Abstract
Signal peptide-CUB-EGF (epidermal growth factor) domain-containing protein 2 (SCUBE2) is a secreted cell-surface glycoprotein. Decreased SCUBE2 expression has been reported in a variety of human cancers, including breast cancer, but its role in gastric cancer (GC) is still unknown. The present study was designed to evaluate the role of SCUBE2 expression in the prognosis of GC patients. SCUBE2 expression in GC tissues was detected by quantitative real-time reverse transcription polymerase chain reaction, Western blotting, and immunohistochemistry. The association between SCUBE2 expression and clinicopathological characteristics was evaluated using the Chi-square test. The Kaplan-Meier method and Cox proportional hazards models were applied to estimate the effect of SCUBE2 expression on survival. Our results show that expression of SCUBE2 in GC tissues is significantly lower than that in adjacent normal gastric mucosa tissues. Loss of SCUBE2 expression was associated with larger tumors (P = 0.001), advanced clinical stage (P = 0.001), T3 or T4 lesion (P = 0.017), lymph node metastasis (P = 0.033), higher histological grade (P = 0.041), and vascular invasion (P = 0.002). Patients with decreased SCUBE2 expression showed poorer recurrence free survival (RFS) and overall survival (OS) than those with higher SCUBE2 expression levels. Furthermore, multivariate analysis indicated that reduced expression of SCUBE2 was an independent prognostic factor predicting poor RFS (HR = 1.764, P = 0.029) and OS (HR = 1.811, P = 0.026). Therefore, expression of SCUBE2 in GC tends to be downregulated, and may serve an important role in predicting the prognosis of GC patients.
Keywords: SCUBE2, gastric cancer, prognosis, survival
Introduction
Globally, stomach cancer ranks fifth in cancer incidence and second in cancer deaths in 2013. In developing countries, however it ranks third in both incidence and mortality [1]. Despite advances in chemotherapy, radiotherapy, surgery, and targeted therapy, the five-year survival rate of patients with advanced gastric cancer (GC) is dismal. In order to improve outcome, new therapeutic strategies are urgently needed.
Signal peptide-CUB-EGF (epidermal growth factor, or EGF) domain-containing protein 2 (SCUBE2) belongs to a secreted, evolutionarily conserved SCUBE protein family, which is composed of three members, SCUBE1 to SCUBE3 [2-7]. SCUBE2 contain ~1000 amino acids and share an organized protein structure of at least five recognizable motifs: an N-terminal signal peptide sequence, followed by nine copies of EGF-like repeats, a spacer region, three cysteine-rich motifs, and one CUB domain at the C terminus [3,4]. SCUBE2 was first identified in zebrafish and confirmed to be an essential mediator of Hedgehog (HH) signaling in zebrafish embryos [7-9].
SCUBE2 is expressed in various tissues and cell types, including heart, lung, testis, primary osteoblasts, bone, and vascular endothelium [2,4,6]. In addition, SCUBE2 expression has been detected in breast, endometrial, colorectal, and oral squamous cancers [3,10-22]. Lin et al. suggested that human SCUBE2 is a novel positive component of Sonic Hedgehog (SHH) signaling, acting upstream of ligand binding at the plasma membrane [23]. Previous studies have shown that SCUBE2 expression is a factor predicting outcome in breast cancer [11,12,14-17]. Overexpression of ectopic SCUBE2 protein resulted in suppression of breast cancer cell proliferation and reduced tumor xenograft growth in nude mice [19]. Moreover, SCUBE2 has growth inhibitory effects through a coordinated regulation of two distinct mechanisms: antagonizing bone morphogenetic protein (BMP) and suppressing the β-catenin pathway [3]. On one hand, the COOH-terminal CUB domain could directly bind to and antagonize BMP activity in an autocrine manner. On the other hand, the NH2-terminal EGF-like repeats could mediate cell-cell homophilic adhesions in a calcium-dependent fashion, interacting with E-cadherin (a master tumor suppressor), and suppressing the β-catenin signaling pathway [3].
Additionally, SCUBE2 plays a key role in suppressing breast carcinoma cell mobility and invasiveness by increasing formation of epithelial E-cadherin-containing adherens junctions to promote epithelial differentiation and drive reversal of epithelial-mesenchymal transition [18]. Other studies have indicated that both estrogen receptor α (ER) and progesterone receptors (PR) protein levels were associated with expression level of SCUBE2 in breast cancer [10]. Likewise, expression of the SCUBE2 gene declined in high-grade endometrial cancer and was associated with the expression of steroid hormone receptors [20]. Recently, a study revealed that decreased expression of SCUBE2 is associated with progression and prognosis in colorectal cancer [21]. However, the role of SCUBE2 in GC remains elusive.
