Abstract
Background/purpose: Mast cells (MCs) play a critical role in the pathogenesis of allergic reactions and inflammatory conditions through the release of inflammatory mediators. T cell immunoglobulin mucin domain (TIM-1) has been reported to express in MCs. The aim of the present study was to examine the expression and analyze the quantification of TIM-1 on tryptase-positive MCs in different stages of human chronic periodontitis using double-immunofluorescence staining. Materials and methods: Individuals who participated in this study were divided into three groups: healthy control gingivae (n = 27), chronic slight periodontitis (n = 34), and chronic severe periodontitis (n = 31). Their gingival specimens were taken and fixed in 10% buffered formalin, stained with hematoxylin and eosin (HE) for histopathology, and stained with double-immunofluorescence (DIF) for identification of tryptase-TIM-1 double-positive MCs in gingival tissues. Results: Compared with healthy controls, the densities (cells/mm2) of tryptase-TIM-1 double-positive MCs were significantly increased in both the chronic slight periodontitis (P < 0.05) and severe periodontitis groups (P < 0.01). However, compared with the chronic slight periodontitis group, both the score of gingival tissue inflammation and the density of tryptase-TIM-1 double-positive MCs in gingival tissue were significantly increased in the severe periodontitis groups (P < 0.05). Conclusion: By incorporating HE with double-immunofluorescence staining in human chronic periodontitis, the significantly increased number of tryptase-TIM-1 double-positive MCs had the similar tendency as the severity of periodontitis inflammation. Based on our results, we suggest that tryptase-TIM-1 double-positive MCs may play an important role in human chronic periodontitis.
Keywords: T-cell immunity, osteoprotegerin ligand, alveolar bone destruction, periodontitis
Introduction
Periodontitis is a complex chronic inflammatory disease that results from interactions between specific subgingival biofilm and the susceptible hosts, defined by loss of periodontal ligament and alveolar bone, and eventually leads to loss of teeth [1]. A subset of endogenous gram-negative periodontal bacteria, including Tannerella forsythia (Tf), Porphyromonas gingivalis (Pg) and Treponema denticola (Td) have been widely considered as major periodontal pathogens [2-4]. These microbes possess numerous potent inflammatory mediators such as lipopolysaccharides, antigens, and other virulence factors aimed at neutralizing local host defenses and destroying periodontal tissues, finally leading to alveolar bone resorption and tooth loss [5,6]. Numerous cytokines have been implicated as mediators of bone resorption in the context of periodontitis, where inflammation-induced bone destruction is a major manifestation [7,8]. However, studies indicate that both Th1 [interferon-gamma (IFN-γ) and tumor necrosis factor (TNF)] and Th2 [interleukin-4 (IL-4), IL-6, IL-10] cytokines play an important role in maintaining alveolar bone homeostasis in chronic periodontitis [9].
MCs are essential tissue resident immune cells which participate in the early recognition of pathogens, are found in most tissues of the body, particularly abundant in locations that are in close contact with the external environment, such as skin, airways, and intestines [10]. MCs are involved in numerous activities ranging from control of the vasculature to tissue injury and repair, allergic inflammation, and host defense [11]. In humans, MCs are classified according to their protease content, with the MCT subclass expressing tryptases only and the MCTC subclass expressing all types of MC proteases, that is, tryptases, chymases, and carboxypeptidase A (MC-CPA) [12]. MCs derived tryptase is a potent chemoattractant for neutrophils, stimulates epithelial cell proliferation, secretion of IL-8 and increases epithelial expression of intercellular adhesion molecule-1 [13]. Within the stimulation from activation of T cells, MCs can undergo degranulation results in rapidly releasing pre-formed mediators present within cytoplasmic granules, including histamine, the proteases tryptase and chymase, and pre-formed tumor necrosis factor-α [10]. Recently, several studies have focused on the role of the immune system in the evolution of periodontal disease, indicating that bacterial antigens trigger an immunopathology reaction and that the innate susceptibility of the patient determines the ultimate outcome of periodontal disease process [14]. Among the inflammation cells found in periodontal tissues, different numbers of MCs have been detected in both healthy and inflamed gingiva, and mostly tryptase positive MCs [11,15]. It has been reported, in inflamed and in healing gingiva, numbers of MCs have been found to be increased, and this indicated that MCs may as key players in gingival homeostasis that may be important in the progression of periodontitis [13].
The T cell/transmembrane, immunoglobulin, and mucin domain protein (Tim) gene family is a relatively newly described group of molecules with a conserved structure [16]. Evidences have indicated that this gene family plays a critical role in regulating immune responses, including allergy, asthma, transplant tolerance, autoimmunity, and the response to viral infections [16,17]. TIM-1, the first family member, was initially identified as the receptor for the hepatitis A virus cellular receptor 1 (HAVCR1) [18], and subsequently identified as a kidney injury molecule (KIM-1) [19]. TIM-1, an important susceptibility gene for asthma and allergy, is preferentially expressed on Th2 cells and functions as a potent costimulatory molecule for T-cell activation [20]. TIM-1 also expressed on cells of the innate immune system, such as MCs, macrophages, and dendritic cells (DCs), but its role in responses by these cell types has not been firmly established [21]. Although ours and others previous studies have shown that MCs play an important role in human periodontitis [11,15], so far limited attention has been given to the regulation of MCs in periodontal diseases. It has been reported that TIM-1 can influence MCs function [22]. However, TIM-1 expression on MCs in human periodontitis has not been reported. Therefore, this study was designed to quantify the tryptase-TIM-1 double-positive MCs in human chronic periodontitis and analyze their possible role in periodontitis progression by using DIF.
