Abstract
Background: Chemotherapy resistance is a great obstacle in effective treatment for metastatic triple negative breast cancer (TNBC). The ability to predict chemotherapy response would allow chemotherapy administration to be directed toward only those patients who would benefit, thus maximizing treatment efficiency. Differentially expressed plasma proteins may serve as putative biomarkers for predicting chemotherapy outcomes. Patients and methods: In this study, 26 plasma samples (10 samples with partial response (S) and 16 samples with progression disease (R)) from patients with metastatic TNBC were measured by Tandem Mass Tag (TMT)-based proteomics analysis to identify differentially expressed proteins between the S and R group. Potential proteinswere validated with enzyme-linked immunosorbent assay (ELISA) in another 67 plasma samples. Results: A total of 320 plasma proteins were identified, and statistical analysis showed that 108 proteins were significantly dysregulated between R and S groups in the screening stage. Bioinformatics revealed relevant pathways and regulatory networks of the differentially expressed proteins. Three differentially expressed proteins were validated by ELISA with 67 samples from TNBC patients. The R group had significantly higher plasma CAMK2A level than the S group (P=0.0074). The ROC curve analysis showed an AUC of 0.708, with sensitivity 48.4% and specificity 86.1%. In multivariate logistic regression analysis, the level of plasma CAMK2A was also significant for chemotherapeutic response (P=0.009, OR=0.152). Furthermore, the patients with higher CAMK2A level had shorter OS than those with lower CAMK2A level, which amounted to 13.9 and 28.9 months, respectively (P=0.034). In the multivariate Cox regression analysis, CAMK2A level still had significant effect on OS (P=0.031, HR=1.865). Conclusion: TMT-based proteomic analysis was able to identify potential biomarkers in plasma that predicted chemotherapy resistance in the metastatic TNBC. The plasma of CAMK2A level may serve as apotential predictive and prognostic biomarker for chemotherapy in metastatic TNBC.
Keywords: Triple negative breast cancer, tandem mass tag, proteomics, plasma, biomarkers
Introduction
Breast cancer is the most common disease and the second leading cause of women death worldwide [1]. In China, the incidence has increased significantly [2]. 30% of early-stage breast cancer developed with metastasis, and about 5% of patients are diagnosed with advanced stage with distant metastasis [3]. Improvements of treatment did not change the prognosis of metastatic breast cancer. The 5-year survival rate of metastatic breast cancer is only 23%, which is much lower than 84-99% in early-stage breast cancer [4].
Around 15-20% of breast cancer cases are classifiedas triple-negative breast cancer (TNBC), named afterthe absence of the expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). Patients with TNBC suffer from poor clinical outcome and shortage of targeted therapy. Chemotherapy plays an important role in the treatment paradigms for metastatic TNBC. Docetaxel is a classical drug for metastatic TNBC. However, chemotherapy resistance will ultimately be developed which impedes the success of chemotherapy. Plasma serves as an important medium that interacts with cells, tissues and organs in the human body. It carries proteins secreted or leaked by different cells in response to pathologic progress. The serum and plasma proteins or peptides have been shown to be good biomarkers for early diagnosis, prognosis, and metastasis in breast cancer [5-7]. Therefore, identification of differentially expressed plasma proteins might offer a rich source of information for development of biomarkers that predictdocetaxel-based chemotherapy resistance from metastatic TNBC patients [8,9]. To date, proteomic technology has been applied to a wide range of cancer studies including analysis of drug resistance [10]. Mass spectrometry (MS)-based proteomics often involves analyzing complex mixtures of proteins derived from cell or tissue lysates or from body fluids on a global scale [11-13]. To our knowledge, no study has been performed to search docetaxel-based chemotherapy resistant markers on plasma samples from metastatic TNBC patients.
In the present study, we applied Tandem Mass Tag (TMT)-based quantitative mass spectrometry to distinguish the differentially expressed proteins between the chemotherapy-sensitive and chemotherapy-resistant plasma from metastatic TNBC patients. The dataset was analyzed using the DAVID and STRING databases, and differentially expressed proteins were further validated by enzyme-linked immunosorbent assay (ELISA). The aim of the study was to identify differences in protein expression to give further insight into the molecular mechanisms on chemotherapy resistance. The association between differentially expressed proteins level and patient survival was also investigated to determine its potential prognostic utility.
Materialsand methods
Patients
The study was approved by the Ethics Com-mittee of the Peking University Cancer Hospital on 12-Dec-2014 and conforms to the principles outlined in the Declaration of Helsinki. Informed consent was obtained from each patient. The plasma of patients with metastatic TNBC was sampled between June 2009 and Dec 2013 from the specimen bank of Beijing Cancer Hospital. The estrogen receptor (ER), progesterone receptor (PR), and HER2 status were the result of the primary tumor. ER and PR status were considered positive when 1% or more tumor cells exhibited nuclear staining for these receptors. HER2 positivity was defined as either as score of 3+ by immunohistochemistry (IHC) or positivity by FISH. All the patients received docetaxel (75 mg/m2 every 21 days for 4-6 cycles) based chemotherapy. Treatment response was assessed after two cycles of chemotherapy by the RECIST criteria 1.1. Patients achieving partial response (PR) were considered aschemotherapy-sensitive (S) group. Patients achieving disease progression (PD) were considered aschemotherapy-resistant (R) group. Demographic and clinicopathologic details of patients were obtained from the medical records of the Department of Breast Oncology (Table 1). Patients with underlyingmedical conditions that could result in systematic alterationin plasma protein levels were excluded. Exclusion conditions included systemic lupus, rheumatoid arthritis, scleroderma, polymyositis, chronic liver disease, chronic renal failure, and diabetes mellitus.
Table 1.
Clinicopathological characteristics of the metastatic breast cancer
| Characteristics | Screening stage n=26 (%) | Validation stage n=67 (%) |
|---|---|---|
| Age (years) median (range) | 55 (35-72) | 53 (30-80) |
| ECOG | ||
| 0, 1 | 25 (96.2) | 63 (94.0) |
| 2 | 1 (3.8) | 4 (6.0) |
| Histology | ||
| IDC | 25 (96.2) | 58 (86.5) |
| ILC | 1 (3.8) | 3 (4.5) |
| Others | 0 | 6 (9.0) |
| Histologic grading | ||
| Grade 1 | 7 (35) | 24 (35.8) |
| Grade 2 | 8 (40) | 31 (46.3) |
| Grade 3 | 5 (25) | 12 (17.9) |
| AJCC stage | ||
| Stage I | 1 (3.8) | 4 (6.0) |
| Stage II | 12 (46.2) | 30 (44.8) |
| Stage III | 8 (30.8) | 24 (35.8) |
| Stage IV | 3 (11.5) | 5 (7.5) |
| Unknown | 2 (7.7) | 4 (6.0) |
| Internalorgan metastasis (liver, lung, brain) | 14 (53.8) | 32 (47.8) |
| More than 2 sites of metastasis | 12 (46.2) | 28 (41.8) |
| Clinical response | ||
| PR | 10 (38.5) | 33 (49.3) |
| PD | 16 (61.5) | 34 (50.7) |
| PFS (months) | 3.2 (2.9-3.6) | 5.1 (2.0-8.2) |
| OS (months) | 21.0 (14.1-28.0) | 18.1 (10.3-25.8) |
Note: ECOG: Eastern Cooperative Oncology Group; IDC: Invasive ductal carcinoma; ILC: Invasive lobular carcinoma; PR: Partial response; PD: Progression disease; PFS: Progression free survival; OS: Overall survival.