In this study, we evaluated the expression status of SCUBE2 in human GC tissues using immunohistochemistry (IHC), Western blotting, and quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR). We then explored the relationship between SCUBE2 expression and pathological data. Furthermore, we analyzed the impact of SCUBE2 expression on the recurrence free survival (RFS) and overall survival (OS) of GC patients.
Materials and methods
Ethics statement
This study was approved by the Human Ethics and Research Ethics Committees of the First Affiliated Hospital of Nanchang University. Patients were informed that the resected specimens were stored by our hospital and potentially used for scientific research, and that their privacy would be maintained. Follow-up survival data were collected retrospectively through medical record analyses.
Patients and tissue specimens
A total of 124 paraffin-embedded primary GC specimens and 124 matched non-cancerous tissue specimens (excised >5 cm away from the outer edge of the tumor) were collected at the Department of Pathology of the First Affiliated Hospital of Nanchang University, between December 2007 and November 2009. Another 45 pairs of cancerous and adjacent normal gastric mucosa tissues were stored at -80°C following surgery for Western blot analysis. None of the patients received any treatment prior to surgery. All cases were diagnosed with gastric adenocarcinoma by pathological examination after surgery. Tumor-node-metastasis stages were classified according to the American Joint Committee on cancer. Characteristics of these patients are summarized in Table 1.
Table 1.
Association between FAM83B expression and clinicopathological parameters in GC patients
| Parameters | Total | FAM83B expression, n | χ2 | p value | |
|---|---|---|---|---|---|
|
| |||||
| High | Low | ||||
| Gender | |||||
| Male | 74 | 28 | 46 | 1.265 | 0.261 |
| Female | 50 | 24 | 26 | ||
| Age (years) | |||||
| <60 | 63 | 29 | 34 | 0.883 | 0.348 |
| ≥60 | 61 | 23 | 38 | ||
| Location of tumor | |||||
| The proximal 1/2 | 31 | 9 | 22 | 2.826 | 0.093 |
| The distal 1/2 | 93 | 43 | 50 | ||
| Preoperative CEA (ng/ml) | |||||
| <5 | 88 | 39 | 49 | 0.707 | 0.401 |
| ≥5 | 36 | 13 | 23 | ||
| Tumor size (cm) | |||||
| <5 | 85 | 44 | 41 | 10.723 | 0.001 |
| ≥5 | 39 | 8 | 31 | ||
| Clinical stage | |||||
| I-II | 42 | 26 | 16 | 10.401 | 0.001 |
| III-IV | 82 | 26 | 56 | ||
| Depth of invasion | |||||
| T1-T2 | 38 | 22 | 16 | 5.731 | 0.017 |
| T3-T4 | 86 | 30 | 56 | ||
| Lymph node status | |||||
| N0-N1 | 60 | 31 | 29 | 4.521 | 0.033 |
| N2-N3 | 64 | 21 | 43 | ||
| Distant metastasis | |||||
| No | 111 | 49 | 62 | 2.121 | 0.145 |
| Yes | 13 | 3 | 10 | ||
| Histological grade | |||||
| Well | 9 | 7 | 2 | 6.366 | 0.041 |
| Moderately | 34 | 16 | 18 | ||
| Poor | 81 | 29 | 52 | ||
| Nerve invasion | |||||
| No | 51 | 24 | 27 | 0.934 | 0.334 |
| Yes | 73 | 28 | 45 | ||
| Vascular invasion | |||||
| No | 68 | 37 | 31 | 9.625 | 0.002 |
| Yes | 56 | 15 | 41 | ||
Bold numbers indicate a significant association among the variables.
Quantitative real-time reverse transcription polymerase chain reaction analysis
In brief, total RNA was extracted from collected samples using TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instructions. RNA concentrations were estimated by reading the spectrophotometric absorbance at 260 nm. qRT-PCR was carried out according to the standard protocol on a Real-Time PCR system with SYBR Green detection. β-actin was used as an endogenous control to normalize for differences in the amount of total RNA in each sample. Primer sequences for the amplification of human genes were used as follows: SCUBE2 (89 bp, forward primer: 5’-ACCCAGTGTAAAACTTCATCCA-3’; reverse primer: 5’-TTTGACCTGGAGGTGAAGGC-3’), and β-actin (295 bp, forward primer: 5’-TCACCCACACTGTGCCCATCATCGA-3’; reverse primer: 5’-CAGCGGAACCGCTCATTGCCAATGG-3’).
Western blot analysis
RIPA lysis buffer was used to extract total proteins from cancerous and adjacent normal mucosa specimens. Equal amounts of protein were separated by 8% sodium dodecyl sulfate polyacrylamide gel and transferred onto nitrocellulose membranes. After being blocked with 5% skim milk, the membranes were incubated overnight at 4°C with primary antibodies against SCUBE2 (1:125, Abcam, USA) or β-actin (1:1000, Zhongshanjinqiao, China), and then incubated for 1 h with horseradish peroxidase (HRP)-conjugated secondary antibodies at room temperature (~20°C). The immunoreactive bands were detected using an enhanced chemiluminescence system. The band density of SCUBE2 was measured with ImageJ software and standardized to β-actin.