Materials and methods
Periodontal tissues
The gingival biopsies were obtained from the patients attending the Department of Periodontology, Liwan Stomatological Teaching Hospital of Jinan University from July 2010 to September 2013. Total 92 gingival tissue specimens were obtained from age 25 to 65, including 47 males (age 46 ± 13.4) and 45 females (age 46 ± 14.2) without diabetes and other system diseases. Periodontitis were diagnosed, according to the 1999 American Academy of Periodontology classification, by measuring probing pocket depth (PD), clinical attachment loss (AL) and bleeding on probing (BOP), and by radiography examination. There were 34 patients with chronic slight periodontitis [gingival inflammation, BOP (+), PD of 3-4 mm, AL of ≤ 3 mm and alveolar bone absorption < one third of root]; 31 patients with chronic severe periodontitis (PD of > 6 mm, AL of < 5 mm, and alveolar bone absorption > two thirds of root); and 27 clinically periodontal healthy subjects [gingival healthy, PD of ≤ 3 mm, BOP (-), and no radiographic evidence of alveolar bone loss]. All subjects in slight and severe periodontitis groups had been ruled out the active stage of chronic periodontitis. The clinically healthy specimens were harvested from teeth extracted for orthodontic reasons, general premolars. Specimens of slight periodontitis were obtained from patients undergoing clinical crown lengthening surgery, and the specimens of severe periodontitis were obtained from tooth extractions that had severe clinical attachment loss and alveolar bone destruction.
Histological staining and grading
The specimens were fixed in 10% neutral-buffered formaldehyde solution for a minimum of 48 h before being embedded in paraffin. Sections (four-micrometer thick) were cut, mounted on glass slides, dewaxed, rehydrated, and stained using standard methods with haematoxylin and eosin (HE) (Sigma-Aldrich), and evaluated for inflammatory pathology. The grade of pathology inflammatory severity of periodontal tissues was semi-quantitatively assessed on a scale assigned scores from 0 to 3: 0 = no inflammation; 1 = slight inflammation; 2 = moderate inflammation; and 3 = severe inflammation [23]. The grade of inflammation of 10 specimens was measured blindly by two pathologists and the average grade of inflammation was calculated. The sections were visualized and photographed by a Leica DM2500B microscope (Wetzlar, Germany).
Toluidine-blue (TB) staining
Toluidine blue staining was used in this study to identify MCs in periodontal tissues. 4-μm-thick sections were dewaxed, rehydrated, and stained with 0.5% toluidine blue (Sigma-Aldrich) for 2 h. MCs were identified by deep bluepurple staining.
Immunohistochemistry (IHC) staining
Immunohistochemistry was carried out using the streptavidin-biotin-peroxidase complex (SABC) method. Sections (4-μm) were dewaxed and rehydrated in distilled water. Heat-induced antigen retrieval was carried out in an 800-W microwave oven for 30 min. Endogenous peroxidase activity was blocked by incubation with 0.3% hydrogen peroxide in methanol for 10 min at 37°C. Non-specific binding as blocked by incubation in PBS containing 10% normal goat serum and 1% bovine serum albumin (BSA) (pH 7.4) for 15 min at room temperature. Sections were incubated with anti-MC tryptase mouse monoclonal antibody (AA1, IgG1; 1 mg/ml, 1:200 dilution; Abcam, UK) overnight at 4°C. Sections incubated with secondary antibodies only were used as isotype controls. IHC was then detected with a SABC kit and developed with diaminobenzidine tetrahydrochloride (Zhongshan Goldenbridge Technology, Beijing, China). The sections were counterstained with hematoxylin followed by light microscopy. Tryptase-positive MCs were identified by darkbrown staining.
Double-immunofluorescence (DIF) staining
Periodontal tissue sections (4-μm) were dewaxed and rehydrated in distilled water. Heat-induced antigen retrieval was carried out in an 800-W microwave oven for 30 min. Endogenous peroxidase activity was blocked by incubation with 0.3% hydrogen peroxide in methanol for 10 min at 37°C. Non-specific binding as blocked by incubation in PBS containing 10% normal goat serum and 1% bovine serum albumin (BSA) (pH 7.4) for 60 min at room temperature. Sections were incubated with anti-MC tryptase mouse monoclonal antibody (AA1, IgG1; 1 mg/ml, 1:200 dilution; Abcam, UK) and anti-TIM-1 rabbit polyclonal antibody (200 μg/ml; 1:100 dilution; Boster, China) together overnight at 4°C. Slides were then rinsed three times with PBS (pH 7.4) and exposed to secondary antibodies, e.g., anti-mouse IgG (H+L), F(ab’)2 fragment (Alexa Fluor®488 Conjugate) (2 mg/ml, 1:200 dilution; Cell Signalling Technology, USA) and anti-rabbit IgG (H+L), F(ab’)2 fragment (Alexa Fluor®555) (2 mg/ml, 1:200 dilution; Cell Signalling Technology, USA) together for 60 min at room temperature in a dark chamber. The slides were washed three times with PBS (pH 7.4) for 30 min at room temperature and mounted by antifade polyvinylpyrrolidone mounting medium (Beyotime, China) in a dark chamber. Tryptase-positive MCs were identified by green fluorescent, while TIM-1-positive appear red fluorescent which were located in the nucleus or cytoplasm, once tryptase and TIM-1 superimposed in one vision that would appear yellow fluorescent. Two pathologists observed the sections by immunofluorescence microscopy, counted out tryptase and TIM-1 double-positive MCs, and measured the area of sections, got the number of cells in the each square millimetre (cells/mm2), and recorded the average value.
