Abstract
Overexpression of the prolyl isomerase PIN1 is involved in tumorigenesis, but the role of PIN1 in cervical cancer is unclear. In this study, we examined PIN1 protein expression by immunohistochemistry in 221 paraffin-embedded samples from cervical cancer patients, cervical intraepithelial neoplasia patients, and control tissues, and found that high expression of PIN1 was significantly associated with lymph node metastasis (P=0.002), advanced stage according to the International Federation of Gynecology and Obstetrics guidelines (P=0.026). When endogenous PIN1 expression was knocked down using siRNA, cell proliferation, colony formation, migration, and invasion were inhibited in the SiHa cervical cancer cell line. Additionally, PIN1 knockdown increased E-cadherin and β-catenin expression, and decreased expression of N-cadherin and Vimentin, suggesting that PIN1 can promote epithelial-mesenchymal transition (EMT). These results indicate that the Overexpression of the prolyl isomerase PIN1 in cervical cancer indicates tumor-Promotive properties of PIN1 that may be a marker of poor prognosis in cervical cancer patients, and the molecular determinants of epithelial polarity which have tumorigenesis enhancing impact, might through EMT.
Keywords: Prolyl isomerase Pin1, cervical cancer
Introduction
Cervical cancer is the leading cause of cancer-related deaths in women in developing countries, with an estimated 265,700 annual worldwide deaths [1,2]. Cervical squamous cell carcinoma (CSCC) accounts for approximately 80% of cervical cancer. Reports have suggested that high-risk types of human papillomavirus (HPV) infection are strongly associated with CSCC development, but HPV infection is not a reliable factor for diagnosis and predicting prognosis [3]. The prognosis of patients with cervical cancer is largely dependent on clinical stage [4]; the survival rate of patients with stage I cancer is 80-98%, but is significantly reduced to 50% when lymph node metastasis occurs [5]. For cervical cancer cells to metastasize to distant sites or the lymphatic system, they must develop the ability to migrate and invade by disrupting cell polarity and intercellular contacts, breaking down the extracellular matrix, and promoting endothelial cell migration and capillary formation. Therefore, identifying appropriate biomarkers and mechanisms involved in cervical carcinoma progression are important to predict lymph node metastasis and prognosis in cervical cancer.
In order for tumor cells to metastasize, they may undergo epithelial-mesenchymal transition (EMT), by which cells lose cell-to-cell adhesion, apical-basolateral polarity, and epithelial molecular markers, while acquiring plasticity, a spindle-cell shape morphology, and mesenchymal markers [6]. Thus, EMT is thought to facilitate cancer cell motility and invasion, and plays a pivotal role in metastasis of a variety of human cancer, including cervical cancer [7,8].
The prolyl isomerase PIN1 is a widely expressed phosphorylation-specific peptidyl prolyl isomerase (PPIase) that regulates phosphorylation signaling. It functions by catalyzing the conversion of specific phosphorylated motifs between two distinct conformations of its protein substrates [9,10]. Consequently, it has important roles in cellular activities since a number of protein kinases have Serine/Threonine-Proline (Ser/Thr-Pro) phosphorylation motifs. In fact, many oncogenes and tumor suppressors are phosphorylated on Proline residues, triggering signaling pathways involving Pro-directed phosphorylation.
Studies have demonstrated that PIN1 is broadly overexpressed in human cancers and overexpression is associated with poor prognosis [11-13]. Additionally, PIN1 plays a pivotal role in tumor progression by influencing various biological processes, including growth-signal responses, cell-cycle progression, and immune responses [14-17]. The role of PIN1 in cancer is further supported by recent findings that PIN1 targets Ser63/73-Pro in c-Jun and multiple pSer/Thr-Pro motifs in c-Fos. C-Jun and Fos are key components of activator protein 1 (AP-1) and function as homodimers or heterodimers, which increase AP-1 transcriptional activity [14,18]. The transcription factor AP-1 is involved in many cellular processes, including cell differentiation, proliferation, apoptosis, and oncogenic transformation, and plays a role in metastasis and angiogenesis. AP-1 function is regulated at different levels, including protein level, stability, and post-translational modifications (including phosphorylation) [19-21].
In this study, we focused on investigate the expression of Pin1 and its clinical significance and biological function in cervical cancer and also explored the potential association with EMT and elucidate the related signal mechanisms in cervical cancer cells.
