Abstract
Management of prostate cancer, especially advanced prostate cancer, remains clinically challenging and requires the identification of new biomarkers and therapeutic targets that can be exploited to improve patient outcome. Galectin-3 (gal-3) is a carbohydrate-binding protein involved in cancer progression and metastasis, including prostate tissues. Gal-3 function is regulated by proteolytic cleavage and the cleaved gal-3 is implicated in tumor progression. This study is the first to determine gal-3 expressions with two monoclonal anti-gal-3 antibodies in prostate tissues to distinguish expression patterns between intact and cleaved gal-3 and analyze their clinical relevance. Our results showed gal-3 cleavage occurred in prostate cancer but not normal prostate. Gal-3 presented in tumor tissues was mainly the cleaved form that can be detected by the anti-gal-3 antibody targeting C terminal. The cleaved gal-3, but not the intact gal-3, was increased in prostate cancer compared to normal prostate tissues and positively associated with malignance, tumor progression and metastasis. In addition, the expression of cleaved gal-3 was closely related to PSA level, indicating a PSA-mediated degradation of intact gal-3 in prostate cancer. In summary, our findings suggested the cleaved gal-3 could be a valuable diagnostic biomarker and a therapeutic target for the treatment of prostate cancer, especially advanced metastatic prostate cancer.
Keywords: Galectin-3, prostate cancer, cleaved, monoclonal antibody, clinical
Introduction
Prostate cancer (PCa) is one of the most common types of cancer in men worldwide and has recently become one of the leading causes of cancer death. Prostate cancer alone accounts for almost 1 in 5 new diagnoses of all cancer cases in men and approximately 26,730 deaths in United States in 2017 [1,2]. While early-stage prostate cancer is well treated by surgical resection or radiotherapy, advanced-stage prostate cancer, especially metastatic prostate cancer, has limited therapeutic options. Therefore, there is a continuous search for better diagnostic markers and therapeutic targets for this disease.
Galectin-3 (gal-3) is a member of the β-galactoside-binding lectin (galectin) family, comprising 250 amino acid residues [3]. Gal-3 is synthesized in the cytoplasm and is then either transported to the nucleus and other cell organelles, or secreted into the extracellular space through non-classical mechanisms [4]. Unlike other galectins that are consisted of exclusive carbohydrate recognition domains (CRD), gal-3 is the only chimera galectin that contains three distinct structural domains: a short NH2-terminal domain containing a phosphorylation site, a repeated collagen α-like sequence, and a C-terminal domain containing a single CRD composed of 140 amino acids [5,6]. The N-terminal domain is associated with self-association of gal-3 from monomer to oligomer, whereas the CRD is responsible for gal-3’s lectin activity, and specifically recognizes and binds to glycoprotein oligosaccharides expressed on the cell surface or within the extracellular matrix [4,7]. This ability of CRD is related to gal-3’s function. Besides, the collagen-like domain of gal-3 is susceptible to rapid and efficient cleavage by proteases [4].
Due to its unique molecular structure, gal-3 was reported to be a substrate for enzymatic cleavage by MMPs, and prostate specific antigen (PSA) at Gly32-Ala33, Ala62-Tyr63, and Tyr107-Gly108 [6,8,9]. Gal-3 cleavage was critical for tumor progression. In breast cancers, the cleaved form of gal-3 modulated angiogenesis, tumor growth and was associated with malignancy in primary lesions [8,9]. In prostate cancer, the cleaved gal-3 was implicated in cell migration and bone metastasis [6,10,11]. It is likely that gal-3 cleavage was a characterized event in bone metastatic niche of prostate cancer. However, the precise role of cleaved gal-3 in the prostate tissue and its implication for clinical pathological parameters remains obscure. In an attempt to further our understanding of the significance of cleaved gal-3 during cancer progression, we measured the expression of gal-3 with two antibodies targeting different domains of gal-3, in prostate tissue, in order to distinguish expression patterns between intact and cleaved gal-3. One antibody targeting C-terminal fragment of gal-3 was a novel monoclonal antibody named anti-gal-3C, the other one was a commercial N-terminal monoclonal antibody named anti-gal-3N. Anti-gal-3C recognizes the epitopes in or around the CRD, which could detected the intact gal-3 and the cleaved C-terminal region of gal-3, while anti-gal-3N only recognizes the intact gal-3 [12]. Our data showed that the positive staining with anti-gal-3C increased, whereas the positive staining with anti-gal-3N decreased, during prostate cancer progression, suggesting gal-3 was cleaved during the cancer progression. In addition, the cleaved gal-3 was significantly associated with clinical parameters. This is the first time gal-3 was detected with a monoclonal antibody specifically targeting C-terminal fragment of gal-3 in prostate cancer and correlated with clinical parameters.