Plasma samples
Four milliliters of peripheral blood were collected before chemotherapy. Blood was collected in EDTA-containing tubes (BD Diagnostics, La Jolla, CA) and centrifuged at 2000 rpm for 15 min within 1 h of collection to remove cellular components. Plasma samples were divided into aliquots and stored at -80°C until use. Specimens showing hemolysis were excluded.
Protein tryptic digestion
Theplasma samples were diluted 10-fold with 50 mM NH4HCO3 pH 8.5. The diluted plasma protein concentrations were measured by the BCA Protein Assay Kit (Thermo Fisher Scientific). Proteins were reduced with 5 mM dithiothreitol for 30 min at 37°C, andalkylated with 15 mM iodoacetamide for 40 min at room temperaturein the dark. Protein samples were digested with sequencing grade modified trypsin (Promega) (ratio of trypsin to protein 1:50) at 37°C overnight. Digests were acidified by addition of 10% trifluoroacetic acid (TFA) to 0.5% finalconcentration and the peptides were desalted on Sep-Pak tC18 cartridges (Waters, Milford, MA, USA) and concentrated in a centrifugal evaporator (Thermo Fisher Scientific).
Peptide TMT labeling
Fifty micrograms of digested peptides from each sample group were used for amine-reactive TMT6-plex labelingaccording to themanufacturer’s protocol (Thermo Fisher Scientific, USA). Briefly, peptides were resuspended in 100 μl of 0.1 M TEAB buffer pH 8.5 and were labeled with TMT6-plex tags which were dissolved in 41 μl of anhydrous acetonitrile (ACN)and added to each sample with briefly mixing. Reactions were incubated at RTfor 1 h, and then quenched by the addition of 10 μl of 5% hydroxylamine for 15 min and then acidified by the addition of 10 μl 100% formic acid. Each TMT-labeled samplewas mixed equally and the mixed peptides were desalted with Sep-Pak C18 cartridges.
Isoelectric focusing fractionation of TMT-labeled peptides
For TMT-labeled samples, 200 μg peptide mixtures were dissolved in buffer containing 5% glycerol and 2% immobilized pH gradient buffer (pH 3-10; GE Healthcare) and loaded into 24 wells over an Immobiline DryStrip (24 cm, pH 3-10; GE Healthcare). The peptide mixtures were fractionated by peptide IEF on a 3100 OFFGEL fractionator (Agilent Technologies) according to the manufacturer’s instructions. A total of 24 fractions were acidified and desalted with C18 Stagetips. The eluted peptides were dried in a vacuum centrifuge.
LC-MS/MS analysis
All experiments were performed using an EASY-nLC 1000 ultra-high pressure system (Thermo Fisher Scientific) coupled to a LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific)according to a previous report [11,12]. Briefly, TMT labeled peptides were separated on 15 cm in-house packed HPLC-columns (100 μmi.d., 360 μmo.d.) with ReproSil-Pur C18-AQ 3 μm resin by Dr. Maisch GmbH. For all measurements, peptides were loaded in buffer A (0.1% formic acid) and eluted with a linear 100 min gradient of 5-30% of buffer B (0.1% formic acid, 100% acetonitrile). The flow rate was kept at 300 nL/min. Mass spectrometry instrument methods for TMT-labeled sample analysis consisted of MS1 survey scans (1 × 106 target value; 30,000 resolution; 300-1,600 Th). The MS/MS spectra of the 3 most intense ions were acquired by higher-energy c-trap dissociation (HCD, normalized collision energy, 70%; activation time, 40 ms) in the Orbitrap at a mass resolution of 7500, and collision induced dissociation (CID, normalized collision energy, 35%; activation time, 30 ms) in the ion trap. Dynamic exclusion duration was set to 30 s with a maximum exclusion list of 500. The data were acquired using Xcalibur 2.2 (Thermo Fisher Scientific).
Protein identification and quantification
TMT data sets were processed separately using the Proteome discoverer software (Version 1.4, Thermo Fisher Scientific). Searches were performed against a non-redundant concatenated human database protein sequence database (UniProt, 2014. 12. 18) containing both forward and reverse protein sequences. The search parameters were as follows: trypsin as the digesting enzyme; 2 miscleavages allowed; carbamidomethylation (C) as the fixed modification; oxidation (M) and TMT6-plex labels at the lysine residues and N-terminus as variable modifications; 10 ppm for MS tolerance; and 0.5 Da for MS/MS tolerance. The false discovery rate for peptides and proteins was set at 0.01, and at least one unique peptide was required for protein identification.
Bioinformatic analysis
The differentially expressed proteins were entered into the DAVID database for functional analysis. Protein-protein interaction (PPI) networks construction and KEGG pathway enrichment analysis were performed using the STRING database (Search Tool for the Retrieval of Interacting Genes/Proteins, Version 10.5) at the website: http://string-db.org/.
ELISA validation
An ELISA assay was applied to validate changes of selected proteins to confirm the TMT proteomics results. Human CAMK2A and CKB ELISA Kit were purchased from LifeSpan Biosciences (USA); 14-3-3 Gamma ELISA Kit was purchased from MBL Life Science (Japan). All ELISA assays were performed according to manufacturer’s protocols.
Statistical analysis
Patients’ demographic and clinicopathologic characteristics were summarized through descriptive analysis. Continuous variables were reported through median and range, whereas categorical variables were described through frequency distributionPFS and OS curves and were calculated by the Kaplan-Meier method and compared by the log rank test. Multivariate Cox regression analysis was used to estimate hazard ratios for PFS and OS. All statistics were calculated using statistical package for the social sciences (SPSS) 18.0 software. P<0.05 was considered to indicate a statistically significant difference. For the discovery stage a 1.3-fold change was used as a combined threshold to define biologically regulated proteins.
Results
Analytical strategy for plasma proteome identification with different docetaxel-based chemo-therapy responses
The analytical strategy of the TMT-based quantitative proteomics approach used in the study is shown in Figure 1. It was divided into two main stages. First was the biomarker discovery stage consisting of plasma sample preparation, protein expression analysis, and bioinformatics analysis (PPI, GO and KEGG); the second stage was the validation stage consisting of ELISA assay validation and combination analysis with clinicopathologic factors.
Figure 1.