Statistical analysis
All of the periodontal measurements were done in a blind fashion on coded specimens, and the quantitative measurements were made twice. Statistical analysis of the data was performed by using SPSS software for windows (version17.0, SPSS Inc., Chicago, IL, USA). Differences in periodontal histopathological examination data between different groups were investigated using the Kruskal-Wallis H. The densities of tryptase-TIM-1 double-positive MCs were analyzed by Nemenyi test. A P value of < 0.05 was considered statistically significant.
Results
Histopathological analysis
The specimens of periodontal tissues from different groups were examined histologically. As shown in Figure 1A, no obvious inflammatory infiltrate was found in healthy gingival tissues. The specimens of slight periodontitis group showed a slight inflammatory cell infiltration (Figure 1B), while the severe periodontitis group presented an intense inflammatory cell infiltration (Figure 1C). Semiquantitative analysis of the severity of inflammation of the specimens from different groups was performed. As shown in Figure 2, compared with healthy controls, the histological scores of periodontal tissues from both slight periodontitis (P < 0.05) and severe periodontitis (P < 0.01) groups were significantly higher. In addition, the histological score of periodontal tissues from severe periodontitis group was significantly higher than that from slight periodontitis group (P < 0.05).
Figure 1.
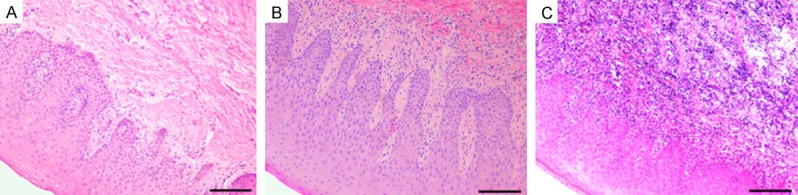
Histology of human gingival tissues. A: Healthy control group, no obvious inflammatory infiltrate was found; B: Chronic slight periodontitis group, scattered inflammatory cells infiltration; C: Chronic severe periodontitis group, an intense inflammatory cell infiltration. HE staining, original magnification × 200, scale bars = 100 μm.
Figure 2.
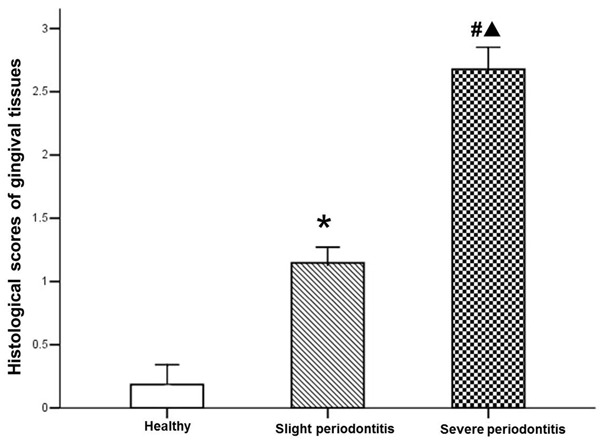
Histological score analysis of per group. Data are represented as mean ± SEM. Significant differences between groups are analyzed by Kruskal-Wallis H and Nemenyi test. *P < 0.05, #P < 0.01 compared to healthy control; ▲P < 0.05 compared to chronic slight periodontitis.
MCs stained with TB and tryptase-positive MCs stained with IHC
Stained with toluidine blue, MCs were identified in tissue sections from their characteristic granular, deep blue-purple metachromatic appearance against blue orthochromatic background tissue. To further confirm the presence of tryptase-positive MCs expression on gingival tissue, all groups were stained by IHC and tryptase-positive MCs were identified by dark-brown staining. As shown in Figure 3, the distribution of MCs by TB staining was quite similar to tryptase-positive MCs by IHC staining, and the coloration and degranulation by IHC staining were much more obvious (P < 0.05). Both only a few positively stained MCs and tryptase-positive MCs with undegranulation were observed in the healthy gingival tissues (Figure 3A), whereas more MCs and tryptase-positive MCs with degranulation were observed in the slight periodontitis group (Figure 3B) and even more MCs and tryptase-positive MCs with strong degranulation were observed in the severe periodontitis group (Figure 3C). As shown in Figure 4, compared with healthy controls, the histological scores of periodontal tissues from both slight periodontitis (P < 0.05) and severe periodontitis (P < 0.01) groups were significantly higher. In addition, the histological score of periodontal tissues from severe periodontitis group was significantly higher than that from slight periodontitis group (P < 0.05).
Figure 3.
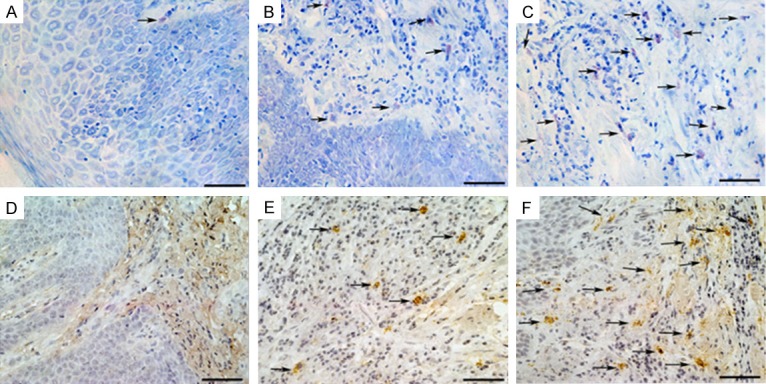
Toluidine Blue staining (A-C) and Immunohistochemistry staining (D-F). (a and d) Healthy control group, MCs with almost without degranulation; (b and e) Chronic slight periodontitis group, scattered MCs infiltration with mild degranulation; (c and f) Chronic severe periodontitis group, an intense MCs infiltration with most disruption and obvious degranulation. TB and IHC staining, original magnification × 400, scale bars = 50 μm.