Materials and methods
Antibodies
Customized monoclonal mouse anti-galectin-3 antibody against the C-terminal region (anti-gal-3C) was created by Dr. Lanqing Huang (The Skip Viragh Center for Pancreatic Cancer Research and Clinical Care and the Sol Goldman Pancreatic Cancer Center at Johns Hopkins). Rat monoclonal antibody against galectin-3 (anti-gal-3N, also known as anti-Mac-2 in other papers) was produced by the hybridoma clone M3/38 (Boehringer Mannheim, Indianapolis, Ind.).
Study patients
66 patients with prostate cancer and 73 patients with benign prostatic hyperplasia (BPH) were recruited in the First Affiliated Hospital of Guangxi Medical University, China, during the period from January 2014 to December 2015. The mean age of prostate cancer patients and BPH patients was 71.2±8.5 years (ranged from 46 to 85 years) and 69.0±7.7 years (ranged from 56 to 85 years) respectively. The TNM stage classification and gleason score information of each tissue specimen was annotated by the clinicians in the above hospital: for histological grade, 43 patients were gleason 6-7, 23 were gleason 8-9; for pathological stage, 29 were stage I-II, 37 were stage III-IV. This study was approved by the ethical committee of Guangxi Medical University, and carried out in accordance with The Code of Ethics of the World Medical Association. Informed consent was obtained from all human subjects.
Immunohistochemistry
Immunohistochemical staining was performed with streptavidin-peroxidase (SP) two steps. Paraffin-embedded sections on polylysine-coated slides were used for staining. Slides were baked at 65°C for 3 hours, and deparaffinage in xylene then rehydrated in a grade alcohol series. After that, the tissue slides were boiled in 1 mmol/L sodium citrate buffer (pH 6.0) in a pressure cooker for 15 minutes then washed with PBS. Endogenous peroxidase activity was blocked by 0.3% hydrogen peroxide for 15 min at 37°C and washed with PBS, then nonspecific binding of immunoglobulin was minimized by blocking with normal goat serum working fluid for 20 min at 37°C. After incubating with an anti-gal-3C mouse monoclonal antibody (10 μg/ml) overnight at 4°C, sections were washed 3 times in PBS to remove unbound primary antibody, then incubated with appropriate biotinylated secondary antibodies for 15 min at 37°C. Before and after incubating with the avidin-biotin-peroxidase complex for 20 minutes at 37°C, sections were washed in PBS. For Blank control, the primary antibody was replaced by PBS. Visualization was performed by using DAB (ZSBIO, ZLI-9018) chromogen for 5 min. Samples were counterstained by incubating with hematoxylin (BORSTER, AR0005) for 1 min, then dehydrated and mounted. Visualization and documentation were accomplished with a Leica Microsystems.
Evaluation of immunohistochemical staining
Ten high-power field (HPF) (magnification, ×400) of each slide of tumor were evaluated by two independent observers (Jiamin Gao, first author) and Tianyu Li (Co-first author) to semiquantify the percentage of gal-3 immunopositivity following the protocols described previously. The staining results were evaluated according to the immunodetection of stain intensity and area of positive cells. The degree of staining was subdivided as follows: 0, No staining; 1, Focal, weak staining; 2, Linear or cluster, strong staining; 3, Diffuse, intense staining. The positive cells in the observed tissue ranged from 0 to 4 in percentage: 0, No staining; 1, <25%; 2, 26%-50%; 3, 51%-75%; 4, > 75%. The samples were categorized as positive and negative based on the sum of the scores as follows: 0-2, negative (-); 3-4, positive (+); 5-6, positive (++); 7, positive (+++). The score of (-) were considered to be negative for gal-3 expression. Positive (+) was considered as low expression, and positive (++ or +++) was considered as high expression. All cases were reviewed by two pathologists who were blinded.