Immunohistochemical staining
Paraffin-embedded specimens were cut into 4-µm-thick sections and were deparaffinized in dimethylbenzene and rehydrated with a graded ethanol gradient. An antigen retrieval process was performed at high temperature and high pressure with citrate buffer (pH 6.0) before blocking the endogenous peroxidase with 3% hydrogen peroxide solution. The sections were then incubated with rabbit anti-SCUBE2 antibody (1:50, ab17037, Abcam, USA) at 4°C overnight. As a negative control, sections were incubated with phosphate-buffered saline (PBS) instead of the primary antibody. After washing with PBS, the sections were incubated with HRP-conjugated second antibodies (Maixin, Fuzhou, China) for 1 h at room temperature. The sections were stained with DAB solution (Maixin, Fuzhou, China) and counterstained with hematoxylin.
Evaluation of immunostaining
The sections were observed and photographed under a light microscope (Olympus, Tokyo, Japan). All specimens were evaluated and scored independently by two pathologists who did not possess knowledge of the clinical data. Any disagreements were arbitrated by a third pathologist. The immunohistochemistry results of SCUBE2 were evaluated by the immunoreactive scores as previously described. The intensity of stained cells was evaluated and scored as 0 (no staining), 1 (weak staining), 2 (moderate staining), or 3 (strong staining). The percentage of positive cells was graded from 0 to 4: 0% (score 0), <25% (score 1), 25-50% (score 2), 50-75% (score 3), or >75% (score 4). The total immunostaining score was calculated as the percent positivity score × staining intensity score, with results ranging from 0 to 12. For survival data analysis, scores of 0-4 were considered as low expression while scores of 5-12 were defined as high expression.
Statistical analysis
Statistical analyses were performed using SPSS 17.0 software. The association between SCUBE2 expression and clinicopathological parameters was evaluated using Pearson’s χ2 test. Kaplan-Meier survival analysis was applied to evaluate the prognostic relevance of SCUBE2 and the survival difference between groups was assessed by log-rank test. Univariate and multivariate survival analyses were performed using Cox proportional hazard regression models. All tests were two-tailed and p-values of less than 0.05 were considered statistically significant.
Results
Expression of SCUBE2 in GC tissue samples by qRT-PCR and Western blotting
To determine the expression of SCUBE2 in GC, 45 fresh-frozen GC tissue samples and their corresponding adjacent non-cancerous samples were collected. The results of qRT-PCR indicated that SCUBE2 mRNA levels in GC tissues were significantly decreased compared with the corresponding adjacent non-cancerous tissues (Figure 1). Consistently, the results of Western blotting showed that protein levels of SCUBE2 in GC cases were markedly reduced compared with those in the corresponding adjacent non-cancerous tissues (Figure 2).
Figure 1.
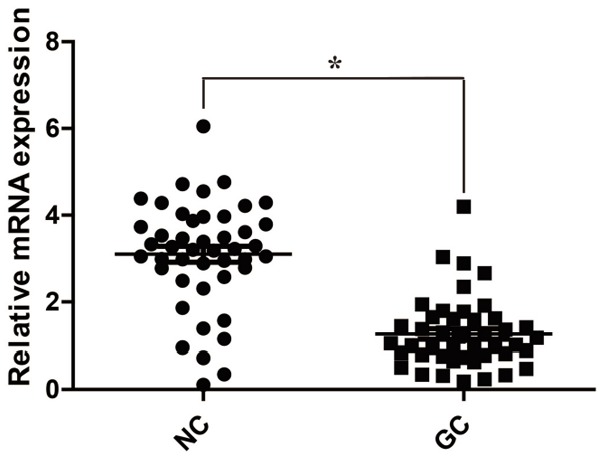
Reduced SCUBE2 mRNA expression in GC. qRT-PCR results indicate that relative expression of SCUBE2 mRNA in GC tissues is significantly decreased compared with that in the adjacent noncancerous tissues. GC: gastric cancer tissues; NC: noncancerous tissues. *P<0.001.
Figure 2.
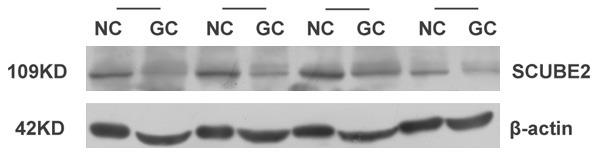
Analysis of SCUBE2 protein expression in GC tissues and adjacent noncancerous tissues by Western blotting. Protein expression of SCUBE2 in GC tissues was significantly lower than that in adjacent noncancerous tissues.