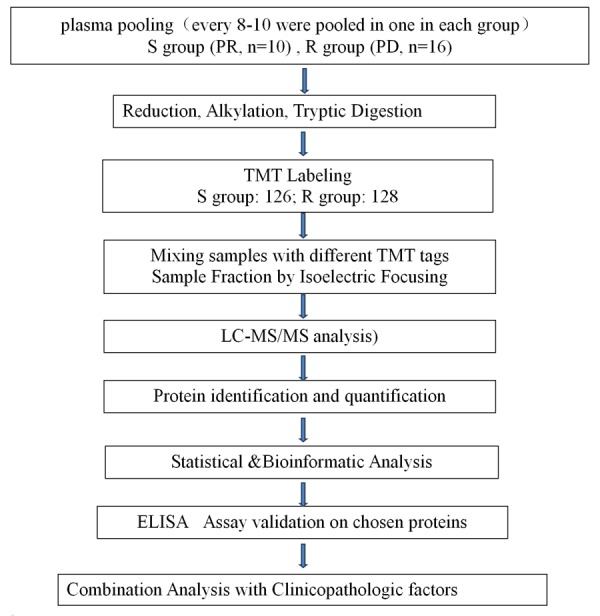
General workflow of the present study. Of a total of 67 plasma samples, 26 samples was used in the screening stage, every 10-16 plasmas in the same group were pooled into one sample. A TMT-based quantitative proteomics analysis for plasma was used to gain a global view of proteome profiling to different responses of docetaxel-based chemotherapy.
Plasma protein expression analysis
A total of 320 non-redundant proteins were identified. Proteins with more than 1.3-fold changes were considered as adifferentially expressed protein. We found a total of 108 significantly dysregulated proteins, among which 65 were up-regulated and 43 were down-regulated in the R group. The details of the 108 proteins, including Uniprotprotein ID, gene name, protein name, ratio (R/S) are listed in Table 2.
Table 2.
Summary of the proteins identified as differentially expressed using the TMT-based quantitative proteomics approach
| Accession No. | Gene Name | Protein Name | Ratio (R/S) |
|---|---|---|---|
| P67936 | TPM4 | Tropomyosin alpha-4 chain | 0.186 |
| H3BT58 | COTL1 | Coactosin-like protein | 0.274 |
| Q5TCU6 | TLN1 | Talin-1 OS=Homo sapiens | 0.348 |
| K7EJ44 | PFN1 | Profilin-1 | 0.354 |
| P05060 | CHGB | Secretogranin-1 | 0.453 |
| Q92496-2 | CFHR4 | Complement factor H-related protein 4 | 0.509 |
| P11226 | MBL2 | Mannose-binding protein C | 0.549 |
| P04275 | VWF | Von Willebrand factor | 0.578 |
| P02776 | PF4 | Platelet factor 4 | 0.582 |
| Q9NU22 | MDN1 | Midasin | 0.584 |
| Q96IY4-2 | CPB2 | Carboxypeptidase B2 | 0.591 |
| P02679-2 | FGG | Fibrinogen gamma chain | 0.605 |
| Q6UY14-2 | ADAMTSL4 | ADAMTS-like protein 4 | 0.636 |
| P62937 | PPIA | Peptidyl-prolyl cis-trans isomerase A | 0.640 |
| E7EUV1 | MUC2 | Mucin-2 | 0.655 |
| C9JEV0 | AZGP1 | Zinc-alpha-2-glycoprotein | 0.658 |
| E9PQD6 | SAA1 | Serum amyloid A protein | 0.665 |
| P01833 | PIGR | Polymeric immunoglobulin receptor | 0.679 |
| Q04756 | HGFAC | Hepatocyte growth factor activator | 0.680 |
| P02675 | FGB | Fibrinogen beta chain | 0.682 |
| P14151 | SELL | L-selectin | 0.687 |
| K7ERI9 | APOC1 | Apolipoprotein C-I, basic form | 0.692 |
| E9PKC6 | CD44 | CD44 antigen | 0.700 |
| P02671 | FGA | Fibrinogen alpha chain | 0.709 |
| P02763 | ORM1 | Alpha-1-acid glycoprotein 1 | 0.710 |
| P02743 | APCS | Serum amyloid P-component | 0.720 |
| F5H5I5 | ABCB9 | ATP-binding cassette sub-family B member 9 | 0.738 |
| P05090 | APOD | Apolipoprotein D | 0.741 |
| P55056 | APOC4 | Apolipoprotein C-IV | 0.742 |
| P04211 | IGLV7-43 | Ig lambda chain V region 4A | 0.744 |
| R4GMN9 | NCAM1 | Neural cell adhesion molecule 1 | 0.744 |
| Q13131-2 | PRKAA1 | 5’-AMP-activated protein kinase catalytic subunit alpha-1 | 0.747 |
| O95445-2 | APOM | Apolipoprotein M | 0.749 |
| B4DJK0 | SRSF5 | Serine/arginine-rich splicing factor 5 | 0.751 |
| P0C0L5 | C4B | Complement C4-B | 0.754 |
| I3L1J1 | SHBG | Sex hormone-binding globulin | 0.755 |
| P01008 | SERPINC1 | Antithrombin-III | 0.756 |
| Q9Y6R7 | FCGBP | IgGFc-binding protein | 0.756 |
| P01042 | KNG1 | Kininogen-1 | 0.766 |
| P0CG05 | IGLC2 | Ig lambda-2 chain C regions | 0.766 |
| P69905 | HBA1 | Hemoglobin subunit alpha | 0.766 |
| P55072 | VCP | Transitional endoplasmic reticulum ATPase | 0.768 |
| P68871 | HBB | Hemoglobin subunit beta | 0.769 |
| Q16352 | INA | Alpha-internexin | 1.316 |
| Q92777-2 | SYN2 | Isoform IIb of Synapsin-2 | 1.321 |
| P18135 | IGKV3-20 | Ig kappa chain V-III region HAH | 1.321 |
| P07196 | NEFL | Neurofilament light polypeptide | 1.323 |
| Q16555-2 | DPYSL2 | Isoform 2 of Dihydropyrimidinase-related protein 2 | 1.329 |
| P62328 | TMSB4X | Thymosin beta-4 | 1.337 |
| P01877 | IGHA2 | Ig alpha-2 chain C region | 1.348 |
| P25705-2 | ATP5A1 | Isoform 2 of ATP synthase subunit alpha, mitochondrial | 1.351 |
| C9JPG5 | SEMA3F | Semaphorin-3F | 1.377 |
| P98160 | HSPG2 | Basement membrane-specific heparan sulfate proteoglycan core protein | 1.379 |
| E2QRF9 | GMNN | Geminin | 1.382 |
| P01608 | IGKV1D-33 | Ig kappa chain V-I region Roy | 1.389 |
| P61026 | RAB10 | Ras-related protein Rab-10 | 1.392 |
| P40197 | GP5 | Platelet glycoprotein V | 1.408 |
| P08519 | LPA | Apolipoprotein(a) | 1.408 |
| M0R1V7 | UBA52 | Ubiquitin-60S ribosomal protein L40 | 1.422 |
| P01857 | IGHG1 | Ig gamma-1 chain C region | 1.429 |
| P23083 | IGHV1-2 | Ig heavy chain V-I region V35 | 1.442 |