Statistical analyses
All results were presented as the mean ± SD for three or more individual experiments. The relationship between the protein expression of gal-3 protein expression and different clinicopathological characteristics was analyzed using Chi-square test (χ2) and Fisher’s exact test. When the confidence interval was defined as 95% and the P values <0.05, the differences were considered statistically significant. Statistical analyses were performed with SPSS software (version 23.0, SPSS Inc. Chicago, IL, USA). The diagrams were drawn by using GraphPad Prism 5.
Results
Expression of the gal-3 in human prostate tissues detected by two different antibodies
To understand the cleavage of gal-3 in human prostate cancer, we examined the prostate tissues with two antibodies recognizing different fragments of gal-3 as described in material and methods.
As shown in Figure 1, the expression of gal-3 stained with anti-gal-3C antibody was predominantly localized in the cytoplasm referred to the positive control ovarian tissue (Figure 1A). In contrast, gal-3 stained with anti-gal-3N antibody was localized in both the cytoplasm and nucleus in BPH tissues and early stage cancer tissues, but transferred into cytoplasm in advanced prostate cancer tissues (Figure 2 and Table 3). The expression of gal-3 varied in different prostate tissues. In BPH tissues, anti-gal-3C showed weak staining (Figure 1B) whereas anti-gal-3N displayed intense staining (Figure 2B), indicating that gal-3 is not cleaved in normal prostate. In prostate tumor tissues, gal-3 was intensely stained in cytoplasm with anti-gal-3C antibody (Figure 1C-F) and the intensity of tissue staining for Gal-3 in prostate cancer was significantly higher than that in BPH tissues (45.45% vs. 10.96%, P<0.001) (Table 1). However, as for anti-gal-3N antibody, there was high density staining in early stage tumor tissues, but little gal-3 staining in advanced tumor samples (Figure 2C-F). These results indicated that gal-3 cleavage occurred in prostate tumor tissues and mainly in advanced tumor tissues. It is noteworthy that the gal-3 staining with anti-gal-3C antibody was more intense in advanced stage tissues compared to those in early stages, meanwhile staining with anti-gal-3N antibody showed opposite results, suggesting the cleaved form of gal-3 was increased during tumor progression.
Figure 1.
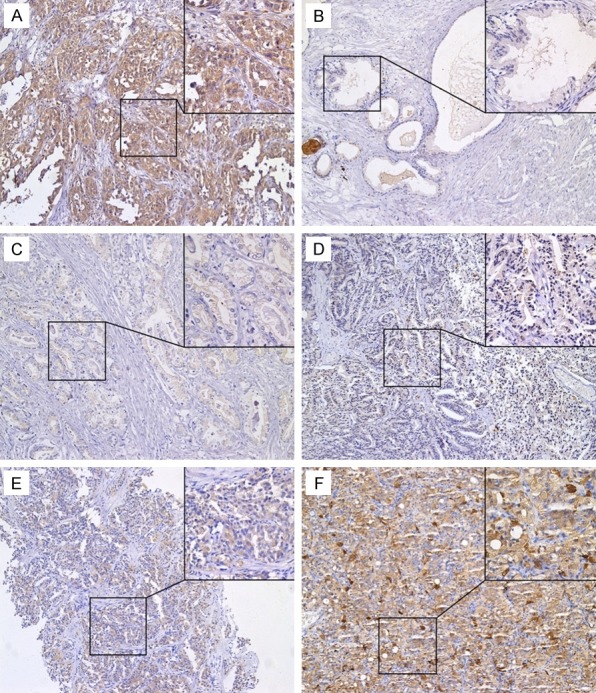
The expression of intact galectin-3 and cleaved galectin-3 in prostate specimens as determined by immunohistochemical staining using anti-gal-3C monoclonal antibody. Representative immunohistochemistry results were shown. Original magnification, ×100, ×400. A. The human ovarian cancer tissue (positive control); B. Benign prostatic hyperplasia; C-F. Prostate cancer; C. Gleason score 6 (T2N0M0, Stage II, aged 60 years); D. Gleason score 7 (T2N0M0, Stage II, aged 65 years); E. Gleason score 8 (T3N0M0, Stage III, aged 64 years); F. Gleason score 9 (T4N1M0, Stage IV, aged 74 years).