Figure 4.
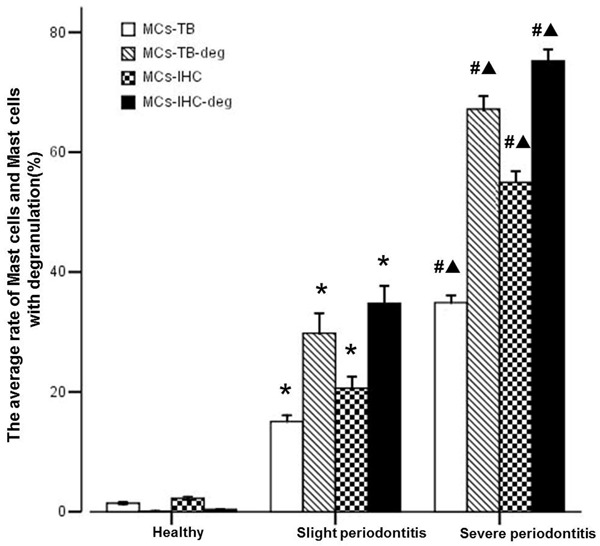
Analyze of the average rate of MCs and MCs with degranulation of per group. Data are represented as mean ± SEM. Significant differences between groups are analyzed by Tamhane’s T2 test. *P < 0.05, #P < 0.01 compared to healthy control; ▲P < 0.05 compared to chronic slight periodontitis.
Tryptase-TIM-1 double-positive MCs stained with DIF
As shown in Figures 5, 6 and 7 tryptase-positive MCs (green fluorescent), TIM-1-positive (red fluorescent), the nucleus (bule fluorescent), and tryptase-TIM-1 double-positive MCs (yellow fluorescent) were identified by DIF staining. A few tryptase-positive MCs (Figure 5A) and tryptase-TIM-1 double-positive MCs (Figure 5C) with membrane integrity and did not see obvious degranulation were observed in healthy gingival tissues (Figure 6); while more tryptase-positive MCs (Figure 5D) and tryptase-TIM-1 double-positive MCs (Figure 5F) were observed in slight periodontitis gingival tissues, and even more tryptase-positive MCs (Figure 5G) and tryptase-TIM-1 double-positive MCs (Figure 5I) with strong degranulation were observed in severe periodontitis gingival tissues (Figure 7). To further confirm the observations, semi-quantitative analysis of tryptase-TIM-1 double-positive MCs in periodontal tissue sections from the three different groups were performed (Figure 8). Compared with healthy control group, there were significantly higher densities (the number of MCs/mm2) of MCs in slight periodontitis group (P < 0.05) and even significantly higher densities of MCs in severe periodontitis group (P < 0.01). Furthermore, the MCs density of severe periodontitis group was significantly higher than that of slight periodontitis group (P < 0.05). Negative controls, in which the primary antibody was omitted, did not show tissue staining (data not shown).
Figure 5.
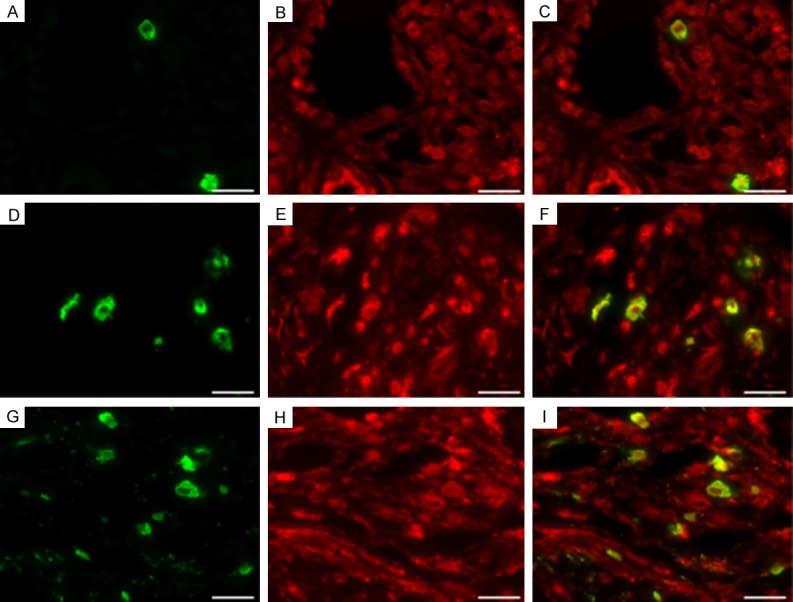
Representative photomicrographs of MC infiltration with tryptase-TIM-1 immunofluorescence double-staining. A few tryptase-positive MCs (A) and tryptase-TIM-1 double-positive MCs (C) in healthy gingival tissues with integrated membrane and unapparent degranulation, more tryptase-positive MCs (D) and tryptase-TIM-1 double-positive MCs (F) in chronic slight periodontitis gingival tissues with a portion of membranolysis and degranulation, and even more tryptase-positive MCs (G) and tryptase-TIM-1 double-positive MCs (I) with strong degranulation in chronic severe periodontitis gingival tissues. (B, E and H) are tryptase-TIM-1 immunofluorescence double-staining. DIF staining, original magnification × 1000, scale bars = 20 μm.