| M0R116 | ATP1A3 | Sodium/potassium-transporting ATPase subunit alpha-3 | 1.468 |
| Q01082-3 | SPTBN1 | Spectrin beta chain | 1.475 |
| H7C2G3 | C21orf33 | ES1 protein homolog, mitochondrial (Fragment) | 1.510 |
| Q13509 | TUBB3 | Tubulin beta-3 chain | 1.528 |
| B8ZZ54 | HSPE1 | 10 kDa heat shock protein, mitochondrial | 1.531 |
| P08238 | HSP90AB1 | Heat shock protein HSP 90-beta | 1.532 |
| P68366-2 | TUBA4A | Isoform 2 of Tubulin alpha-4A chain | 1.536 |
| E9PKE3 | HSPA8 | Heat shock cognate 71 kDa protein | 1.557 |
| Q08380 | LGALS3BP | Galectin-3-binding protein | 1.558 |
| P06753-7 | TPM3 | Tropomyosin alpha-3 chain | 1.563 |
| B7Z1R5 | ATP6V1A | V-type proton ATPase catalytic subunit A | 1.567 |
| Q13813-2 | SPTAN1 | Isoform 2 of Spectrin alpha chain, non-erythrocytic 1 | 1.568 |
| Q13885 | TUBB2A | Tubulin beta-2A chain | 1.574 |
| P62158 | CALM1 | Calmodulin | 1.604 |
| H3BQ34 | PKM | Pyruvate kinase | 1.607 |
| Q99798 | ACO2 | Aconitate hydratase, mitochondrial | 1.623 |
| P63104 | YWHAZ | 14-3-3 protein zeta/delta | 1.630 |
| E9PMR5 | MBP | Myelin basic protein | 1.630 |
| H3BMQ8 | ALDOA | Fructose-bisphosphate aldolase A | 1.637 |
| P61981 | YWHAG | 14-3-3 protein gamma | 1.637 |
| P04271 | S100B | Protein S100-B | 1.702 |
| I7HJJ0 | SLC25A6 | ADP/ATP translocase 3 (Fragment) | 1.702 |
| Q71U36-2 | TUBA1A | Isoform 2 of Tubulin alpha-1A chain | 1.703 |
| K7EKU0 | GPATCH8 | G patch domain-containing protein 8 | 1.721 |
| H7BZC1 | HPCAL1 | Hippocalcin-like protein 1 | 1.729 |
| K7EKH6 | GFAP | Glial fibrillary acidic protein | 1.740 |
| B7Z2X9 | ENO2 | Gamma-enolase | 1.744 |
| P01861 | IGHG4 | Ig gamma-4 chain C region | 1.751 |
| O95236-3 | APOL3 | Apolipoprotein L3 | 1.774 |
| D6REX5 | SEPP1 | Selenoprotein P (Fragment) | 1.803 |
| P27348 | YWHAQ | 14-3-3 protein theta | 1.816 |
| F5H5G7 | LDHC | L-lactate dehydrogenase | 1.816 |
| C9JZ20 | PHB | Prohibitin | 1.838 |
| Q8NEX6 | WFDC11 | Protein WFDC11 | 1.887 |
| P12277 | CKB | Creatine kinase B-type | 1.916 |
| U3KPZ0 | TPI1 | Triosephosphate isomerase | 1.965 |
| D6R9Z7 | COX7C | Cytochrome c oxidase subunit 7C, mitochondrial | 1.981 |
| H7C394 | CAMK2B | Calcium/calmodulin-dependent protein kinase type II subunit beta | 2.078 |
| Q00610-2 | CLTC | Isoform 2 of Clathrin heavy chain 1 | 2.125 |
| O43423 | ANP32C | Acidic leucine-rich nuclear phosphoprotein 32 family member C | 2.141 |
| Q9UQM7 | CAMK2A | Calcium/calmodulin-dependent protein kinase type II subunit alpha | 2.170 |
| B1AKQ8 | GNB1 | Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 | 2.451 |
| P62258 | YWHAE | 14-3-3 protein epsilon | 2.479 |
| P15104 | GLUL | Glutamine synthetase | 3.370 |
| E5RJH4 | PPP3CC | Serine/threonine-protein phosphatase 2B catalytic subunit gamma isoform | 3.768 |
| K7EKH5 | ALDOC | Fructose-bisphosphate aldolase C (Fragment) | 4.683 |
| P62805 | HIST1H4A | Histone H4 | 5.765 |
Functional classification and protein-protein interaction analysis of differentially expressed proteins
To analyze the function of proteins, a protein-protein interaction network was constructed for 108 differentially expressed proteins by retrieving the known interactions between each protein (Figure 2). The 108 significantly dysregulated proteins were then interrogated and mapped to KEGG pathways (Table 3), and the three significantly enriched pathways were complement and coagulation cascades, biosynthesis of amino acids, and oocyte meiosis. To further extend our knowledge about the change of plasma proteins between R and S groups, GO analysis was performed to reveal the molecular function, biological process, and cellular component associated with the 108 significantly differentially expressed proteins. As shown in Table 3, the significantly regulated proteins were highly correlated with regulation of biological quality, platelet degranulation and activation, response to wounding etc. In terms of biological process, they were highly correlated with participating in the processes of protein binding, structural constituents of the cytoskeleton and carbohydrate derivative binding etc. In terms of molecular function, they were highly correlated with the extracellular region and vesicles.
Figure 2.
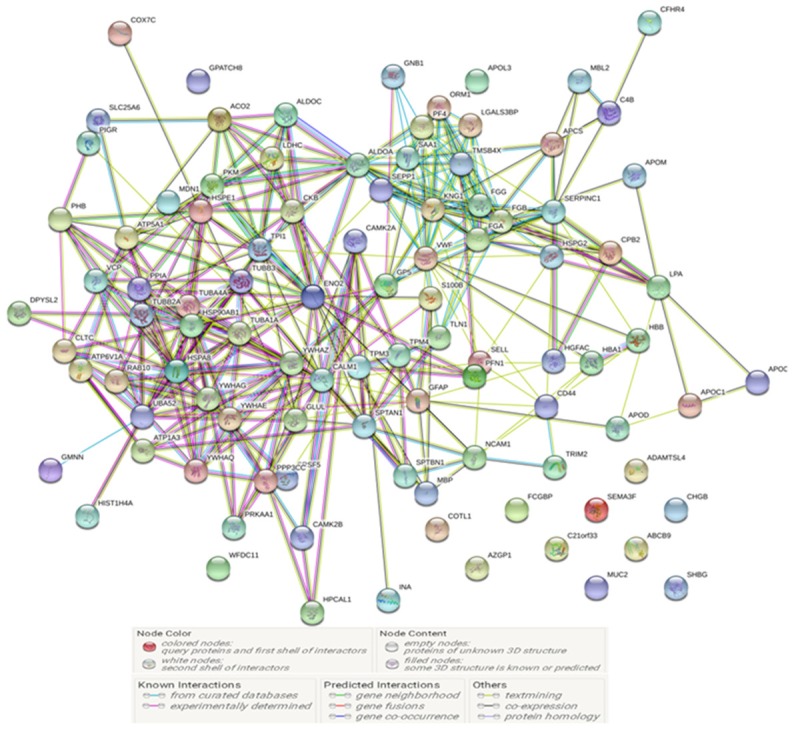
The protein-protein interaction network constructed from the 108 dysregulated proteins using the online tool STRING v10.5. Each edge represents a type of interaction between the linked nodes.