Figure 2.

The expression of intact galectin-3 in prostate specimens as determined by immunohistochemical staining using anti-gal-3N monoclonal antibody. Representative immunohistochemistry results were shown. Original magnification, ×100, ×400. A. The human ovarian cancer tissue (positive control); B. Benign prostatic hyperplasia (BPH); C-F. Prostate cancer; C. T1N0M0, Stage I, aged 71 years; D. T2N0M0, Stage II, aged 84 years; E. T3N0M0, Stage III, aged 83 years; F. T4N0M0, Stage IV, aged 76 years.
Table 3.
Expression of gal-3 (detected by anti-gal-3N antibody) in benign prostatic hyperplasia (BPH) tissues
| Tissue | Case n | Anti-gal-3N staining n (%) | |
|---|---|---|---|
|
| |||
| Cytoplasm and nucleus | Cytoplasm | ||
| BPH | 11 | 11 (99.9) | 0 (0) |
Table 1.
Expression of gal-3 (detected by using anti-gal-3C antibody) in different types of prostate tissues
| Prostate tissue | Case n | Anti-gal-3C staining n (%) | P | |
|---|---|---|---|---|
|
| ||||
| Low expression | High expression | |||
| PCa | 66 | 36 (54.55) | 30 (45.45) | <0.001 |
| BPH | 73 | 65 (89.04) | 8 (10.96) | |
Relationship between the expression of the gal-3 and clinic pathological features in prostate cancer
While the intensity of gal-3 staining with anti-gal-3C antibody was showed to increase with prostate tumor progression, the clinical relevance of gal-3 expression of remains unclear. Thus we analyzed the relationship between the gal-3 expression determined by the anti-gal-3C antibody and clinical parameters.
Among the prostate cancer tissues tested, the percentage of gal-3-positive expression in cases with gleason score 8-9 was significantly higher than cases with gleason score 6-7 (91.30% vs. 20.93%, P<0.001) (Table 2), suggesting increased gal-3 is related to malignance of prostate cancer. Likewise, a higher percentage of gal-3 positive staining was observed in cases of TNM stage III-IV compared to cases of TNM stage I-II (56.76% vs. 31.03%, P=0.037) (Table 2). In contrast, anti-gal-3N staining was significantly more intense in tissues of TNM stage I-II compared to those of TNM III-IV (Table 4), indicating the intact gal-3 was reduced with tumor progression. All these results suggested that the cleaved gal-3, but not the intact gal-3, was positively associated with tumor progression. More interestingly, the positivity rate of gal-3 in cases with total PSA≥20 ng/ml was significantly higher than those with total PSA<20 ng/ml (52.94% vs. 20.00%, P=0.024), indicating gal-3 cleavage might be associated with PSA level and distant metastases. There was no association between gal-3 expression and age, the ratio of free PSA to total PSA in prostate cancer (Table 2). Taken together, these data suggested the cleaved gal-3 was implicated with malignance and tumor progression in prostate cancer.
Table 2.
Relationship between the expression of gal-3 (determined by using anti-gal-3C antibody) and clinicopathological features in prostate cancer
| Variables | Case n | Anti-gal-3C staining n (%) | P | |
|---|---|---|---|---|
|
| ||||
| Low expression | High expression | |||
| Age (years) | ||||
| <70 | 24 | 10 (41.67) | 14 (58.33) | 0.112 |
| ≥70 | 42 | 26 (61.90) | 16 (38.10) | |
| Free PSA (ng/ml)/Total PSA (ng/ml) | ||||
| ≥0.16 | 15 | 10 (66.67) | 5 (33.33) | 0.283 |
| <0.16 | 51 | 26 (50.98) | 25 (49.02) | |
| Total PSA (ng/ml) | ||||
| <20 | 15 | 12 (80.00) | 3 (20.00) | 0.024 |
| ≥20 | 51 | 24 (47.06) | 27 (52.94) | |
| TNM Stage | ||||
| I-II | 29 | 20 (68.97) | 9 (31.03) | 0.037 |
| III-IV | 37 | 16 (43.24) | 21 (56.76) | |
| Gleason Score | ||||
| 6-7 | 43 | 34 (79.07) | 9 (20.93) | <0.001 |
| 8-9 | 23 | 2 (8.70) | 21 (91.30) | |
Table 4.