Figure 6.
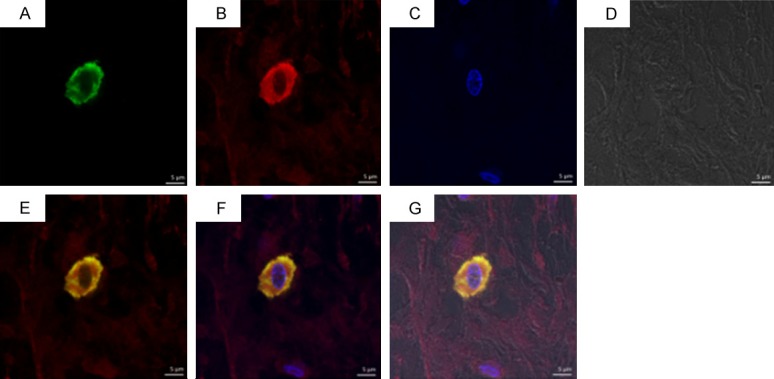
Double immunofluorescence staining of tryptase-TIM-1 in healthy control group with integrated membrane and unapparent degranulation. A: Tryptase+; B: TIM-1+; C: Nucleus; D: Light observation; E: A+B; F: A+B+C; G: A+B+C+D+E. Laser scanning confocal microscope, DIF staining, original magnification × 1260, scale bars = 5 μm.
Figure 7.
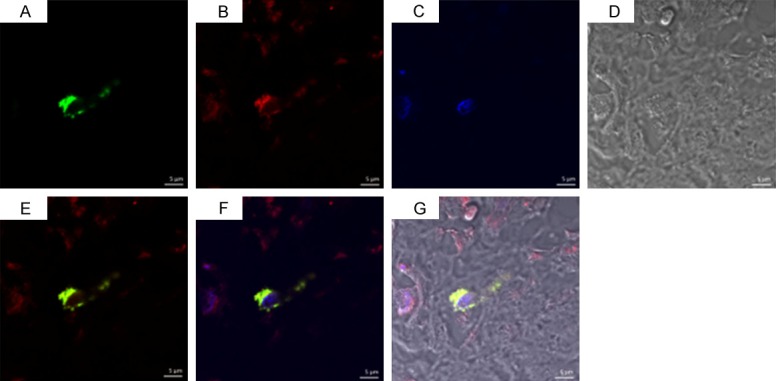
Double immunofluorescence staining of tryptase-TIM-1 in chronic severe periodontitis group with strong degranulation. A: Tryptase+; B: TIM-1+; C: Nucleus; D: Light observation; E: A+B; F: A+B+C; G: A+B+C+D. Laser scanning confocal microscope, DIF staining, original magnification × 1260, scale bars = 5 μm.
Figure 8.
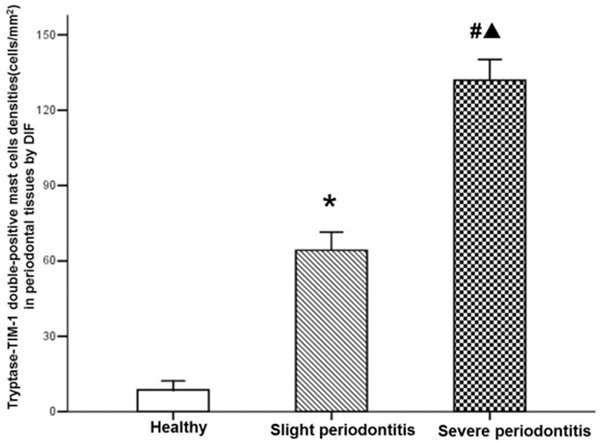
Analyze of the densities of tryptase-TIM-1 double-positive MCs in gingival tissues of per group. Data are represented as mean ± SEM. Significant differences between groups are analyzed by Tamhane’s T2 test. *P < 0.05, #P < 0.01 compared to healthy control; ▲P < 0.05 compared to chronic slight periodontitis.
Discussion
There is no doubt that plaque bacteria are necessary to initiate disease and drive the chronic inflammatory response in the periodontal tissues and the host inflammatory response are responsible for the majority of the hard- and soft-tissue breakdown leading to the clinical signs of periodontitis. T cells, especially Th1/Th2 cells are now believed to be involved in the homeostasis of periodontal tissues, modulation of the inflammatory/immune responses and mediation of the bone loss observed in periodontal disease [24,25]. Th1/Th2 cells paradigm plays an important role in periodontal disease and Th1/Th2 cytokines expression of different proportion in the different period of chronic periodontitis [26]. Some scholars proposed that a Th2 cytokine profile was associated with a stable lesion, while the progressive lesion was characterized by the Th1 profile [27]. In recent years, various mechanisms such as the nature of the antigen, DCs profile, the cytokine milieu in the tissues have all been shown to regulate the Th1/Th2 response in periodontal disease [28]. Research shows that Th1 cytokines is associated with the early process of chronic periodontitis while Th2 cytokines is associated with the late development lesions [24]. At the early process of chronic periodontitis, it has been shown that a strong innate response leads to a Th1 response under the influence of IL-12, IL-2 and IFN-γ, and IFN-γ enhances the phagocytic activity of both neutrophils and macrophages and hence contains the infection; as the lesion goes on, under the influence of IL-4 cytokines, minimal IL-12 production, MCs stimulation and the subsequent production of IL-4 would encourage a Th2 response, B-cell activation and antibody production, if these antibodies are protective and clear infection, the disease will not progress but if they are not protective, as in case of IgG2, the lesion will persist, continued B-cell activation may result in large amounts of IL-1 and hence tissue destruction [24,26,27,29].