Table 3.
Bioinformatics analysis of the proteomic results
| KEGG Pathways | ||||
|
| ||||
| Pathway ID | Pathway description | Observed gene count | False discovery rate | Matching proteins in your network (labels) |
|
| ||||
| 4610 | Complement and coagulation cascades | 9 | 1.02E-08 | C4B, CPB2, FGA, FGB, FGG, KNG1, MBL2, SERPINC1, VWF |
| 1230 | Biosynthesis of amino acids | 7 | 4.18E-06 | ACO2, ALDOA, ALDOC, ENO2, GLUL, PKM, TPI1 |
| 4114 | Oocyte meiosis | 8 | 4.18E-06 | CALM1, CAMK2A, CAMK2B, PPP3CC, YWHAE, YWHAG, YWHAQ, YWHAZ |
|
| ||||
| Biological Process (GO) | ||||
|
| ||||
| Pathway ID | Pathway description | Observed gene count | False discovery rate | Matching proteins in your network (labels) |
|
| ||||
| GO: 0002576 | Platelet degranulation | 13 | 2.36E-12 | ALDOA, CALM1, FGA, FGB, FGG, KNG1, PF4, PFN1, PPIA, TLN1, TMSB4X, TUBA4A, VWF |
| GO: 0030168 | Platelet activation | 17 | 3.05E-12 | ALDOA, CALM1, FGA, FGB, FGG, GNB1, GP5, KNG1, PF4, PFN1, PPIA, SAA1, TLN1, TMSB4X, TUBA4A, VWF, YWHAZ |
| GO: 0065008 | Regulation of biological quality | 46 | 3.05E-12 | ALDOA, APCS, APOC4, ATP1A3, ATP6V1A, AZGP1, CALM1, CAMK2A, CAMK2B, CD44, CKB, CPB2, DPYSL2, FGA, FGB, FGG, GFAP, GLUL, GP5, HIST1H4A, HSP90AB1, HSPA8, KNG1, LPA, MBL2, MUC2, PF4, PFN1, PHB, PIGR, PPIA, PRKAA1, S100B, SAA1, SELL, SEMA3F, SERPINC1, SLC25A6, SPTAN1, SPTBN1, TLN1, TUBA4A, VWF, YWHAE, YWHAG, YWHAZ |
| GO: 0009611 | Response to wounding | 25 | 7.82E-12 | ALDOA, APOD, CALM1, CPB2, FGA, FGB, FGG, GFAP, GNB1, GP5, KNG1, LPA, PF4, PFN1, PKM, PPIA, SAA1, SELL, SERPINC1, SRSF5, TLN1, TMSB4X, TUBA4A, VWF, YWHAZ |
| GO: 0007596 | Blood coagulation | 21 | 4.52E-11 | ALDOA, CALM1, CD44, CPB2, FGA, FGB, FGG, GNB1, GP5, KNG1, PF4, PFN1, PPIA, SAA1, SELL, SERPINC1, TLN1, TMSB4X, TUBA4A, VWF, YWHAZ |
|
| ||||
| Molecular Function (GO) | ||||
|
| ||||
| Pathway ID | Pathway description | Observed gene count | False discovery rate | Matching proteins in your network (labels) |
|
| ||||
| GO: 0005515 | Protein binding | 53 | 2.07E-09 | ABCB9, ADAMTSL4, ALDOA, ALDOC, APCS, ATP1A3, ATP5A1, AZGP1, CALM1, CAMK2A, CAMK2B, CD44, CHGB, CKB, CLTC, COTL1, FGB, FGG, GLUL, GMNN, GNB1, HBB, HIST1H4A, HSP90AB1, HSPA8, HSPE1, HSPG2, KNG1, LPA, MBL2, MDN1, NCAM1, PF4, PFN1, PHB, PKM, PPIA, RAB10, S100B, SAA1, SELL, SERPINC1, SPTAN1, SPTBN1, TLN1, TPI1, UBA52, VCP, VWF, YWHAE, YWHAG, YWHAQ, YWHAZ |
| GO: 0005200 | Structural constituent of cytoskeleton | 9 | 1.90E-06 | GFAP, INA, SPTAN1, SPTBN1, TLN1, TUBA1A, TUBA4A, TUBB2A, TUBB3 |
| GO: 0005198 | Structural molecule activity | 15 | 7.19E-05 | CLTC, FGA, FGB, FGG, GFAP, INA, MBP, SPTAN1, SPTBN1, TLN1, TPM4, TUBA1A, TUBA4A, TUBB2A, TUBB3 |
| GO: 0097367 | Carbohydrate derivative binding | 29 | 9.76E-05 | ABCB9, ATP1A3, ATP5A1, ATP6V1A, AZGP1, CAMK2A, CAMK2B, CD44, CKB, GLUL, HSP90AB1, HSPA8, HSPE1, KNG1, LPA, MDN1, PF4, PKM, PRKAA1, RAB10, SAA1, SELL, SERPINC1, TUBA1A, TUBA4A, TUBB2A, TUBB3, VCP, VWF |
| GO: 0023026 | MHC class II protein complex binding | 4 | 0.000131 | HSP90AB1, HSPA8, PKM, YWHAE |
|
| ||||
| Cellular Component (GO) | ||||
|
| ||||
| Pathway ID | Pathway description | Observed gene count | False discovery rate | Matching proteins in your network (labels) |
|
| ||||
| GO. 0044421 | Extracellular region part | 69 | 1.93E-28 | ADAMTSL4, ALDOA, ALDOC, APCS, APOC1, APOC4, APOD, ATP1A3, ATP5A1, ATP6V1A, AZGP1, CALM1, CD44, CKB, CLTC, COTL1, CPB2, DPYSL2, ENO2, FCGBP, FGA, FGB, FGG, GLUL, GNB1, GP5, HBA1, HGFAC, HIST1H4A, HPCAL1, HSP90AB1, HSPA8, HSPE1, INA, KNG1, LDHC, LGALS3BP, LPA, MBL2, MUC2, NCAM1, ORM1, PF4, PFN1, PHB, PIGR, PKM, PPIA, PPP3CC, RAB10, S100B, SAA1, SEMA3F, SERPINC1, SHBG, SPTAN1, SPTBN1, TLN1, TPM3, TUBA1A, TUBA4A, TUBB2A, TUBB3, VCP, VWF, YWHAE, YWHAG, YWHAQ, YWHAZ |