Relationship between gal-3 expression (determined by anti-gal-3N antibody) and clinicopathological features in prostate cancer
| Variables | Case n | Anti-gal-3N staining n (%) | P | |
|---|---|---|---|---|
|
| ||||
| Low expression | High expression | |||
| TNM stage | ||||
| I-II | 8 | 6 (75.0) | 2 (25.0) | 0.013 |
| III-IV | 10 | 1 (10.0) | 9 (90.0) | |
Discussion
Prostate cancer is one of the leading causes of cancer-related death among men worldwide [1]. Metastasis is the major cause of cancer death due to the limitation of chemotherapy. Therefore, better diagnostic biomarkers and therapeutic targets to diagnose or control prostate cancer is badly needed.
There is growing clinical interest in the role of gal-3 as a potential diagnostic marker and/or a therapeutic target. To our best knowledge, the present study is the first to evaluate gal-3 expression with a monoclonal antibody targeting C-terminal region in comparison with one targeting the N-terminal, and to correlate their expression levels to clinical parameters.
Gal-3 is a carbohydrate-binding protein, and it has been associated with many tumor development processes including cell growth, adhesion, proliferation, and metastasis [13,14]. Gal-3 is known to serve as a tumor suppressor in tumorigenesis and progression in prostate cancer. Knockdown of gal-3 in prostate cancer cells was related with reduced cell proliferation, cell migration and invasion, and tumor formation in nude mice. Inhibition of gal-3 functions with an inhibitor significantly reduced lung metastasis of prostate cancer [15]. Due to its unique chimera structure, gal-3 was susceptible to be cleaved by proteases, such as MMPs and PSA [4]. The function of gal-3 is regulated, at least in part, by proteolytic processing that cleaves the gal-3 CRD from the N-terminal non-lectin domain [16]. Proteolytic cleavage of galectin-3 results in the production of a functional galectin-3 CRD fragment and abolishes the ability of intact gal-3 to crosslink its target ligands [16,17]. Gal-3 cleavage was reported to be critical to the function of gal-3 and play an important role in tumor progression [8-11,16].
In this study, we immunochemically measured the expression of gal-3 in prostate cancers with two antibodies, anti-gal-3C and anti-gal-3N, to distinguish expression patterns between intact and cleaved gal-3. The anti-gal-3C antibody, a novel monoclonal antibody recognizing epitopes in or around the CRD of gal-3, detects both the intact and the cleaved gal-3 (C-terminal region), while the anti-gal-3N antibody binding to the N-terminal of gal-3, detects only the intact gal-3. We investigated the role of gal-3 and its cleaved form during the progression of human prostate cancer by using these two different anti-gal-3 antibodies. In BPH tissues, gal-3 was not cleaved since anti-gal-3N antibody showed intense staining whereas the anti-gal-3C exhibited weak staining. However, the results were opposite in prostate tumor tissues: the anti-gal-3C staining was more intense in tumor tissues compared to BPH tissues, while anti-gal-3N staining was weaker in tumor tissues (especially advanced prostate tumor tissues) compared to BPH tissues. This discrepancy suggests gal-3 cleavage occurs in prostate tumor tissues but not prostate tissues. Our results obtained by using anti-gal-3N antibody showed the gal-3 expression was significantly higher in TNM stage I-II tissues compared to TNM stage III-IV tissues, indicating the intact gal-3 was decreased during tumor progression. This finding was consistent with past studies [12,18-20], which describe a decreased expression of gal-3 during prostate cancer progression when using a monoclonal antibody recognizing only the intact gal-3, indicating that loss of gal-3 expression may be associated with the evolution of the disease. More importantly, our findings obtained with anti-gal-3C antibody indicated that the levels of cleaved gal-3 increased with the progression of the prostate cancer, although the levels of intact gal-3 decreased, which is in agreement with Wang et al’s results [10]. In their study, the cleaved form of gal-3 was detected by a polyclonal antibody that recognized both the intact and cleaved gal-3. What is more, as anti-gal-3N