Materials and methods
Patient samples
We obtained cervical tissue specimens from Uighur women with CSCC and from those who did not have cervical disease, but received hysterectomies in the Department of Gynecology at the First Affiliated Hospital in Medical University of Xinjiang. The median age of patients with cervical cancer was 50.8 years (IQ range 29-65.5 years). Formalin-fixed, paraffin-embedded (FFPE) tissues (n=109) specimens were collected during an initial outpatient visit, gynecologic examination, or after a surgical procedure involving general anesthesia; none of the patients received chemotherapy or radiation prior to surgery. Tumor samples were collected within 30 minutes of surgical resection, and were immediately frozen in liquid nitrogen and stored at -80°C after evaluation by a pathologist. Hematoxylin and eosin staining was also performed to confirm the diagnosis and analyze pathological grade, metastasis, and tumor cell content. All cancers were staged according to the criteria established by the International Federation of Gynecology and Obstetrics (FIGO). Seventy percent of all tumor samples were free of necrosis. We identified 69 FIGO stage IB and 40 FIGO stage IIA tumors, as well as 45 well differentiated, 36 moderately differentiated, and 28 poorly differentiated tumors. Lymph node metastasis was documented in 33 patients. Sixty-four patients with cervical intraepithelial neoplasia (CIN) II-III were selected for this study. Control tissues (n=66) were from patients who did not have cervical lesions or cancer, but had hysterectomies for other reasons (i.e. fibroids, uterine prolapse, adenomyosis, or a combination of fibroids with uterine prolapse) during the same time period.
Cell lines
The human invasive cervical cancer cell line SiHa was purchased from the China Center for Type Culture Collection (Wuhan, China). All cells were cultured in Roswell Park Memorial Institute 1640 medium (Gibco, San Diego, CA), containing 10% fetal bovine serum (Gibco, San Diego, CA) and 1% Penicillin & Streptomycin solution, at 37°C in 5.0% CO2.
Immunohistochemistry (IHC)
IHC staining was performed with primary antibodies against PIN1and c-Jun (1:500 Abcam, Cambridge, MA, USA), N-cadherin, Vimentin, and E-cadherin (1:300 Shanghai Jingtian, Biotechnology, China). Sections (3-mm-thick) were cut from paraffin-embedded tissue blocks. After deparaffinization and rehydration, sections were subjected to antigen retrieval in citrate buffer for 15 min. Endogenous peroxidase activity was quenched with 3% H2O2 and sections were blocked with goat serum for 15 min. Primary antibodies were incubated overnight at 4°C. Following this, sections were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for 10 min, and then in horseradish enzyme-labeled chain avidin solution for 10 min at 37°C. Slides were visualized using DAB as the substrate and counterstained with hematoxylin. Pathologists reviewed the slides and reached a consensus number for each tumor sample. Protein expression levels were quantified using Image Pro plus 6.0 to detect the mean photo density. Briefly, five positive fields in a section were selected at random and separated in Image Pro plus 6.0 to read the optical density. The average values of the five optical densities represented the mean intensity of protein expression in each case.
Transfections
Three PIN1 and c-Jun-targeting small interfering RNAs (siRNAs) and negative control siRNA constructs were synthesized by Genechem (Shanghai, China). The details of the targeting sequences for PIN1-siRNAs are as follows: 5’CATTTGAAGACGCCTCGTT-3’ (PIN1-siRNA1), and 5’-AGAAGATCAAGT CGGGAGA-3’ (PIN1-siRNA2), 5’-GAUGGAAACGACCUUCUAUTT-3’ (c-Jun-siRNA1) and 5’-AAUGAAGUGGCACAGCUGATT-3’ (c-Jun siRNA2) Control siRNA sequences were randomly scrambled sequences.
Western blotting analysis
Total protein was extracted from cells: Protease inhibitors (Boster, Wuhan, China) were added to cell lysates that were maintained on ice for 20 minutes. Samples (50 μg) were boiled for 5 min in sample buffer and then separated on 12% gels by SDS-PAGE. Gels were transferred onto nitrocellulose membranes and blocked for 1 h in 5% skim milk at room temperature with shaking. A primary antibody against PIN1 and c-Jun (Abcam, Cambridge, MA, USA) and EMT-associated proteins (all from Shanghai Jingtian, Biotechnology, China) was added overnight to blots at 4°C. β-actin (1:500; Proteintech, Wuhan, China) was used as a loading control. Blots were washed in PBS-Tween three times, after which the secondary antibody (horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G; Thermo, IL, USA) was added at room temperature for 2 hours. Chemiluminescent substrate (Thermo, IL, USA) was added to visualize bands. Quantity One software was used to quantify the intensity of each band and was normalized to the inten-sity of the internal control β-Actin.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from cancer cells using TRIzol reagent (Ambion Inc., Austin, TX, USA). cDNA was synthesized using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Rockford, IL, USA). qRT-PCR for PIN1 and EMT-associated genes was performed as previously described using the primer sequences shown below; primers were synthesized by Generay (Shanghai, China.) qRT-PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad, CA, USA). Each sample was analyzed in triplicate and quantification of gene expression was determined using the 2-ΔΔCT method.
The primer sequences used in RT-PCR (Table 5)
Table 5.
The primer sequences
| Target | Forward sequence | Reverse sequence |
|---|---|---|
| PIN1 | GATCAACGGCTACATCCAGAA | GGCGTCTTCAAATGGCTTC |
| VIM | GTCCACTGAGTACCGGAGACA | CACGAAGGTGACGAGCCAT |
| CDH1 | AATCTGAAAGCGGCTGATACTG | TGCCCCATTCGTTCAAGTAG |
| CDH2 | ACCAGGTTTGGAATGGGACA | CATGTTGGGTGAAGGGGTG |
| ACTB | GACATCCGCAAAGACCTG | GGAAGGTGGACAGCGAG |
Cell proliferation and apoptotic by flow cytometry
SiHa cells were seeded onto 96-well plates at a density of 2000 cells per well in RPMI 1640 plus 10% calf serum and 1% penicillin/streptomycin. HFC polysaccharide (50~250 μg/mL) was added for 1 h followed by the treatment with 300 μM H2O2 for varying time points (0-24 h). Cell Proliferation distributions was detected by measuring PI-fluorescence with a BD FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA, USA) through an FL-2 filter (585 nm). We recorded 1×104 events/sample. Data were analyzed with Cell Quest.