| GO. 1903561 | Extracellular vesicle | 61 | 1.82E-27 | ALDOA, ALDOC, APCS, APOC1, APOD, ATP1A3, ATP5A1, ATP6V1A, AZGP1, CALM1, CD44, CKB, CLTC, COTL1, CPB2, DPYSL2, ENO2, FCGBP, FGA, FGB, FGG, GLUL, GNB1, GP5, HBA1, HIST1H4A, HPCAL1, HSP90AB1, HSPA8, HSPE1, HSPG2, KNG1, LDHC, LGALS3BP, NCAM1, ORM1, PFN1, PHB, PIGR, PKM, PPIA, PPP3CC, RAB10, SAA1, SEPP1, SERPINC1, SHBG, SPTAN1, SPTBN1, TLN1, TPM3, TUBA1A, TUBA4A, TUBB2A, TUBB3, VCP, VWF, YWHAE, YWHAG, YWHAQ, YWHAZ |
| GO. 0005576 | Extracellular region | 72 | 2.11E-27 | ADAMTSL4, ALDOA, ALDOC, APCS, APOC1, APOC4, APOD, APOL3, ATP1A3, ATP5A1, ATP6V1A, AZGP1, CALM1, CD44, CFHR4, CHGB, CKB, CLTC, COTL1, CPB2, DPYSL2, ENO2, FCGBP, FGA, FGB, FGG, GLUL, GNB1, GP5, HBA1, HIST1H4A, HPCAL1, HSP90AB1, HSPA8, HSPE1, INA, KNG1, LDHC, LGALS3BP, LPA, MBL2, MUC2, NCAM1, ORM1, PF4, PFN1, PHB, PIGR, PKM, PPIA, PPP3CC, RAB10, SAA1, SEMA3F, SERPINC1, SHBG, SPTAN1, SPTBN1, TLN1, TMSB4X, TPM3, TUBA1A, TUBA4A, TUBB2A, TUBB3, VCP, VWF, WFDC11, YWHAE, YWHAG, YWHAQ, YWHAZ |
| GO. 0070062 | Extracellular exosome | 60 | 8.03E-27 | ALDOA, ALDOC, APCS, APOC1, APOD, ATP5A1, ATP6V1A, AZGP1, CALM1, CD44, CKB, CLTC, COTL1, CPB2, DPYSL2, ENO2, FCGBP, FGA, FGB, FGG, GLUL, GNB1, GP5, HBA1, HIST1H4A, HPCAL1, HSP90AB1, HSPA8, HSPE1, HSPG2, KNG1, LDHC, LGALS3BP, NCAM1, ORM1, PFN1, PHB, PIGR, PKM, PPIA, PPP3CC, RAB10, SAA1, SEPP1, SERPINC1, SHBG, SPTAN1, SPTBN1, TLN1, TPM3, TUBA1A, TUBA4A, TUBB2A, TUBB3, VCP, VWF, YWHAE, YWHAG, YWHAQ, YWHAZ |
| GO. 0031988 | Membrane-bounded vesicle | 64 | 7.84E-26 | ALDOA, ALDOC, APCS, APOC1, APOD, ATP5A1, ATP6V1A, AZGP1, CALM1, CAMK2A, CAMK2B, CD44, CHGB, CKB, COTL1, CPB2, DPYSL2, ENO2, FCGBP, FGA, FGB, FGG, GLUL, GNB1, GP5, HBA1, HIST1H4A, HPCAL1, HSP90AB1, HSPA8, HSPE1, HSPG2, KNG1, LDHC, LGALS3BP, NCAM1, ORM1, PF4, PFN1, PHB, PIGR, PKM, PPIA, PPP3CC, RAB10, SAA1, SEPP1, SERPINC1, SHBG, SPTAN1, SPTBN1, TLN1, TMSB4X, TPM3, TUBA1A, TUBA4A, TUBB2A, TUBB3, VCP, VWF, YWHAE, YWHAG, YWHAQ, YWHAZ |
Validation of differential expression of proteins
A total of 67 samples were recruited for ELISA analysis to validate the protein level of CAMK2A (Calcium/calmodulin-dependent protein kinase type II subunit alpha), CKB (Creatine kinase B-type), and YWHAG (14-3-3 protein gamma) in plasma. As shown in the grouped scatter plot in Figure 3, there was a significant difference in the plasma level of CAMK2A between the R and S group (R: 29 vs S: 38, P=0.0074). The CAMK2A level was significantly up-regulated in the R group, which was consistent with the TMT quantification result in the proteomic analysis. Two additional proteins, CKB (R: 16 vs S: 21) and YWHAG (R: 33 vs S: 33) has expression levels that were also up-regulated in the R group, consistent with the proteomics results. However, the difference was not statistically significant (P>0.05). Furthermore, the ROC curve analysis showed that AUC was 0.708 for CAMK2A, 0.596 for CKB, and 0.563 for YWHAG (Figure 3B). The sensitivityand specificity of CAMKK2A was 48.4% and 86.1% respectively.
Figure 3.
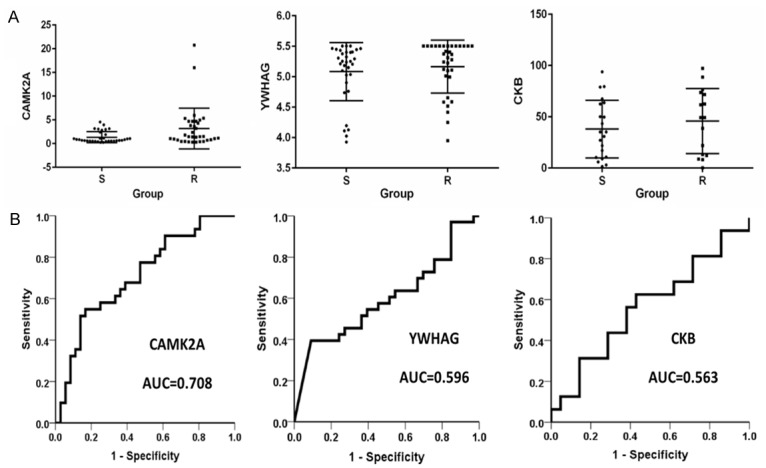
The ELISA test result and ROC analysis of CAMK2A, CKB, and YWHAG in 67 plasma samples. R: resistance group. S: sensitive group.
Combination analysis with clinicopathologic factors
The association of CAMK2A and other clinicopathologic factors with chemotherapy response was analyzedwith univariate and multivariate logistic regression methods. The level of plasma CAMK2A was identified as an independent predictive factor for chemotherapeutic response (P=0.009, OR=0.152). The ORR of the patients with higher CAMK2A level was 35.3% and the patients with lower CAMK2A level was 63.6% (Table 4). In the Log-Rank analysis, patients with higher CAMK2A level had shorter OS than patients with lower CAMK2A level, which amounted to 13.9 and 28.9 months, respectively (P=0.034). While CAMK2A level had no significant effect on PFS, which was 2.9 and 8.6 months for the patients with higher and lower CAMK2A level (P=0.060) (Figure 4). In the multivariate Cox regression analysis, CAMK2A level still had a significant effect on OS (P=0.031, HR=1.865) (Table 5).
Table 4.