Expression of SCUBE2 in GC tissue samples by IHC
To further confirm decreased expression of SCUBE2 in GC, 124 paraffin-embedded samples of paired carcinomatous and the corresponding adjacent non-cancerous GC tissues were collected. The clinicopathological features of the specimens are shown in Table 1. SCUBE2 was predominantly expressed in the cytoplasm of the positively stained cells, and representative images of SCUBE2 in GC tissues and in the corresponding adjacent non-cancerous tissues are shown in Figure 3. The results of IHC showed high expression of SCUBE2 in 62.9% (78/124) of the non-cancerous tissues, but only in 41.9% (52/124) of the GC tissues. According to the final IHC score, SCUBE2 expression in GC tissues was significantly lower than that in non-cancerous tissues (P = 0.001).
Figure 3.
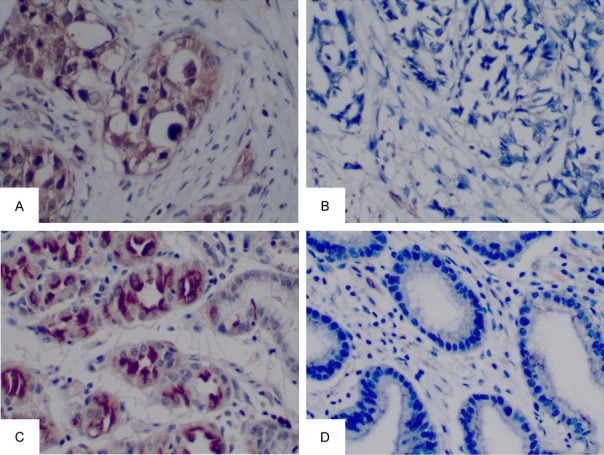
Immunochemical staining for SCUBE2 in GC tissues (A, B) and adjacent noncancerous tissues (C, D). (A) High expression of SCUBE2 in GC. (B) Low expression of SCUBE2 in GC. (C) High expression of SCUBE2 in corresponding adjacent noncancerous tissues. (D) Low expression of SCUBE2 in corresponding adjacent noncancerous tissues (magnification 200×).
Association between SCUBE2 expression and clinicopathological features
To uncover the functions of SCUBE2 in GC, we explored the relationship between SCUBE2 expression and the clinicopathological parameters of GC patients. The results suggested that SCUBE2 expression was closely associated with tumor size (P = 0.001), clinical stage (P = 0.001), depth of invasion (P = 0.017), lymph node status (P = 0.033), histological grade (P = 0.041), and vascular invasion (P = 0.002). In contrast, no linkage was found between SCUBE2 expression and gender (P = 0.261), age (P = 0.348), location of tumor (P = 0.093), pre-operative carcinoembryonic antigen (CEA) (P = 0.401), distant metastasis (P = 0.145), or nerve invasion (P = 0.334).
Relationship between SCUBE2 expression and survival
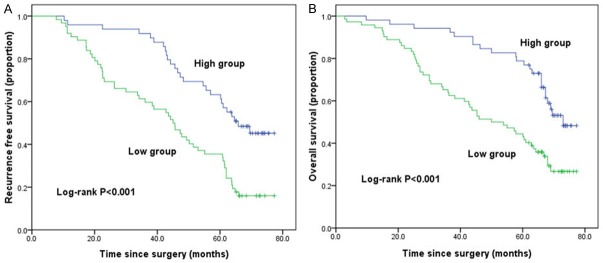
To evaluate the potential prognostic value of SCUBE2, the RFS and OS curves were delineated using the Kaplan-Meier method and compared using the log-rank test (Figure 4). According to the IHC score, patients were divided into two groups defined as low SCUBE2 expression and high SCUBE2 expression. The median recurrence free survival (mRFS) was significantly poorer in patients with low SCUBE2 expression compared to those with high SCUBE2 expression (44.2 vs. 65.8 m, P<0.001, Figure 4A). Furthermore, patients with high SCUBE2 expression had a favorable OS compared to those with low SCUBE2 expression (73 vs. 50 m, P<0.001, Figure 4B).
Figure 4.
Kaplan-Meier analysis of RFS and OS rates grouped according to SCUBE2 expression. GC patients with low SCUBE2 expression had poorer RFS (A, P<0.001) and OS (B, P<0.001) rates than those with high SCUBE2 expression.
Univariate and multivariate analyses of RFS
The data of univariate analysis showed that tumor size, clinical stage, depth of invasion, lymph node status, histological grade, vascular invasion, and SCUBE2 expression were significantly associated with RFS (Table 2). Moreover, multivariate Cox regression analysis confirmed clinical stage, depth of invasion, lymph node status, histological grade, vascular invasion, and SCUBE2 expression as independent prognostic factors of RFS for GC patients.
Table 2.