Control and treated SiHa cells were added at 5×105 cells/mL in binding buffer (10 mM HEPES [(4-(2-hydroxyethyl)-1-piper-azineethanesulfonic acid] [pH 7.4], 140 mM NaCl, 2.5 mM CaCl2). FITC-annexin V (10 μl) in 190 μl of cell suspension was incubated for 10 min at room temperature. Cell mixtures were centrifuged and resuspended in 190 μl binding buffer, and 10 μl PI (1 mg/mL) solution was added. Cells were acquired on a FACS Calibur flow cytometer at 1×104 events/sample. Necrotic cells were defined as positive for both PI and annexin V and were excluded from further analysis.
Wound-healing assay
Cell migration was analyzed by using a wound-healing assay in vitro. SiHa cells infected with PIN1-siRNA were cultured in 6-well plates until they reached 90% confluence. Wounds were inflicted in the cell monolayer with a sterile pipette tip. After 0, 24, 48, and 72 h, cells were observed under a microscope. The distance between the two wounds was measured and expressed as the average percent of wound closure compared to the zero time point.
Transwell membrane-based migration and invasion assay
The effect of PIN1 on the ability of SiHa cells to migrate through a filter or invade through a biological barrier was examined using Transwell insert chambers with 8-μm pore filters (Corning, NY, USA). For Transwell invasion assays, the upper side of an 8-μm pore, 6.5-mm polycarbonate Transwell filter chamber was uniformly coated with Matrigel basement membrane matrix (Corning, NY, USA) for 2 h at 37°C before cells were added. Two hundred and five cells were seeded in the upper chambers with 200 μl serum-free media; the lower chambers were filled with 750 μl complete media. After 24 h, cells that migrated/invaded to the lower surface of the filter were fixed with 4% paraformaldehyde, stained with 0.5% crystal violet, and counted under a microscope.
Statistical analysis
All statistical analyses were performed using SPSS software 17.0 (Chicago, IL, USA). Data were presented as mean ± standard deviation. Statistical analysis was performed using a Student’s t-test or analysis of variance (ANOVA). P-values <0.05 were considered significant.
Results
PIN1 protein overexpression is associated with malignant phenotypes
To investigate whether PIN1 is dysregulated in human cervical cancer, we performed immunohistochemistry on 109 CSCC samples, 64 CIN samples, and 66 normal cervical tissues. As showed in Figure 1, normal cervical epithelium showed very weak PIN1 expression, with a mean (SD) density of 0.101 (0.0101) (Figure 1A1). In CINII, PIN1 was mainly expressed in the atypical cells of the lower third layer (Figure 1A2), with a significantly higher mean (SD) density [0.213 (0.0191)] than that of normal cervical epithelium. The positive expression of PIN1 in CSCC tissues was more obvious, with dark positively stained particles present in the cytoplasm of cancer cells (Figure 1A3). The relationship between PIN1 expression and clinicopathological parameters of CSCC is summarized in Table 1. In addition, we found that PIN1 was significantly associated with lymph node metastasis (P=0.034) and CSCC FIGO Stage (P=0.038). We also found that PIN1 levels in poorly differentiated CSCC tissue were higher than in well-differentiated and moderately differentiated CSCC tissue (P=0.037). The above results demonstrate that PIN1 overexpression is correlated with more advanced CSCC characteristics.
Figure 1.
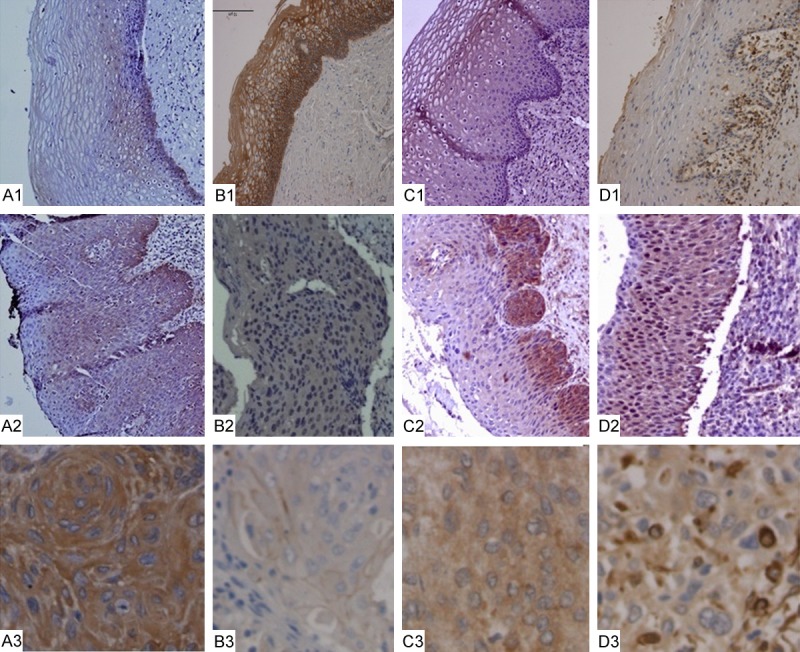
Detection of PIN1 and EMT-related protein expression in normal cervical epithelia, CIN, and CSCC. (A) PIN1 expression detected by immunohistochemistry (IHC) in normal cervical squamous epithelium (A1), CINII (A2), and CSCC (A3). (B) E-cadherin expression by IHC in normal cervical epithelium (B1), CIN (B2), and CSCC (B3). (C, D) Expression of Vimentin and N-cadherin by IHC in normal cervical epithelia (C1, D1), CIN (C2, D2), and CSCC (C3, D3).