The correlation of CAMK2A and other clinicopathologic factors with chemotherapy response rate
| Characteristics | ORR (%) | Single variate | Multivariate | ||
|---|---|---|---|---|---|
|
|
|||||
| P | P | OR | |||
| Age | ≤53 | 44.1 | 0.393 | 0.811 | 1.180 |
| >53 | 54.5 | ||||
| ECOG | 0, 1 | 50.8 | 0.614* | 0.999 | 0.001 |
| 2 | 25.0 | ||||
| Grading | G1 | 50.0 | 0.927 | 0.432 | 1.656 |
| G2-3 | 48.8 | ||||
| AJCC stage at diagnosis | Stage I-III | 51.7 | 0.672* | 0.277 | 3.531 |
| Stage IV | 40.0 | ||||
| Internalorgan metastasis (liver, lung, brain) | Yes | 50.0 | 0.866 | 0.897 | 0.906 |
| No | 47.8 | ||||
| Sites of metastasis ≥3 | Yes | 48.6 | 0.912 | 0.521 | 1.200 |
| No | 50.0 | ||||
| CAMK2A | High | 35.3 | 0.02 | 0.009 | 0.152 |
| Low | 63.6 | ||||
Figure 4.
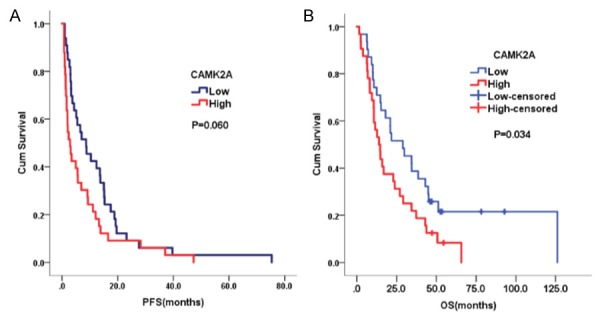
The progression free survival (PFS) and overall survival (OS) of patients with high and low CAMK2A in plasma.
Table 5.
The correlation of CAMK2A and other clinicopathologic factors with overall survival
| Characteristics | OS (median) | Log-rank | Cox Regression | ||
|---|---|---|---|---|---|
|
|
|||||
| P | P | HR | |||
| Age | ≤53 | 14.7 | 0.078 | 0.508 | 0.771 |
| >53 | 22.9 | ||||
| ECOG | 0, 1 | 21.0 | 0.07 | 0.007 | 4.499 |
| 2 | 2.4 | ||||
| Grading | G1 | 29.2 | 0.504 | 0.797 | 1.085 |
| G2-3 | 14.7 | ||||
| AJCC stage at diagnosis | Stage I-III | 16.9 | 0.182 | 0.218 | 2.526 |
| Stage IV | 14.8 | ||||
| Internal organ metastasis (liver, lung, brain) | Yes | 15.9 | 0.081 | 0.368 | 1.484 |
| No | 21.8 | ||||
| Sites of metastasis ≥3 | Yes | 14.8 | 0.046 | 0.409 | 1.349 |
| No | 22.9 | ||||
| CAMK2A | High | 13.9 | 0.034 | 0.031 | 1.865 |
| Low | 28.9 | ||||
Discussion
Quantitative proteomics is driving the discovery of disease-specific targets and biomarkers. UHPLC and mass spectrometry-based proteomics were combined with TMT labeled samples to quantify protein expression changes. Due to its faster separation,greater sensitivity, and resolution, we applied the TMT-based proteomic approach to discover and identify plasma protein biomarkers for predicting docetaxel-basedchemoresistance in the metastatic TNBC patients.
In this study, we found 108 differently expressed proteins which demonstrated at least a 1.3-fold difference between the R and S group withMT-based proteomic approach. Furthermore, we selected 3 proteins (CKB, YWHAG (isoform of 14-3-3 gamma) and CAMKIIA) for further validation. The 4 isoforms of 14-3-3 (epsilon, gamma, theta/tauand zeta/delta) which were upregulated in the R group.The association between chemotherapy resistance in breast cancer and expression of 14-3-3 proteins has been reported previously [10,14-16]. There are seven isoforms of 14-3-3 (beta/alpha, epsilon, gamma, eta, theta/tau, sigma/stratifin and zeta/delta), which are reported to associate with proteins involved in critical processes including cell cycle regulation, intracellular signaling, and apoptosis [17]. Due to the nature of their protein targets, 14-3-3 proteins have been widely associated with cancer, including response to therapeutic agents and it is thought that 14-3-3 proteins promote cell survival by inhibition of apoptosis [18]. In the current study, ELISA assays demonstrated that the expression levels of CKBand YWHAG (isoform of 14-3-3 gamma) were up-regulated in the R group, consistent with the proteomics results. However, the difference was not statistically significant (P>0.05). Calcium/calmodulin-dependent protein kinase (CAMK) is a large family of protein kinases that act as an effector of calcium/calmodulin, which have been classified into myosin light chain kinase, phosphorylase kinase, CAM kinase I, CAM kinase II, EF-2 kinase (CAM kinase III) and CAM kinase IV [19]. Calcium/calmodulin-dependent protein kinase II (CAMKII) is a multifunctional calcium/calmodulin-dependent serine/threonine protein kinase. Recent studies suggest that CaMKII plays important roles in the control of cell cycle progression and cell proliferation [20-22]. Potential connections between Ca2+/CaMKII signaling and multiple signaling pathways have been reported in many cell types. Runbi et al [23] found that mesenchymal stem cell (MSC)-exosomes potentiated chemoresistance in gastric cancer cells in vivo and ex vivo and exerted this role at least in part through the activation of CAMKs (predominantly CAMKII and CAMKIV) and the downstream Raf/MEK/ ERK pathway. The tumor microenvironment is emerging as a significant determinant of a tumor’s response to chemotherapy [24,25] and MSCs have been considered as an important component of the tumor microenvironment. The Raf/MEK/ERK kinase cascade is one of the downstream pathways of the CAMKs.
In our study, the CAMKIIA level was significantly up-regulated in the R group, which is consistent with the TMT quantification result in the proteomic analysis. Furthermore, the ROC curve analysis showed that the AUC was 0.708 for CAMK2A, 0.596 for CKB and 0.563 for YWHAG (Figure 3B). These data indicate that CAMK2A could be potential biomarker to distinguish the R group from the S group of metastatic TNBC patientswith high sensitivity and specificity.When tested for its ability to predict PFS and OS, the median value of the CAMK2A level separated patients into significantly different groups, those with values above the median showing significantly shorter OS than those with values below the median.
Docetaxel is one of the common drugs used to treat metastatic breast cancer which binds to β-tubulin in assembled tubulin, thereby reducing depolymerisation [26]. Chemoresistance is a major factor involved in poor response and reduced overall survival in patients with locally advanced and metastatic breast cancer. Chemoresistance is a very challenging and complex phenomenon involving a number of complex mechanisms. The most established in vitro mechanism for resistance is overexpression of drug efflux proteins. The best known drug efflux proteins are members of the ATP-binding cassette (ABC) superfamily, including P-glycoprotein (Pgp), multidrug resistance associated protein 1 (MRP-1), and breast cancer resistance protein (BCRP), which export anticancer agents out of cells [27,28]. However, the results in clinical studies are controversial. Some studies showed no correlation between ABC transporter expression level and response to either paclitaxel or docetaxel treatment in breast cancer patients [29].
This study has some limitations. First, the samples of the study were limited and a larger number of patients are required to confirm the prognostic significance. Second, the data were retrospectively collected from prospectively maintained database. Third, we did not explore all the dysregulated proteins identified in the proteomics analysis. The combination of proteins might have been more predictive.