Univariate and multivariate analyses of RFS
| Parameters | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | p | HR | 95% CI | p | |
| Gender | ||||||
| Female vs. Male | 0.860 | 0.546-1.353 | 0.514 | |||
| Age (years) | ||||||
| ≥60 years vs. <60 years | 1.303 | 0.831-2.044 | 0.249 | |||
| Location of tumor | ||||||
| The distal 1/2 vs. The proximal 1/2 | 0.850 | 0.511-1.415 | 0.533 | |||
| Preoperative CEA (ng/ml) | ||||||
| ≥5 ng/ml vs. <5 ng/ml | 1.593 | 0.986-2.573 | 0.057 | |||
| Tumor size (cm) | ||||||
| ≥5 cm vs. <5 cm | 2.486 | 1.566-3.948 | <0.001 | 1.108 | 0.654-1.877 | 0.704 |
| Clinical stage | ||||||
| III-IV vs. I-II | 5.821 | 3.287-10.309 | <0.001 | 2.628 | 1.223-5.646 | 0.013 |
| Depth of invasion | ||||||
| T3-T4 vs. T1-T2 | 5.001 | 2.763-9.050 | <0.001 | 2.860 | 1.335-6.127 | 0.007 |
| Lymph node status | ||||||
| N2-N3 vs. N0-N1 | 2.921 | 1.840-4.639 | <0.001 | 2.113 | 1.218-3.668 | 0.008 |
| Histological grade | ||||||
| Poor vs. Well + Moderately | 1.966 | 1.213-3.186 | 0.006 | 1.834 | 1.046-3.217 | 0.034 |
| Nerve invasion | ||||||
| Yes vs. No | 1.249 | 0.795-1.961 | 0.335 | |||
| Vascular invasion | ||||||
| Yes vs. No | 2.709 | 1.725-4.255 | <0.001 | 1.842 | 1.132-2.999 | 0.014 |
| SCUBE2 expression | ||||||
| Low vs. High | 2.468 | 1.535-3.969 | <0.001 | 1.746 | 1.060-2.875 | 0.029 |
HR hazard ratio, CI confidence interval. Bold numbersindicate statistical significance, P<0.05.
Univariate and multivariate analyses of OS
We also utilized Cox proportional hazard regression models to perform univariate and multivariate analyses of OS for GC patients. The results showed that OS was correlated with tumor size, clinical stage, depth of invasion, lymph node status, distant metastasis, histological grade, vascular invasion, and SCUBE2 expression (Table 3). Furthermore, clinical stage, depth of invasion, lymph node status, distant metastasis, histological grade, vascular invasion, and SCUBE2 expression were independent prognostic factors of OS for GC patients.
Table 3.
Univariate and multivariate analyses of OS
| Parameters | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | p | HR | 95% CI | p | |
| Gender | ||||||
| Female vs. Male | 0.922 | 0.578-1.470 | 0.733 | |||
| Age (years) | ||||||
| ≥60 years vs. <60 years | 0.852 | 0.538-1.348 | 0.493 | |||
| Location of tumor | ||||||
| The distal 1/2 vs. The proximal 1/2 | 0.774 | 0.462-1.297 | 0.330 | |||
| Preoperative CEA (ng/ml) | ||||||
| ≥5 ng/ml vs. <5 ng/ml | 1.460 | 0.900-2.369 | 0.125 | |||
| Tumor size (cm) | ||||||
| ≥5 cm vs. <5 cm | 2.598 | 1.623-4.157 | <0.001 | 1.398 | 0.803-2.432 | 0.236 |
| Clinical stage | ||||||
| III-IV vs. I-II | 7.942 | 3.888-16.223 | <0.001 | 2.717 | 1.024-7.209 | 0.045 |
| Depth of invasion | ||||||
| T3-T4 vs. T1-T2 | 7.631 | 3.595-16.199 | <0.001 | 2.756 | 1.029-7.381 | 0.044 |
| Lymph node status | ||||||
| N2-N3 vs. N0-N1 | 3.176 | 1.931-5.225 | <0.001 | 1.971 | 1.107-3.510 | 0.021 |
| Distant metastasis | ||||||
| Yes vs. No | 80.695 | 26.893-242.131 | <0.001 | 82.496 | 24.567-277.021 | <0.001 |
| Histological grade | ||||||
| Poor vs. Well + Moderately | 3.526 | 1.933-6.434 | <0.001 | 2.529 | 1.306-4.899 | 0.006 |
| Nerve invasion | ||||||
| Yes vs. No | 1.409 | 0.876-2.268 | 0.157 | |||
| Vascular invasion | ||||||
| Yes vs. No | 2.343 | 1.469-3.738 | <0.001 | 1.690 | 1.006-2.839 | 0.048 |
| SCUBE2 expression | ||||||
| Low vs. High | 2.461 | 1.496-4.050 | <0.001 | 1.811 | 1.075-3.051 | 0.026 |
HR hazard ratio, CI confidence interval. Bold numbersindicate statistical significance, P <0.05.