showed very weak staining while anti-gal-3C displayed intense staining in advanced tumor tissues, we speculate the gal-3 detected in advanced prostate cancer tissues is mainly the cleaved form of gal-3, which can be detected by our novel anti-gal-3C monoclonal antibody. Our previous results showed that the location of gal-3 was in the cytoplasm in advanced prostate cancer, suggesting the cleaved form of gal-3 was mostly localized in cytoplasm. Taken together, our findings showed that gal-3 cleavage may occur in prostate cancer tissues but not normal prostate tissues. Although the intact gal-3 decreased, the cleaved gal-3 increased with prostate cancer progression. The form of gal-3 existed in advanced tumor tissue was mainly the cleaved form of gal-3, which can be detected by a C-terminal region monoclonal antibody.
Gal-3 is known to be involved in the progression and metastasis of various cancers, including prostate adenocarcinoma. An evaluation of gal-3 expression in tissue microarrays prepared from prostate cancer patients demonstrated that the decrease of gal-3 staining intensity correlated with biochemical recurrence [21]. However, the clinical significance of gal-3 still remains elusive. In the present study, the relationship between the expression of gal-3 (detected by anti-gal-3C antibody) and the clinical and pathological features of prostate cancer was investigated. We found that the percentage of gal-3-positive expression in cases with gleason score 8-9 was significantly higher than cases with gleason score 6-7, indicating increased expression of gal-3 is associated with malignance of prostate cancer. Besides, a more intense staining of anti-gal-3C in cases of TNM stage III-IV was observed when compared to that of TNM stage I-II, suggesting the increased expression of gal-3 is associated with prostate cancer progression. Approximately 90% of patients with advanced prostate cancer develop osseous metastases, which are difficult to eradicate [22]. Thus, the strong expression of gal-3 examined by anti-gal-3C in advanced-stage prostate cancer might be closely correlated with tumor metastasis. These findings are consistent with previous studies [11,23-26], which demonstrated that increased expression of gal-3 is associated with high metastatic potential. On the basis of the results above, we could conclude that the increased expression of gal-3 is mostly the cleaved form of gal-3 since the intact gal-3 is down-regulated in prostate cancer with progressive stages. Thereby, the significant correlation between gal-3 expression and pathological features is mainly concerned with the cleaved gal-3 but not the intact gal-3. Collectively, our results suggested the cleaved gal-3, but not the intact gal-3, promotes prostate cancer progression and is implicated with malignance, tumor progression and metastasis of prostate cancer.
It is important to note that the expression of gal-3 determined by anti-gal-3C antibody was significantly higher in case with PSA≥20 ng/ml compared to cases with PSA<20 ng/ml. This result was in accordance with the previous finding that gal-3 level was positively associated with PSA level in prostate cancer patients [27]. Recently, it was shown that gal-3 can be cleaved by PSA after Tyr107 [16]. PSA is a well-known prostate cancer biomarker, rising in serum of prostate cancer patients. In this context, we propose that the decrease of intact gal-3 in advanced prostate cancer might be attributed to PSA-mediated degradation of intact gal-3, which resulting in an increase of the cleaved form of gal-3. Our hypothesis was supported by Saraswati S’ investigation [16]. Saraswati S et al demonstrated that PSA cleaves gal-3 after Tyr107, and this cleavage destroys gal-3 multivalency while preserving its carbohydrate binding activity [16]. That might be the reason why the cleaved form of gal-3 can only be detected by C-terminal region antibody but not N-terminal region antibody. However, PSA is not the only protease that cleaves gal-3, and other proteases (such as MMP2, MMP9) are known to cleave intact gal-3 and leave the C-terminal region of gal-3 [17]. Therefore, further study is needed to indentify which protease is responsible for gal-3 cleavage in prostate cancer.