In summary, using a TMT-Based Proteomics Analysis of plasma samples, we were able to identify differentially expressed proteins predictive of chemotherapy resistance in the metastatic TNBC. The plasma CAMK2A level may serve as apotential predictive and prognosis biomarker for chemotherapy and deserves further prospective trials for validation.
Acknowledgements
This work was supported in part by grant from the National Natural Science Foundation of China (No. 81160214) and Beijing Natural Science Foundation of China (No. 7143173).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Zheng R, Zeng H, Zhang S, Chen T, Chen W. National estimates of cancer prevalence in China, 2011. Cancer Lett. 2016;370:33–38. doi: 10.1016/j.canlet.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 4.Schneider AP 2nd, Zainer CM, Kubat CK, Mullen NK, Windisch AK. The breast cancer epidemic: 10 facts. Linacre Q. 2014;81:244–277. doi: 10.1179/2050854914Y.0000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Opstal-van Winden AW, Vermeulen RC, Peeters PH, Beijnen JH, van Gils CH. Early diagnostic protein biomarkers for breast cancer: how far have we come? Breast Cancer Res Treat. 2012;134:1–12. doi: 10.1007/s10549-011-1907-2. [DOI] [PubMed] [Google Scholar]
- 6.Bohm D, Keller K, Wehrwein N, Lebrecht A, Schmidt M, Kölbl H, Grus FH. Serum proteome profiling of primary breast cancer indicates a specific biomarker profile. Oncol Rep. 2011;26:1051–1056. doi: 10.3892/or.2011.1420. [DOI] [PubMed] [Google Scholar]
- 7.Lei L, Wang XJ, Zheng ZG, Huang J, Cao WM, Chen ZH, Shao XY, Cai JF, Ye WW, Lu HY. Identification of serum protein markers for breast cancer relapse with SELDI-TOF MS. Anat Rec (Hoboken) 2011;294:941–944. doi: 10.1002/ar.21399. [DOI] [PubMed] [Google Scholar]
- 8.Duffy MJ. Serum tumor markers in breast cancer: are they of clinical value? Clin Chem. 2006;52:345–351. doi: 10.1373/clinchem.2005.059832. [DOI] [PubMed] [Google Scholar]
- 9.Hanash SM, Pitteri SJ, Faca VM. Mining the plasma proteome for cancer biomarkers. Nature. 2008;452:571–579. doi: 10.1038/nature06916. [DOI] [PubMed] [Google Scholar]
- 10.Chuthapisith S, Layfield R, Kerr ID, Hughes C, Eremin O. Proteomic profiling of MCF-7 breast cancer cells with chemoresistance to different types of anti-cancer drugs. Int J Oncol. 2007;30:1545–1551. [PubMed] [Google Scholar]
- 11.Aebersold R, Mann M. Mass spectrometry-based proteomics. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 12.Yates JR 3rd, Gilchrist A, Howell KE, Bergeron JJ. Proteomics of organelles and large cellular structures. Nat Rev Mol Cell Biol. 2005;6:702–714. doi: 10.1038/nrm1711. [DOI] [PubMed] [Google Scholar]
- 13.Walther TC, Mann M. Mass spectrometry-based proteomics in cell biology. J Cell Biol. 2010;190:491–500. doi: 10.1083/jcb.201004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodgkinson VC, ELFadl D, Agarwal V, Garimella V, Russell C, Long ED, Fox JN, McManus PL, Mahapatra TK, Kneeshaw PJ, Drew PJ, Lind MJ, Cawkwell L. Proteomic identification of predictive biomarkers of resistance to neoadjuvant chemotherapy in luminal breast cancer: a possible role for 14-3-3 theta/tau and tBID? J Proteomics. 2012;75:1276–1283. doi: 10.1016/j.jprot.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Hodgkinson VC, Eagle GL, Drew PJ, Lind MJ, Cawkwell L. Biomarkers of chemotherapy resistance in breast cancer identified by proteomics: current status. Cancer Lett. 2010;294:13–24. doi: 10.1016/j.canlet.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Liu H, Han B, Zhang JT. Identification of 14-3-3sigma as a contributor to drug resistance in human breast cancer cells using functional proteomic analysis. Cancer Res. 2006;66:3248–3255. doi: 10.1158/0008-5472.CAN-05-3801. [DOI] [PubMed] [Google Scholar]
- 17.Tzivion G, Gupta VS, Kaplun L, Balan V. 14-3-3 proteins as potential oncogenes. Semin Cancer Biol. 2006;16:203–213. doi: 10.1016/j.semcancer.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Masters SC, Subramanian RR, Truong A, Yang H, Fujii K, Zhang H, Fu H. Survival-promoting functions of 14-3-3 proteins. Biochem Soc Trans. 2002;30:360–365. doi: 10.1042/bst0300360. [DOI] [PubMed] [Google Scholar]
- 19.Nairn AC, Picciotto MR. Calcium/calmodulin-dependent protein kinases. Semin Cancer Biol. 1994;5:295–303. [PubMed] [Google Scholar]
- 20.Ducibella T, Schultz RM, Ozil JP. Role of calcium signals in early development. Semin Cell Dev Biol. 2006;17:324–32. doi: 10.1016/j.semcdb.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Zayzafoon M. Calcium/calmodulin signaling controls osteoblast growth and differentiation. J Cell Biochem. 2006;97:56–70. doi: 10.1002/jcb.20675. [DOI] [PubMed] [Google Scholar]
- 22.Colomer J, Means AR. Physiological roles of the Ca2+/CaM-dependent protein kinase cascade in health and disease. Subcell Biochem. 2007;45:169–214. doi: 10.1007/978-1-4020-6191-2_7. [DOI] [PubMed] [Google Scholar]
- 23.Ji R, Zhang B, Zhang X, Xue J, Yuan X, Yan Y, Wang M, Zhu W, Qian H, Xu W. Exosomes derived from human mesenchymal stem cells confer drug resistance in gastric cancer. Cell Cycle. 2015;14:2473–2483. doi: 10.1080/15384101.2015.1005530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chien J, Kuang R, Landen C, Shridhar V. Platinum-sensitive recurrence in ovarian cancer: the role of tumor microenvironment. Front Oncol. 2013;3:251. doi: 10.3389/fonc.2013.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mao Y, Keller ET, Garfield DH, Shen K, Wang J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2013;32:303–315. doi: 10.1007/s10555-012-9415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 27.McGrogan BT, Gilmartin B, Carney DN, McCann A. Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta. 2008;1785:96–132. doi: 10.1016/j.bbcan.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Zelnak A. Overcoming taxane and anthracycline resistance. Breast J. 2010;16:309–312. doi: 10.1111/j.1524-4741.2010.00911.x. [DOI] [PubMed] [Google Scholar]
- 29.Kanzaki A, Toi M, Nakayama K, Bando H, Mutoh M, Uchida T, Fukumoto M, Takebayashi Y. Expression of multidrug resistance-related transporters in human breast carcinoma. Jpn J Cancer Res. 2001;92:452–458. doi: 10.1111/j.1349-7006.2001.tb01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