Discussion
SCUBE2, a member of the SCUBE family, is a secreted cell-surface glycoprotein that exists widely in normal or cancerous tissue. Recent studies have shown that decreased expression of SCUBE2 is associated with poor prognosis in breast, endometrial, colorectal, and oral squamous cancer [11,12,14-16,18-22]. Human SCUBE2 can specifically interact with the cholesterol anchor of SHH and the SHH receptor PTCH1 (Patched-1), and enhance SHH signaling activity within the cholesterol-rich raft microdomains of the plasma membranes [23,24]. SHH signaling has been extensively studied for its role in GC. Kim et al. suggested that SHH overexpression may be a marker of favorable prognosis in GC [25]. In contrast, another study confirmed that a high level of SHH mRNA was associated with poor prognosis [26]. Moreover, SHH signaling could promote motility, invasiveness, metastasis, and proliferation of GC cells [27-29]. Beyond these reports, the function of SCUBE2 in GC tumorigenesis and progression remains controversial.
In this study, qRT-PCR and Western blotting showed that SCUBE2 expression was markedly decreased in GC tissues in comparison to that in non-cancerous tissues at both the mRNA and protein levels. Moreover, we used IHC staining to examine the expression level of SCUBE2 in 124 GC patients, and the results were consistent with the findings of Western blotting. According to our IHC results, the SCUBE2 protein was predominantly allocated in the cytoplasm. This finding was different from that reported in previous studies where SCUBE2 was expressed on the cell surface [2,19,23], and this phenomenon requires further investigation. In light of previously published data, decreased SCUBE2 expression was significantly associated with advanced clinical stage, T4 or T3 lesion, lymph node metastasis, distant metastasis, and higher histological grade in patients with colorectal cancer [21]. Similarly, the present study found that reduced SCUBE2 expression was markedly correlated with larger tumor, advanced clinical stage, T4 or T3 lesion, lymph node metastasis, higher histological grade, and vascular invasion, but no information confirming the relationship between SCUBE2 and distant metastasis was available in our study.
The Kaplan-Meier method was utilized to evaluate the impact of SCUBE2 expression on RFS and OS of GC patients. The results show that both RFS and OS of GC patients with decreased SCUBE2 expression were significantly poorer than those in patients with high levels of SCUBE2 expression. In addition, multivariate analysis performed with the Cox proportional hazard regression model confirmed that SCUBE2 is an independent prognostic factor for RFS and OS in GC patients, along with depth of invasion, lymph node status, clinical stage, histological grade, and vascular invasion. Furthermore, distant metastasis was also shown to be an independent prognostic factor for OS.
In conclusion, our results show that SCUBE2 is frequently downregulated in gastric carcinoma and is related to tumor size, clinical stage, depth of invasion, lymph node status, histological grade, and vascular invasion. Moreover, loss of SCUBE2 expression was found to be an independent predictor of poor prognosis. Therefore, our data demonstrated that SCUBE2 may be used as a novel drug target for GC.
Acknowledgements
The study was supported by the National Natural Science Foundation of China (No. 81660402), and a grant from the Science and Technology Department of Jiangxi Province (No. 20171BBH80027).
Disclosure of conflict of interest
None.
References
- 1.Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, Wolfe C, Hamadeh RR, Moore A, Werdecker A, Gessner BD, Te Ao B, McMahon B, Karimkhani C, Yu C, Cooke GS, Schwebel DC, Carpenter DO, Pereira DM, Nash D, Kazi DS, De Leo D, Plass D, Ukwaja KN, Thurston GD, Yun Jin K, Simard EP, Mills E, Park EK, Catala-Lopez F, deVeber G, Gotay C, Khan G, Hosgood HD 3rd, Santos IS, Leasher JL, Singh J, Leigh J, Jonas JB, Sanabria J, Beardsley J, Jacobsen KH, Takahashi K, Franklin RC, Ronfani L, Montico M, Naldi L, Tonelli M, Geleijnse J, Petzold M, Shrime MG, Younis M, Yonemoto N, Breitborde N, Yip P, Pourmalek F, Lotufo PA, Esteghamati A, Hankey GJ, Ali R, Lunevicius R, Malekzadeh