized infants younger than one year. Respir Med. 2015;109:751–757. doi: 10.1016/j.rmed.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Othman N, Isaacs D, Kesson A. Mycoplasma pneumoniae infections in Australian children. J Paediatr Child Health. 2005;41:671–676. doi: 10.1111/j.1440-1754.2005.00757.x. [DOI] [PubMed] [Google Scholar]
- 9.Ferwerda A, Moll HA, de Groot R. Respiratory tract infections by mycoplasma pneumoniae in children: a review of diagnostic and therapeutic measures. Eur J Pediatr. 2001;160:483–491. doi: 10.1007/s004310100775. [DOI] [PubMed] [Google Scholar]
- 10.Michelow IC, Olsen K, Lozano J, Rollins NK, Duffy LB, Ziegler T, Kauppila J, Leinonen M, McCracken GH Jr. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics. 2004;113:701–707. doi: 10.1542/peds.113.4.701. [DOI] [PubMed] [Google Scholar]
- 11.Chen CJ, Lin PY, Tsai MH, Huang CG, Tsao KC, Wong KS, Chang LY, Chiu CH, Lin TY, Huang YC. Etiology of community-acquired pneumonia in hospitalized children in northern Taiwan. Pediatr Infect Dis J. 2012;31:e196–201. doi: 10.1097/INF.0b013e31826eb5a7. [DOI] [PubMed] [Google Scholar]
- 12.Juvén T, Mertsola J, Waris M, Leinonen M, Meurman O, Roivainen M, Eskola J, Saikku P, Ruuskanen O. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J. 2000;19:293–298. doi: 10.1097/00006454-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Chiang WC, Teoh OH, Chong CY, Goh A, Tang JP, Chay OM. Epidemiology, clinical characteristics and antimicrobial resistance patterns of community-acquired pneumonia in 1702 hospitalized children in Singapore. Respirology. 2007;12:254–261. doi: 10.1111/j.1440-1843.2006.01036.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen K, Jia R, Li L, Yang C, Shi Y. The aetiology of community associated pneumonia in children in Nanjing, China and aetiological patterns associated with age and season. BMC Public Health. 2015;15:113. doi: 10.1186/s12889-015-1422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Defilippi A, Silvestri M, Tacchella A, Giacchino R, Melioli G, Di Marco E, Cirillo C, Di Pietro P, Rossi GA. Epidemiology and clinical features of mycoplasma pneumoniae infection in children. Respir Med. 2008;102:1762–1768. doi: 10.1016/j.rmed.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 16.Sorensen CM, Schonning K, Rosenfeldt V. Clinical characteristics of children with mycoplasma pneumoniae infection hospitalized during the Danish 2010-2012 epidemic. Dan Med J. 2013;60:A4632. [PubMed] [Google Scholar]
- 17.Xia Y, Wu CK, Tang YY, Cao J. Differences in the clinical features of mycoplasma pneumoniae pneumonia among children of different ages. Zhongguo Dang Dai Er Ke Za Zhi. 2013;15:179–182. [PubMed] [Google Scholar]
- 18.Youn YS, Lee KY, Hwang JY, Rhim JW, Kang JH, Lee JS, Kim JC. Difference of clinical features in childhood mycoplasma pneumoniae pneumonia. BMC Pediatr. 2010;10:48. doi: 10.1186/1471-2431-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma YJ, Wang SM, Cho YH, Shen CF, Liu CC, Chi H, Huang YC, Huang LM, Huang YC, Lin HC, Ho YH, Mu JJ. Clinical and epidemiological characteristics in children with community-acquired mycoplasma pneumonia in Taiwan: a nationwide surveillance. J Microbiol Immunol Infect. 2015;48:632–638. doi: 10.1016/j.jmii.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Ji W, Sun JY, Chen ZR. Role of platelet activation and platelet-related inflammatory factor in pneumonia children with wheezing. Jiangsu Med J. 2010;36:55–56. [Google Scholar]
- 21.Paats MS, Bergen IM, Hanselaar WE, Groeninx van Zoelen EC, Hoogsteden HC, Hendriks RW, van der Eerden MM. Local and systemic cytokine profiles in nonsevere and severe community-acquired pneumonia. Eur Respir J. 2013;41:1378–1385. doi: 10.1183/09031936.00060112. [DOI] [PubMed] [Google Scholar]
- 22.Guo Q, Li HY, Zhou YP, Li M, Chen XK, Peng HL, Yu HQ, Liang LH, Zhao QZ, Jiang M. Associations of radiological features in mycoplasma pneumoniae pneumonia. Arch Med Sci. 2014;10:725–732. doi: 10.5114/aoms.2014.44863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi IS, Byeon JH, Yoo Y, Lee KC, Choung JT. Increased serum interleukin-5 and vascular endothelial growth factor in children with acute mycoplasma pneumonia and wheeze. Pediatr Pulm. 2009;44:423–428. doi: 10.1002/ppul.20961. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Hao C, Ji W, Yan Y, Shao X, Xu J. Bronchiolitis associated with mycoplasma pneumoniae in infants in Suzhou China between 2010 and 2012. Sci Rep. 2015;5:7846. doi: 10.1038/srep07846. [DOI] [PMC free article] [PubMed] [Google Scholar]