Table 1.
Correlation between Pin1 expression and clinicopathological characteristics in patients with cervical cancer
| Characteristics | n | Pin1 Expression Mean (SD) | P |
|---|---|---|---|
| Normal mucous epithelia | 66 | 0.101 (0.0101) | |
| CINII-III | 46 | 0.213 (0.0191) | |
| CSCC | 109 | 0.498 (0.0313) | 0.009 |
| Tumor size, cm | |||
| <4 | 71 | 0.191 (0.0524) | |
| ≥4 | 38 | 0.201 (0.0485) | 0.471 |
| Differentiation | |||
| Well | 45 | 0.181 (0.041) | |
| Moderate | 36 | 0.183 (0.0434) | 0.513 |
| Poor | 28 | 0.209 (0.0471) | 0.037 |
| Lymph node metastasis | |||
| Negative | 76 | 0.188 (0.0400) | |
| Positive | 33 | 0.211 (0.0393) | 0.034 |
| FIGO Stage | |||
| <IB | 69 | 0.189 (0.0524) | |
| ≥IB | 40 | 0.201 (0.0471) | 0.038 |
PIN1 overexpression in cervical cancer is associated with EMT markers
To investigate the relationship between PIN1 overexpression and EMT, we characterized the expression of EMT and polarity markers in patient tissues. We found membrane expression of E-cadherin that trended in decreasing expression levels from normal cervical tissue (Figure 1B1), to CIN (Figure 1B2), to CSCC (Figure 1B3). However, we also found positive cytoplasmic expression of N-cadherin and Vimentin in CIN and CSCC (Figure 1C, 1D). We noted that N-cadherin and Vimentin expression tended to increase as cervical lesions became more aggressive. The mean (SD) expression levels of E-cadherin, N-cadherin, and Vimentin in different groups are summarized in Table 2. A Pearson correlation test revealed that PIN1 expression was positively correlated with Vimentin and N-cadherin expression (r=0.421, P=0.009; r=0.650, P=0.005), and negatively correlated with E-cadherin expression (r=-2.98, P=0.037).
Table 2.
Expression of E-cadherin, β-catentin, N-cadherin and Vimentin proteins in patients with CIN and cervical cancer
| Normal cervical epithelia (N=66) | CINII-III (N=46) | CSCC (N=109) | P | |
|---|---|---|---|---|
| β-catentin expression Mean (SD) | 0.411 (0.0493) | 0.211 (0.0393) | 0.163 (0.0191) | 0.023 |
| E-cadherin expression Mean (SD) | 0.409 (0.0472) | 0.301 (0.0376) | 0.198 (0.0385) | 0.009 |
| N-cadherin expression Mean (SD) | 0.201 (0.0403) | 0.297 (0.0487) | 0.497 (0.0665) | 0.032 |
| Vimentin expression Mean (SD) | 0. 203 (0.0390) | 0.313 (0.0398) | 0.499 (0.0671) | 0.008 |
(r=0.421, P=0.009; r=0.650, P=0.005), and a negative correlation of Pin1 expression with E-cadherin and β-catentin expression (r=-2.98, P=0.037; r=-3.09, P=0.029), respectively.