R, Dellavalle R, Weintraub R, Lucas R, Hay R, Rojas-Rueda D, Westerman R, Sepanlou SG, Nolte S, Patten S, Weichenthal S, Abera SF, Fereshtehnejad SM, Shiue I, Driscoll T, Vasankari T, Alsharif U, Rahimi-Movaghar V, Vlassov VV, Marcenes WS, Mekonnen W, Melaku YA, Yano Y, Artaman A, Campos I, MacLachlan J, Mueller U, Kim D, Trillini M, Eshrati B, Williams HC, Shibuya K, Dandona R, Murthy K, Cowie B, Amare AT, Antonio CA, Castaneda-Orjuela C, van Gool CH, Violante F, Oh IH, Deribe K, Soreide K, Knibbs L, Kereselidze M, Green M, Cardenas R, Roy N, Tillmann T, Li Y, Krueger H, Monasta L, Dey S, Sheikhbahaei S, Hafezi-Nejad N, Kumar GA, Sreeramareddy CT, Dandona L, Wang H, Vollset SE, Mokdad A, Salomon JA, Lozano R, Vos T, Forouzanfar M, Lopez A, Murray C, Naghavi M. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1:505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang RB, Ng CK, Wasserman SM, Colman SD, Shenoy S, Mehraban F, Komuves LG, Tomlinson JE, Topper JN. Identification of a novel family of cell-surface proteins expressed in human vascular endothelium. J Biol Chem. 2002;277:46364–46373. doi: 10.1074/jbc.M207410200. [DOI] [PubMed] [Google Scholar]
- 3.Lin YC, Chen CC, Cheng CJ, Yang RB. Domain and functional analysis of a novel breast tumor suppressor protein, SCUBE2. J Biol Chem. 2011;286:27039–27047. doi: 10.1074/jbc.M111.244418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu BT, Su YH, Tsai MT, Wasserman SM, Topper JN, Yang RB. A novel secreted, cell-surface glycoprotein containing multiple epidermal growth factor-like repeats and one CUB domain is highly expressed in primary osteoblasts and bones. J Biol Chem. 2004;279:37485–37490. doi: 10.1074/jbc.M405912200. [DOI] [PubMed] [Google Scholar]
- 5.Grimmond S, Larder R, Van Hateren N, Siggers P, Hulsebos TJ, Arkell R, Greenfield A. Cloning, mapping, and expression analysis of a gene encoding a novel mammalian EGF-related protein (SCUBE1) Genomics. 2000;70:74–81. doi: 10.1006/geno.2000.6370. [DOI] [PubMed] [Google Scholar]
- 6.Grimmond S, Larder R, Van Hateren N, Siggers P, Morse S, Hacker T, Arkell R, Greenfield A. Expression of a novel mammalian epidermal growth factor-related gene during mouse neural development. Mech Dev. 2001;102:209–211. doi: 10.1016/s0925-4773(00)00586-4. [DOI] [PubMed] [Google Scholar]
- 7.Woods IG, Talbot WS. The you gene encodes an EGF-CUB protein essential for hedgehog signaling in zebrafish. PLoS Biol. 2005;3:e66. doi: 10.1371/journal.pbio.0030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollway GE, Maule J, Gautier P, Evans TM, Keenan DG, Lohs C, Fischer D, Wicking C, Currie PD. Scube2 mediates Hedgehog signalling in the zebrafish embryo. Dev Biol. 2006;294:104–118. doi: 10.1016/j.ydbio.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 9.Kawakami A, Nojima Y, Toyoda A, Takahoko M, Satoh M, Tanaka H, Wada H, Masai I, Terasaki H, Sakaki Y, Takeda H, Okamoto H. The zebrafish-secreted matrix protein you/scube2 is implicated in long-range regulation of hedgehog signaling. Curr Biol. 2005;15:480–488. doi: 10.1016/j.cub.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Andres SA, Wittliff JL. Co-expression of genes with estrogen receptor-alpha and progesterone receptor in human breast carcinoma tissue. Horm Mol Biol Clin Investig. 2012;12:377–390. doi: 10.1515/hmbci-2012-0025. [DOI] [PubMed] [Google Scholar]
- 11.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Navarro I, Gamez-Pozo A, Pinto A, Hardisson D, Madero R, Lopez R, San Jose B, Zamora P, Redondo A, Feliu J, Cejas P, Gonzalez Baron M, Angel Fresno Vara J, Espinosa E. An 8-gene qRT-PCR-based gene expression score that has prognostic value in early breast cancer. BMC Cancer. 2010;10:336. doi: 10.1186/1471-2407-10-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calza S, Hall P, Auer G, Bjohle J, Klaar S, Kronenwett U, Liu ET, Miller L, Ploner A, Smeds J, Bergh J, Pawitan Y. Intrinsic molecular signature of breast cancer in a population-based cohort of 412 patients. Breast Cancer Res. 2006;8:R34. doi: 10.1186/bcr1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habel LA, Shak S, Jacobs MK, Capra A, Alexander C, Pho M, Baker J, Walker M, Watson D, Hackett J, Blick NT, Greenberg D, Fehrenbacher L, Langholz B, Quesenberry CP. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res. 2006;8:R25. doi: 10.1186/bcr1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parris TZ, Kovacs A, Aziz L, Hajizadeh S, Nemes S, Semaan M, Forssell-Aronsson E, Karlsson P, Helou K. Additive effect of the AZGP1, PIP, S100A8 and UBE2C molecular biomarkers improves outcome prediction in breast carcinoma. Int J Cancer. 2014;134:1617–1629. doi: 10.1002/ijc.28497. [DOI] [PubMed] [Google Scholar]