MCs synthesize and secrete a wide variety of mediators, modulating the functions of nearby cells, and initiate complex physiological changes. The significant contribution of MCs mediator to tissue damage and the propagation of inflammatory responses make control of MCs function vital to the management of many inflammatory diseases [30]. Studies suggest MCs can secrete both Th2-type and Th1-type cytokines, influencing the differentiation of T cells, which indicate that MCs are involved in immune regulation [11,31]. Besides, MCs release vascular active amine and preformed tumor necrosis factor-α both increase vascular permeability, improve the expression of intercellular adhesion molecule-1 (ICAM-1) and P-selectin in endothelial cell, the subsequent sticking and migration of polymorphonuclear leukocytes (PMNs) into the gingival tissues and release the lysosomal enzyme; as a inflammatory response, matrix metalloproteinases (MMPs) locally rise, MMPs and lysosomal enzyme destroy the extracellular matrix of gingival tissue, resulting the invasion of lymphocytes and macrophages [26]. Key mediators that are pre-formed in MCs are the serine proteases tryptase, chymase, and cathepsin G, histamine, heparin, serotonin, acid hydrolases, and the cytokines TNF-α and IL-16; following activation, MCs can synthesize a range of mediators, including the interleukins IL-1, IL-3, IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, and IL-16, together with platelet-activating factor (PAF) and prostaglandin (PG), resulting the destruction of the periodontal tissue and the absorption of alveolar bone [32].
Tim gene family is a relatively newly described group of molecules which consists of Type I transmembrane proteins with a unique structure and is associated with or implicated in several important immunological processes, including the regulation of allergy and asthma, and also the regulation of autoimmunity and transplantation immunity [16,33,34]. The members of Tim gene family including TIM-1, TIM-3 and TIM-4 are conserved both in mice and human [35]. TIM-1 and TIM-3 are differentially expressed by effector Th2 and Th1 cells; TIM-1 enhances Th2 cell activation and TIM-3 down-regulates Th1 cell function [22,36-38]. TIM-1 serves as a costimulatory molecule for T cells, particularly on Th2 cells, enhancing T cell proliferation and cytokine production [16]. Studies have demonstrated TIM-1 expressed on MCs, macrophages and DCs; and recombinant mouse TIM-4 (rmTIM-4), a ligand for TIM-1, can affect IL-4, IL-10, IL-17 and IFN-g production, the TIM-1-TIM-4 interaction is important in regulating the proliferation of T cells [37,39]. T cells require signals for optimal activation, the first provided by T cell receptor (TCR) triggering; TIM-1 provides a strong costimulatory signal for T cell activation and cytokine production that augments TCR signaling [37,40]. Moreover, epidemiological studies have shown that the prevalence of allergy and asthma was significantly lower in HAV seropositive individuals compared with HAV seronegative individuals, suggesting that HAV may directly affect Th2 mediated immune system through TIM-1 [20]. Treatment of the mice with anti-TIM-1 during the initial exposure to ovalbumin (OVA) prevented tolerance induction, and OVA challenge induced severe allergen-induced airway-hyper-reactivity (AHR) that was accompanied by considerable airway inflammation [37]. Thus, TIM-1 costimulation prevents of peripheral T cell tolerance and in the development of AHR and airway inflammation after antigen challenge. Anti-TIM-1 mAbs recognizing distinct epitopes differentially modulated inflammation, the recognizing mapped revealing that mAbs to the IgV domains of TIM-1 has therapeutic activity when exon 4 of the mucin/stalk domains exacerbated immune responses [41]. After IgE (+) Ag stimulation TIM-1 expression was down-regulated on bone marrow derived cultured MCs (BMCMCs), using rmTIM-4 can promote Th2 cytokines production without enhancing degranulation in BMCMCs stimulated with IgE (+) Ag, suggesting that TIM-1 may be able to influence T-cell-mediated immune responses in part through effects on MCs [22]. Anyway, the role of TIM-1 in human is not fully understood and only a limited amount of data is available, the underlying mechanisms have not been well-explored.
In the present study, we focused on the TIM-1 expressions on MCs in gingival tissues of human chronic periodontitis with different severities. Our results showed that, the density of tryptase-TIM-1 double-positive MCs and degranulation of MCs in the chronic severe periodontitis group was significantly higher than that in the chronic slight periodontitis group; most importantly, the increase of the number of tryptase-TIM-1 double-positive MCs and degranulation of MCs had the similar tendency as the severity of periodontitis inflammation, suggesting that tryptase-TIM-1 double-positive MCs may secrete various of biological medium and cytokines participate in the immune-regulation (including periodontal tissue destruction and damage repair) of chronic periodontitis. To the best of our knowledge, it is the first time to provide evidence for the presence of TIM-1 on MCs in human gingival tissues with chronic periodontitis by DIF staining. Although little information is currently available regarding the role of tryptase-TIM-1 double-positive MCs immune function in human periodontal disease, TIM-1 which has a double action in inflammation may be an attractive target for clinical intervention, thus, illuminating the immunologic mechanism of TIM-1 on MCs in periodontitis may make it possible for control of this disease.