PIN1 knockdown inhibits proliferation and promote apoptosis of SiHa cervical cancer cells
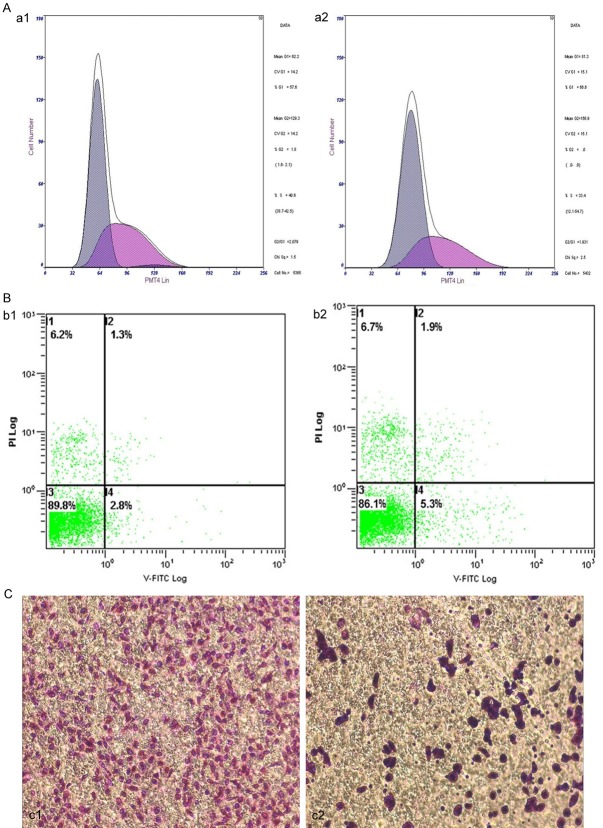
The above results demonstrate that PIN1 overexpression is correlated with tumor progression. Thus, we wanted to explore whether PIN1 knockdown could inhibit growth of cervical cancer cells. siRNAs were designed against PIN1 and transfected into the SiHa cervical cancer cell line. Western blot analysis verified that PIN1 protein was downregulated in SiHa cells transfected with siRNA against PIN1 (Figure 2A) compared with the vector control and normal groups (Figure 2B). Cell proliferation assays showed that proliferation of SiHa cells transfected with PIN1 siRNA was significantly impaired compared with control cells (Figure 3A), The percentage of SiHa cells in G0/G1 phase significant increased (64.60%±4.06%) 48 h after PIN1 knockdown compared with the percentage of control cells in G0/G1 (53.27%±2.11%). The percentage of PIN1 shRNA-transfected cells in S phase was significantly decreased (33.97%±1.82%) compared with of control (42.93%±1.95% Table 3). indicating that PIN1 expression stimulates SiHa cell proliferation. To determine the effect of PIN1 knockdown on the apoptotic changes of SiHa cells, we conducted a flow cytometry analysis. There were 8.13%±0.55% of SiHa cells that demonstrated apoptotic changes 48 h after PIN1 knockdown; this was a significant increase compared with control (2.23%±0.45%) (Figure 3B; Table 4).
Figure 2.
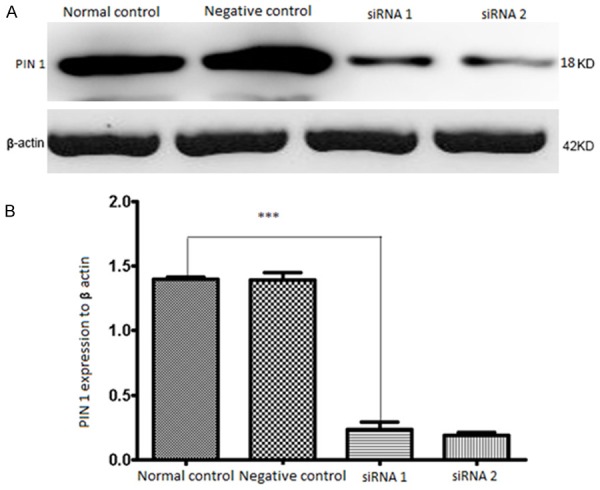
The levels of PIN1 protein detected by Western blotting after transfection for 48 h. A. The relative expression of PIN1 was displayed, which normalized to β-actin. B. There is a statistically significant difference between the group transfected with PIN1-shRNA and normal control. ***P<0.001.
Figure 3.
PIN1 positively modulates cervical cancer cells malignant phenotypes. (A, B and C) Cell Proliferation, apoptosis and invasion in Siha cells, respectively (a1, b1 and c1 Normal controls). (a2, b2 and c2) A Knockdown of PIN1 decreased cell proliferation, increased cell apoptosis, decreased cell invasion, which significantly decreased malignant phenotypes of Siha cells. All experiments were performed at least three times.
Table 3.
Changing Siha cells proliferation after PIN1 siRNA vector transfect 48 hours (x̅±s, n=3)
| G0/G1 (%) | S (%) | G2/M (%) | |
|---|---|---|---|
| Normal control | 54.47±3.01 | 41.60±1.56 | 3.93±3.19 |
| Negative control | 53.27±2.11 | 42.93±1.95 | 3.73±3.61 |
| PIN1 siRNA1 | 63.83±3.29Δ | 35.87±2.79Δ | 0.27±0.46 |
| PIN1 siRNA2 | 64.60±4.06Δ | 33.97±1.82Δ | 1.40±2.42 |
compared with control group, P<0.01.
Table 4.
Changing apoptosis rate of Siha cell lines in response to altered PIN1 expression by transfect PIN1 siRNA vector after 48 hours (x̅±s, n=3)
| Apoptosis rate of Siha cell (%) | |
|---|---|
| Normal control | 2.23±0.74 |
| Negative control | 2.23±0.45 |
| PIN1 siRNA1 | 4.60±0.61Δ |
| PIN1 siRNA2 | 8.13±0.55Δ |
compared with control group, P<0.01.