In conclusion, this is the first study to detect the cleaved gal-3 with a novel C-terminal region monoclonal antibody in prostate cancer and correlated it to pathological parameters. We are the first to report that the level of cleaved gal-3 in the prostate, was positively associated with malignancy and tumor progression. Intact gal-3 is not an ideal therapeutic target because it is cleaved in advanced tumor, while the cleaved gal-3 is high expressed in prostate cancer especially advanced cancer. Thus, this study provides evidence that the cleaved gal-3 has the potential to be a clinical diagnostic biomarkers and therapeutic target for the treatment of prostate cancer, especially advanced prostate cancer.
Acknowledgements
The authors wish to thank Dr. Lanqing Huang for his contribution in the production of the antibodies. This research was supported by Guangxi Natural Science foundation (project number 2015GXNSFBA139161 & 2014GXNSFBA118201) and National Natural Science Foundation of China (project number 81472414 & 81560152).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Maia MC, Hansen AR. A comprehensive review of immunotherapies in prostate cancer. Crit Rev Oncol Hematol. 2017;113:292–303. doi: 10.1016/j.critrevonc.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Balan V, Gao X, Reddy PG, Kho D, Tait L, Raz A. The significance of galectin-3 as a new basal cell marker in prostate cancer. Cell Death Dis. 2013;4:e753. doi: 10.1038/cddis.2013.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao X, Liu J, Liu X, Li L, Zheng J. Cleavage and phosphorylation: important post-translational modifications of galectin-3. Cancer Metastasis Rev. 2017;36:367–374. doi: 10.1007/s10555-017-9666-0. [DOI] [PubMed] [Google Scholar]
- 5.Nakajima K, Kho DH, Yanagawa T, Harazono Y, Gao X, Hogan V, Raz A. Galectin-3 inhibits osteoblast differentiation through notch signaling. Neoplasia. 2014;16:939–949. doi: 10.1016/j.neo.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balan V, Nangia-Makker P, Kho DH, Wang Y, Raz A. Tyrosine-phosphorylated galectin-3 protein is resistant to prostate-specific antigen (PSA) cleavage. J Biol Chem. 2012;287:5192–5198. doi: 10.1074/jbc.C111.331686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760:616–635. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Nangia-Makker P, Wang Y, Raz T, Tait L, Balan V, Hogan V, Raz A. Cleavage of galectin-3 by matrix metalloproteases induces angiogenesis in breast cancer. Int J Cancer. 2010;127:2530–2541. doi: 10.1002/ijc.25254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nangia-Makker P, Raz T, Tait L, Hogan V, Fridman R, Raz A. Galectin-3 cleavage: a novel surrogate marker for matrix metalloproteinase activity in growing breast cancers. Cancer Res. 2007;67:11760–11768. doi: 10.1158/0008-5472.CAN-07-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Nangia-Makker P, Tait L, Balan V, Hogan V, Pienta KJ, Raz A. Regulation of prostate cancer progression by Galectin-3. Am J Pathol. 2009;174:1515–1523. doi: 10.2353/ajpath.2009.080816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakajima K, Kho DH, Yanagawa T, Harazono Y, Hogan V, Chen W, Ali-Fehmi R, Mehra R, Raz A. Galectin-3 cleavage alters bone remodeling: different outcomes in breast and prostate cancer skeletal metastasis. Cancer Res. 2016;76:1391–1402. doi: 10.1158/0008-5472.CAN-15-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellerhorst JA, Stephens LC, Nguyen T, Xu XC. Effects of galectin-3 expression on growth and tumorigenicity of the prostate cancer cell line LNCaP. Prostate. 2002;50:64–70. doi: 10.1002/pros.10033. [DOI] [PubMed] [Google Scholar]