Acknowledgements
Science and Technology Planning Project of Guangdong Province, China (No.2013B021800043; No.2014A020212212).
Disclosure of conflict of interest
None.
References
- 1.Feng Z, Weinberg A. Role of bacteria in health and disease of periodontal tissues. Periodontol 2000. 2006;40:50–76. doi: 10.1111/j.1600-0757.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- 2.Takeuchi Y, Umeda M, Sakamoto M, Benno Y, Huang Y, Ishikawa I. Treponema socranskii, treponema denticola, and porphyromonas gingivalis are associated with severity of periodontal tissue destruction. J Periodontol. 2001;72:1354–1363. doi: 10.1902/jop.2001.72.10.1354. [DOI] [PubMed] [Google Scholar]
- 3.Kumar PS, Griffen AL, Barton JA, Paster BJ, Moeschberger ML, Leys EJ. New bacterial species associated with chronic periodontitis. J Dent Res. 2003;82:338–344. doi: 10.1177/154405910308200503. [DOI] [PubMed] [Google Scholar]
- 4.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 5.Chapple IL, Milward MR, Ling-Mountford N, Weston P, Carter K, Askey K, Dallal GE, De Spirt S, Sies H, Patel D, Matthews JB. Adjunctive daily supplementation with encapsulated fruit, vegetable and berry juice powder concentrates and clinical periodontal outcomes: a doubleblind RCT. J Clin Periodontol. 2012;39:62–72. doi: 10.1111/j.1600-051X.2011.01793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yakob M, Kari K, Tervahartiala T, Sorsa T, Söder PÖ, Meurman JH, Söder B. Associations of periodontal microorganisms with salivary proteins and MMP-8 in gingival crevicular fluid. J Clin Periodontol. 2012;39:256–263. doi: 10.1111/j.1600-051X.2011.01813.x. [DOI] [PubMed] [Google Scholar]
- 7.McCauley LK, Nohutcu RM. Mediators of periodontal osseous destruction and remodeling: principles and implications for diagnosis and therapy. J Periodontol. 2002;73:1377–1391. doi: 10.1902/jop.2002.73.11.1377. [DOI] [PubMed] [Google Scholar]
- 8.Baker PJ. The role of immune responses in bone loss during periodontal disease. Microbes Infect. 2000;2:1181–1192. doi: 10.1016/s1286-4579(00)01272-7. [DOI] [PubMed] [Google Scholar]
- 9.Alayan J, Ivanovski S, Farah CS. Alveolar bone loss in T helper 1/T helper 2 cytokinedeficient mice. J Periodontal Res. 2007;42:97–103. doi: 10.1111/j.1600-0765.2006.00920.x. [DOI] [PubMed] [Google Scholar]
- 10.Urb M, Sheppard DC. The role of mast cells in the defence against pathogens. PLoS Pathog. 2012;8:e1002619. doi: 10.1371/journal.ppat.1002619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batista A, Rodini C, Lara V. Quantification of mast cells in different stages of human periodontal disease. Oral Dis. 2005;11:249–254. doi: 10.1111/j.1601-0825.2005.01113.x. [DOI] [PubMed] [Google Scholar]
- 12.Pejler G, Rönnberg E, Waern I, Wernersson S. Mast cell proteases: multifaceted regulators of inflammatory disease. Blood. 2010;115:4981–4990. doi: 10.1182/blood-2010-01-257287. [DOI] [PubMed] [Google Scholar]
- 13.Steinsvoll S, Helgeland K, Schenck K. Mast cells--a role in periodontal diseases? J Clin Periodontol. 2004;31:413–419. doi: 10.1111/j.1600-051X.2004.00516.x. [DOI] [PubMed] [Google Scholar]
- 14.Gemmell E, Yamazaki K, Seymour GJ. Destructive periodontitis lesions are determined by the nature of the lymphocytic response. Crit Rev Oral Biol Med. 2002;13:17–34. doi: 10.1177/154411130201300104. [DOI] [PubMed] [Google Scholar]
- 15.Huang S, Lu F, Chen Y, Huang B, Liu M. Mast cell degranulation in human periodontitis. J Periodontol. 2013;84:248–255. doi: 10.1902/jop.2012.120066. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Manzanet R, DeKruyff R, Kuchroo VK, Umetsu DT. The costimulatory role of TIM molecules. Immunol Rev. 2009;229:259–270. doi: 10.1111/j.1600-065X.2009.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su EW, Lin JY, Kane LP. TIM-1 and TIM-3 proteins in immune regulation. Cytokine. 2008;44:9–13. doi: 10.1016/j.cyto.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feigelstock D, Thompson P, Mattoo P, Zhang Y, Kaplan GG. The human homolog of HAVcr-1 codes for a hepatitis a virus cellular receptor. J Virol. 1998;72:6621–6628. doi: 10.1128/jvi.72.8.6621-6628.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ichimura T. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is upregulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 20.Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010;235:172–189. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtiss ML, Gorman JV, Businga TR, Traver G, Singh M, Meyerholz DK, Kline JN, Murphy AJ, Valenzuela DM, Colgan JD, Rothman PB, Cassel SL. Tim-1 regulates Th2 responses in an airway hypersensitivity model. Eur J Immunol. 2012;42:651–661. doi: 10.1002/eji.201141581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakae S, Iikura M, Suto H, Akiba H, Umetsu DT, Dekruyff RH, Saito H, Galli SJ. TIM-1 and TIM-3 enhancement of Th2 cytokine production by mast cells. Blood. 2007;110:2565–2568. doi: 10.1182/blood-2006-11-058800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang S, Lu F, Zhang Z, Yang X, Chen Y. The role of psychologic stress-induced hypoxia-inducible factor-1alpha in rat experimental periodontitis. J Periodontol. 2011;82:934–941. doi: 10.1902/jop.2010.100610. [DOI] [PubMed] [Google Scholar]