Downregulation of PIN1 suppresses migration and invasion of SiHa cervical cancer cells
The above analysis showed that PIN1 overexpression is associated with lymph node metastasis, poor tumor differentiation, and higher FIGO stage, suggesting that PIN1 might promote cervical cancer progression and metastasis. Cancer cells metastasize once they acquire the ability to migrate and invade. Thus, we carried out in vitro experiments to determine whether PIN1 enhances migration and invasion of cervical cancer cells. Transwell migration assay demonstrated that PIN1 knockdown decreased the number of cells that migrated through Transwell filters (Figure 3C). Additionally, a wound-healing assay showed that reduced expression of PIN1 by siRNA knockdown led to slower migration in both cervical cancer cell lines (Figure 4A, 4B). Together, these results suggest that Cell migration abilities was inhibited after PIN1 down-expression compared with control, and Siha cells with reduced expression of PIN1 were inhibited the migration ability.
Figure 4.
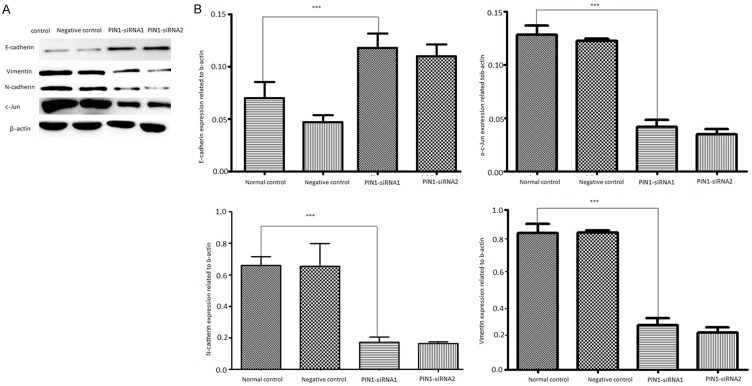
Knockdown of PIN1 expression in SiHa cervical cancer cells. A, B. Cells were transfected with PIN1-specific siRNAs for 48 h and lysed for western blot analyze E-cadherin, N-cadherin, Vimentin expression. β-actin was used as an internal control.
Pin1 knockdown decreases epithelial-mesenchymal transition
Studies illustrate that before metastasis, cancer cells may have EMT that involves both transcriptional suppression of epithelial genes and activation of mesenchymal genes. Based on this knowledge and our results above, we hypothesize that Pin1 can induce EMT during its promotion of migration and invasion. Consequently, we first explored the markers of EMT with western blot for epithelial marker and mesenchymal markers. The result showed that Pin1 knockdown upregulated E-cadherin and repressed N-cadherin and Vimentin (Figure 4), which clearly indicates inhibition of EMT in cervical cancer cells.
Discussion
Metastasis is the leading cause of cancer-related death. Invasion represents the first step of the migration of cancer cells away from the primary site [22,23]. As a novel regulator, Pin1 has become an essential oncogene regulating multiple aspects of cancer initiation and progression, including malignant transformation, cell survival, angiogenesis, and EMT. Therefore, Pin1 may be a potential target for cell type specific therapeutic strategies, as an assistant or as a primary approach, that may have clinical implications in cancer.
On a histological level, we detected the expression levels of Pin1 in normal cervical epithelium, CINII, and cervical carcinoma. From normal cervical epithelium to CINII, the expression of Pin1 was gradually increased, and an overexpression of Pin1 was found in CSCC tissues, indicating the participation of Pin1 in the Initiation and development of CSCC. This result is consistent with other reports on Pin1 that an aberrant elevation of Pin1 expression has been found to involve progression of human cancers [24-27]. In this study, Pin1 overexpression was found to be significantly associated with lymph node metastasis and advanced FIGO stage in CSCC, suggesting Pin1 plays an oncogenic role in cervical cancer.
our results partly consistent with the studies of Poonam J et al reported that a high level of Pin1 associated with FIGO stage, tumor size and frequency of lymph node involvement of cervical cancer [28], but we did not find the correlation of Pin1 with tumor size in cervical cancer. The Pin1 expression was not significantly different between the CSCC tissues of well differentiation and the moderate differentiation, but its level in the cancer tissues of poorly differentiation was, interestingly, higher than its level in the cancer tissues of well and moderate differentiation. The discrepancies may be due to different histological origin of cervical cancer and may also reflected its involvement in the cancer progression.
To evaluate the relationship between Pin1 and EMT of CSCC, the expression levels of E-cadherin, N-cadherin and Vimentin, in CSCC tissues was also determine. As a result, a clearly positive expression of Vimentin and N-cadherin and relatively weak expression of E-cadherin was detected in CSCC tissues, representing that there existed EMT of the carcinoma cells. Importantly, the expression level of Pin1 was found to be positively correlated with Vimentin and N-cadherin expression and negatively correlated with E-cadherin expression, which suggested that Pin1 promoted the EMT of CSCC.