- 13.Fukumori T, Kanayama HO, Raz A. The role of galectin-3 in cancer drug resistance. Drug Resist Updat. 2007;10:101–108. doi: 10.1016/j.drup.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Balan V, Kho D, Hogan V, Nangia-Makker P, Raz A. Galectin-3 regulates p21 stability in human prostate cancer cells. Oncogene. 2013;32:5058–5065. doi: 10.1038/onc.2012.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pienta KJ, Naik H, Akhtar A, Yamazaki K, Replogle TS, Lehr J, Donat TL, Tait L, Hogan V, Raz A. Inhibition of spontaneous metastasis in a rat prostate cancer model by oral administration of modified citrus pectin. J Natl Cancer Inst. 1995;87:348–353. doi: 10.1093/jnci/87.5.348. [DOI] [PubMed] [Google Scholar]
- 16.Saraswati S, Block AS, Davidson MK, Rank RG, Mahadevan M, Diekman AB. Galectin-3 is a substrate for prostate specific antigen (PSA) in human seminal plasma. Prostate. 2011;71:197–208. doi: 10.1002/pros.21236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochieng J, Fridman R, Nangia-Makker P, Kleiner DE, Liotta LA, Stetler-Stevenson WG, Raz A. Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry. 1994;33:14109–14114. doi: 10.1021/bi00251a020. [DOI] [PubMed] [Google Scholar]
- 18.Knapp JS, Lokeshwar SD, Vogel U, Hennenlotter J, Schwentner C, Kramer MW, Stenzl A, Merseburger AS. Galectin-3 expression in prostate cancer and benign prostate tissues: correlation with biochemical recurrence. World J Urol. 2013;31:351–358. doi: 10.1007/s00345-012-0925-y. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed H, Cappello F, Rodolico V, Vasta GR. Evidence of heavy methylation in the galectin 3 promoter in early stages of prostate adenocarcinoma: development and validation of a methylated marker for early diagnosis of prostate cancer. Transl Oncol. 2009;2:146–156. doi: 10.1593/tlo.09118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merseburger AS, Kramer MW, Hennenlotter J, Simon P, Knapp J, Hartmann JT, Stenzl A, Serth J, Kuczyk MA. Involvement of decreased galectin-3 expression in the pathogenesis and progression of prostate cancer. Prostate. 2008;68:72–77. doi: 10.1002/pros.20688. [DOI] [PubMed] [Google Scholar]
- 21.Laderach DJ, Gentilini L, Jaworski FM, Compagno D. Galectins as new prognostic markers and potential therapeutic targets for advanced prostate cancers. Prostate Cancer. 2013;2013:519436. doi: 10.1155/2013/519436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed H. Promoter methylation in prostate cancer and its application for the early detection of prostate cancer using serum and urine samples. Biomark Cancer. 2010;2010:17–33. doi: 10.4137/BIC.S3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bresalier RS, Yan PS, Byrd JC, Lotan R, Raz A. Expression of the endogenous galactose-binding protein galectin-3 correlates with the malignant potential of tumors in the central nervous system. Cancer. 1997;80:776–787. [PubMed] [Google Scholar]
- 24.Xu XC, el-Naggar AK, Lotan R. Differential expression of galectin-1 and galectin-3 in thyroid tumors. Potential diagnostic implications. Am J Pathol. 1995;147:815–822. [PMC free article] [PubMed] [Google Scholar]
- 25.Schoeppner HL, Raz A, Ho SB, Bresalier RS. Expression of an endogenous galactose-binding lectin correlates with neoplastic progression in the colon. Cancer. 1995;75:2818–2826. doi: 10.1002/1097-0142(19950615)75:12<2818::aid-cncr2820751206>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez PL, Merino MJ, Gomez M, Campo E, Medina T, Castronovo V, Sanjuan X, Cardesa A, Liu FT, Sobel ME. Galectin-3 and laminin expression in neoplastic and non-neoplastic thyroid tissue. J Pathol. 1997;181:80–86. doi: 10.1002/(SICI)1096-9896(199701)181:1<80::AID-PATH699>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 27.Nakajima K, Heilbrun LK, Hogan V, Smith D, Heath E, Raz A. Positive associations between galectin-3 and PSA levels in prostate cancer patients: a prospective clinical study-I. Oncotarget. 2016;7:82266–82272. doi: 10.18632/oncotarget.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]