- 16.Andres SA, Brock GN, Wittliff JL. Interrogating differences in expression of targeted gene sets to predict breast cancer outcome. BMC Cancer. 2013;13:326. doi: 10.1186/1471-2407-13-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parris TZ, Danielsson A, Nemes S, Kovacs A, Delle U, Fallenius G, Mollerstrom E, Karlsson P, Helou K. Clinical implications of gene dosage and gene expression patterns in diploid breast carcinoma. Clin Cancer Res. 2010;16:3860–3874. doi: 10.1158/1078-0432.CCR-10-0889. [DOI] [PubMed] [Google Scholar]
- 18.Lin YC, Lee YC, Li LH, Cheng CJ, Yang RB. Tumor suppressor SCUBE2 inhibits breast-cancer cell migration and invasion through the reversal of epithelial-mesenchymal transition. J Cell Sci. 2014;127:85–100. doi: 10.1242/jcs.132779. [DOI] [PubMed] [Google Scholar]
- 19.Cheng CJ, Lin YC, Tsai MT, Chen CS, Hsieh MC, Chen CL, Yang RB. SCUBE2 suppresses breast tumor cell proliferation and confers a favorable prognosis in invasive breast cancer. Cancer Res. 2009;69:3634–3641. doi: 10.1158/0008-5472.CAN-08-3615. [DOI] [PubMed] [Google Scholar]
- 20.Skrzypczak M, Lattrich C, Haring J, Schuler S, Ortmann O, Treeck O. Expression of SCUBE2 gene declines in high grade endometrial cancer and associates with expression of steroid hormone receptors and tumor suppressor PTEN. Gynecol Endocrinol. 2013;29:1031–1035. doi: 10.3109/09513590.2013.829441. [DOI] [PubMed] [Google Scholar]
- 21.Song Q, Li C, Feng X, Yu A, Tang H, Peng Z, Wang X. Decreased expression of SCUBE2 is associated with progression and prognosis in colorectal cancer. Oncol Rep. 2015;33:1956–1964. doi: 10.3892/or.2015.3790. [DOI] [PubMed] [Google Scholar]
- 22.Parris TZ, Aziz L, Kovacs A, Hajizadeh S, Nemes S, Semaan M, Chen CY, Karlsson P, Helou K. Clinical relevance of breast cancer-related genes as potential biomarkers for oral squamous cell carcinoma. BMC Cancer. 2014;14:324. doi: 10.1186/1471-2407-14-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai MT, Cheng CJ, Lin YC, Chen CC, Wu AR, Wu MT, Hsu CC, Yang RB. Isolation and characterization of a secreted, cell-surface glycoprotein SCUBE2 from humans. Biochem J. 2009;422:119–128. doi: 10.1042/BJ20090341. [DOI] [PubMed] [Google Scholar]
- 24.Tukachinsky H, Kuzmickas RP, Jao CY, Liu J, Salic A. Dispatched and scube mediate the efficient secretion of the cholesterol-modified hedgehog ligand. Cell Rep. 2012;2:308–320. doi: 10.1016/j.celrep.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JY, Ko GH, Lee YJ, Ha WS, Choi SK, Jung EJ, Jeong CY, Ju YT, Jeong SH, Hong SC. Prognostic value of sonic hedgehog protein expression in gastric cancer. Jpn J Clin Oncol. 2012;42:1054–1059. doi: 10.1093/jjco/hys137. [DOI] [PubMed] [Google Scholar]
- 26.Saze Z, Terashima M, Kogure M, Ohsuka F, Suzuki H, Gotoh M. Activation of the sonic hedgehog pathway and its prognostic impact in patients with gastric cancer. Dig Surg. 2012;29:115–123. doi: 10.1159/000336949. [DOI] [PubMed] [Google Scholar]
- 27.Wan J, Zhou J, Zhao H, Wang M, Wei Z, Gao H, Wang Y, Cui H. Sonic hedgehog pathway contributes to gastric cancer cell growth and proliferation. Biores Open Access. 2014;3:53–59. doi: 10.1089/biores.2014.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoo YA, Kang MH, Kim JS, Oh SC. Sonic hedgehog signaling promotes motility and invasiveness of gastric cancer cells through TGF-beta-mediated activation of the ALK5-Smad 3 pathway. Carcinogenesis. 2008;29:480–490. doi: 10.1093/carcin/bgm281. [DOI] [PubMed] [Google Scholar]
- 29.Yoo YA, Kang MH, Lee HJ, Kim BH, Park JK, Kim HK, Kim JS, Oh SC. Sonic hedgehog pathway promotes metastasis and lymphangiogenesis via activation of Akt, EMT, and MMP-9 pathway in gastric cancer. Cancer Res. 2011;71:7061–7070. doi: 10.1158/0008-5472.CAN-11-1338. [DOI] [PubMed] [Google Scholar]