Although several studies on Pin1 in cervical cancer have been reported, little is known about the biological function and mechanism of Pin1 in cervical cancer. Until now, only Tao W group reported a study of the Pin1 role in the chemo-resistance of cervical cancer [29]. In present study, to further verify the results of the histological level, we altered the Pin1 expression levels in SiHa cells with gene transfection and siRNA techniques to assess its biological functions such as promotion of proliferation, migration, invasion and EMT of cervical cancer on a cellular level. prospectively, we found that overexpression of Pin1 led to an up-regulation of Vimentin and N-cadherin companied with a down-regulation of E-cadherin both on mRNA and protein level in SiHa cells. While, the knockdown of Pin1 led to opposite results. These results showed that Pin1 could enhance the expression of Vimentin and N-cadherin and inhibit the expression of E-cadherin in SiHa cells, signifying that Pin1 was involved in the EMT of cervical carcinoma because loss of epithelial feature and obtain mesenchymal characteristic are known as the vital steps of EMT. Our results were in consistent with the reports Sakuma Y research group and Kim MR et al, which demonstrated the involvement of Pin1 in EMT of lung adenocarcinoma and breast cancer [30,31]. However, the molecular mechanisms related to the association of Pin1 with EMT in cervical cancer have never been investigated.
In summary, our results demonstrated that Pin1 is overexpressed in cervical cancer and is a potential prognostic biomarker of cervical cancer, which Promotes Progression of Cervical Squamous Cell Carcinoma related with Epithelial-Mesenchymal Transition, but the detailed mechanisms need to further study.
Acknowledgements
This work was funded by the Xinjiang Natural Science Foundation of China (215211C059). The funding source had no role in the study design, data collection or analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Koh WJ, Greer BE, Abu-Rustum NR, Apte SM, Campos SM, Cho KR, Chu C, Cohn D, Crispens MA, Dorigo O, Eifel PJ, Fisher CM, Frederick P, Gaffney DK, Han E, Huh WK, Lurain JR 3rd, Mutch D, Fader AN, Remmenga SW, Reynolds RK, Teng N, Tillmanns T, Valea FA, Yashar CM, McMillian NR, Scavone JL. Cervical cancer, version 2. 2015. J Natl Compr Canc Netw. 2015;13:395–404. doi: 10.6004/jnccn.2015.0055. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J. Clin. Oncol. 2008;26:5802–5812. doi: 10.1200/JCO.2008.16.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gien LT, Covens A. Lymph node assessment in cervical cancer: prognostic and therapeutic implications. J Surg Oncol. 2009;99:242–247. doi: 10.1002/jso.21199. [DOI] [PubMed] [Google Scholar]
- 5.Benedetti Panici P, Basile S, Angioli R. Pelvic and aortic lymphadenectomy in cervical cancer: the standardization of surgical procedure and its clinical impact. Gynecol Oncol. 2009;113:284–290. doi: 10.1016/j.ygyno.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol. 2014;16:488–494. doi: 10.1038/ncb2976. [DOI] [PubMed] [Google Scholar]
- 7.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 8.Lee MY, Chou CY, Tang MJ, Shen MR. Epithelial-mesenchymal transition in cervical cancer: correlation with tumor progression, epidermal growth factor receptor overexpression, and snail up-regulation. Clin Cancer Res. 2008;14:4743–4750. doi: 10.1158/1078-0432.CCR-08-0234. [DOI] [PubMed] [Google Scholar]
- 9.Wulf G, Finn G, Suizu F, Lu KP. Phosphorylationspecific prolyl isomerization: is there an underlying theme? Nat Cell Biol. 2005;7:435–441. doi: 10.1038/ncb0505-435. [DOI] [PubMed] [Google Scholar]
- 10.Lu KP, Hanes SD, Hunter T. A human peptidylprolyl isomerase essential for regulation of mitosis. Nature. 1996;380:544–547. doi: 10.1038/380544a0. [DOI] [PubMed] [Google Scholar]
- 11.Xu M, Cheung CC, Chow C, Lun SW, Cheung ST, Lo KW. Overexpression of PIN1 enhances cancer growth and aggressiveness with Cyclin D1 induction in EBV-associated nasopharyngeal carcinoma. PLoS One. 2016;11:e0156833. doi: 10.1371/journal.pone.0156833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atabay KD, Yildiz MT, Avsar T, Karabay A, Kiliç T. Knockdown of Pin1 leads to reduced angiogenic potential and tumorigenicity in glioblastoma cells. Oncol Lett. 2015;10:2385–2389. doi: 10.3892/ol.2015.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi HJ, Kim JY, Lim SC, Kim G, Yun HJ, Choi HS. Dipeptidyl peptidase 4 promotes epithelial cell transformation and breast tumourigenesis via induction of PIN1 gene expression. Br J Pharmacol. 2015;172:5096–109. doi: 10.1111/bph.13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chae U, Park SJ, Kim B, Suo W, Min JS, Lee JH, Park SH, Lee AH, Lu KP, Lee DS, Min SH. Critical role of XBP1 in cancer signaling is regulated by PIN1. Biochem J. 2016;473:2603–10. doi: 10.1042/BCJ20160482. [DOI] [PubMed] [Google Scholar]
- 15.Kutay DA, Mehmet TY, Timucin A, Arzu K, Turker K. Knockdown of Pin1 leads to reduced angiogenic potential and tumorigenicity in glioblastoma cells. Oncol Lett. 2015;10:2385–2389. doi: 10.3892/ol.2015.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu KP, Zhou XZ. The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signalling and disease. Nature. 2007;8:904–916. doi: 10.1038/nrm2261. [DOI] [PubMed] [Google Scholar]
- 17.Sakuma Y, Nishikiori H, Hirai S, Yamaguchi M, Yamada G, Watanabe A, Hasegawa T, Kojima T, Niki T, Takahashi H. Prolyl isomerase Pin1 promotes survival in EGFR-mutant lung adenocarcinoma cells with an epithelial-mesenchymal transition phenotype. Lab Invest. 2016;96:391–8. doi: 10.1038/labinvest.2015.155. [DOI] [PubMed] [Google Scholar]
- 18.Beretta GL, De Cesare M, Albano L, Magnifico A, Carenini N, Corna E, Perego P, Gatti L. Targeting peptidyl-prolyl isomerase pin1 to inhibit tumor cell aggressiveness. Tumori. 2016;102:144–149. doi: 10.5301/tj.5000471. [DOI] [PubMed] [Google Scholar]
- 19.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Bergami P, Lau E, Ronai Z. Emerging roles of ATF2 and the dynamic AP1 network in cancer. Nat Rev Cancer. 2010;10:65–76. doi: 10.1038/nrc2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Dam H, Castellazzi M. Distinct roles of Jun: Fos and Jun: ATF dimers in oncogenesis. Oncogene. 2001;20:2453–2464. doi: 10.1038/sj.onc.1204239. [DOI] [PubMed] [Google Scholar]
- 22.Nam EH, Lee Y, Moon B, Lee JW, Kim S. Twist1 and AP-1 cooperatively upregulate integrin α5 expression to induce invasion and the epithelial-mesenchymal transition. Carcinogenesis. 2015;36:327–337. doi: 10.1093/carcin/bgv005. [DOI] [PubMed] [Google Scholar]
- 23.Kim MR, Choi HS, Heo TH, Hwang SW, Kang KW. Induction of vascular endothelial growth factor by peptidyl-prolyl isomerase Pin1 in breast cancer cells. Biochem Biophys Res Commun. 2008;369:547–553. doi: 10.1016/j.bbrc.2008.02.045. [DOI] [PubMed] [Google Scholar]
- 24.Gao J, Yan Q, Wang J, Liu S, Yang X. Epithelial-to-mesenchymal transition induced by TGF-β1 is mediated by AP1-dependent EpCAM expression in MCF-7 cells. J Cell Physiol. 2015;230:775–82. doi: 10.1002/jcp.24802. [DOI] [PubMed] [Google Scholar]
- 25.Zhang C, Ding XP, Zhao QN, Yang XJ, An SM, Wang H, Xu L, Zhu L, Chen HZ. Role of α7-nicotinic acetylcholine receptor in nicotine-induced invasion and epithelial-to-mesenchymal transition in human non-small cell lung cancer cells. Oncotarget. 2016;7:59199–59208. doi: 10.18632/oncotarget.10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark AG, Vignjevic DM. Modes of cancer cell invasion and the role of the microenvironment. Curr Opin Cell Biol. 2015;36:13–22. doi: 10.1016/j.ceb.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Li K, Ma T, Cai J, Huang M, Guo H, Zhou D, Luan S, Yang J, Liu D, Jing Y, Zhao L. Conjugates of 18β-glycyrrhetinic acid derivatives with 3-(1H-benzo[d] imidazol-2-yl)propanoic acid as Pin1 inhibitors displaying anti-prostate cancer ability. Bioorg Med Chem. 2017;25:5441–5451. doi: 10.1016/j.bmc.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Z, Zhang H, Lang F, Liu G, Gao D, Li B, Liu Y. Pin1 promotes prostate cancer cell proliferation and migration through activation of Wnt/β-catenin signaling. Clin Transl Oncol. 2016;18:792–7. doi: 10.1007/s12094-015-1431-7. [DOI] [PubMed] [Google Scholar]
- 29.Shinoda K, Kuboki S, Shimizu H, Ohtsuka M, Kato A, Yoshitomi H, Furukawa K, Miyazaki M. Pin1 facilitates NF-κB activation and promotes tumour progression in human hepatocellular carcinoma. Br J Cancer. 2015;113:1323–31. doi: 10.1038/bjc.2015.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi HJ, Kim JY, Lim SC, Kim G, Yun HJ, Choi HS. Dipeptidyl peptidase 4 promotes epithelial cell transformation and breast tumourigenesis via induction of PIN1 gene expression. Br J Pharmacol. 2015;172:5096–109. doi: 10.1111/bph.13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poonam J, Sudha S, Indrani D, Richa T, Gayatri R. Peptidyl-prolyl isomerase Pin1-mediated abrogation of APC-β-catenin interaction in squamous cell carcinoma of cervix. Rom J Morphol Embryol. 2014;55:83–90. [PubMed] [Google Scholar]