- 24.Gemmell E, Yamazaki K, Seymour GJ. The role of T cells in periodontal disease: homeostasis and autoimmunity. Periodontol 2000. 2007;43:14–40. doi: 10.1111/j.1600-0757.2006.00173.x. [DOI] [PubMed] [Google Scholar]
- 25.Teng YT, Nguyen H, Gao XJ, Teng YT, Nguyen H, Gao X, Kong YY, Gorczynski RM, Singh B, Ellen RP, Penninger JM. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J Clin Invest. 2000;106:R59–R67. doi: 10.1172/jci10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohlrich EJ, Cullinan MP, Seymour GJ. The immunopathogenesis of periodontal disease. Aust Dent J. 2009;54:S2–S10. doi: 10.1111/j.1834-7819.2009.01139.x. [DOI] [PubMed] [Google Scholar]
- 27.Arun KV, Talwar A, Kumar TS. T-helper cells in the etiopathogenesis of periodontal disease: a mini review. J Indian Soc Periodontol. 2011;15:4–10. doi: 10.4103/0972-124X.82255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pulendran B, Kumar P, Cutler CW, Mohamadzadeh M, Van Dyke T, Banchereau J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J Immunol. 2001;167:5067–5076. doi: 10.4049/jimmunol.167.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gemmell E, Seymour GJ. Modulation of immune responses to periodontal bacteria. Curr Opin Periodontol. 1994:28–38. [PubMed] [Google Scholar]
- 30.Forsythe P, Defus AD. Inhibition of calpain is a component of nitric oxide-induced downregulation of human mast cell adhesion. J Immunol. 2003;170:287–293. doi: 10.4049/jimmunol.170.1.287. [DOI] [PubMed] [Google Scholar]
- 31.de Pater-Huijsen FL, de Riemer MJ, Reijneke RM, Pompen M, Lutter R, Jansen HM, Out TA. Products from human mast cell line cells enhance the production of interferon-gamma by CD8+ and CD4+ T cells. Immunology. 2002;106:11–19. doi: 10.1046/j.1365-2567.2002.01411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh LJ. Mast cells and oral inflammation. Crit Rev Oral Biol Med. 2003;14:188–198. doi: 10.1177/154411130301400304. [DOI] [PubMed] [Google Scholar]
- 33.McIntire JJ, Umetsu SE, Akbari O, Potter M, Kuchroo VK, Barsh GS, Freeman GJ, Umetsu DT, DeKruyff RH. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat Immunol. 2001;2:1109–1116. doi: 10.1038/ni739. [DOI] [PubMed] [Google Scholar]
- 34.Mariat C, Degauque N, Balasubramanian S, Kenny J, DeKruyff RH, Umetsu DT, Kuchroo V, Zheng XX, Strom TB. Tim-1 signaling substitutes for conventional signal 1 and requires costimulation to induce T cell proliferation. J Immunol. 2009;182:1379–1385. doi: 10.4049/jimmunol.182.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balasubramanian S, Kota SK, Kuchroo VK, Humphreys BD, Strom TB. TIM family proteins promote the lysosomal degradation of the nuclear receptor NUR77. Sci Signal. 2012;5:ra90. doi: 10.1126/scisignal.2003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao S, Najafian N, Reddy J, Albin M, Zhu C, Jensen E, Imitola J, Korn T, Anderson AC, Zhang Z, Gutierrez C, Moll T, Sobel RA, Umetsu DT, Yagita H, Akiba H, Strom T, Sayegh MH, DeKruyff RH, Khoury SJ, Kuchroo VK. Differential engagement of Tim-1 during activation can positively or negatively costimulate T cell expansion and effector function. J Exp Med. 2007;204:1691–1702. doi: 10.1084/jem.20062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Umetsu SE, Lee WL, McIntire JJ, Downey L, Sanjanwala B, Akbari O, Berry GJ, Nagumo H, Freeman GJ, Umetsu DT, DeKruyff RH. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat Immunol. 2005;6:447–454. doi: 10.1038/ni1186. [DOI] [PubMed] [Google Scholar]
- 38.Huang S, Lu F, Li J, Lan T, Huang B, Yin X, Jin H. Quantification of tryptase-TIM-3 doublepositive mast cells in human chronic periodontitis. Arch Oral Biol. 2014;59:654–61. doi: 10.1016/j.archoralbio.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Meyers JH, Chakravarti S, Schlesinger D, Illes Z, Waldner H, Umetsu SE, Kenny J, Zheng XX, Umetsu DT, DeKruyff RH, Strom TB, Kuchroo VK. TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat Immunol. 2005;6:455–464. doi: 10.1038/ni1185. [DOI] [PubMed] [Google Scholar]
- 40.de Souza AJ, Oriss TB, O’malley KJ, Ray A, Kane LP. T cell Ig and mucin 1 (TIM-1) is expressed on in vivo-activated T cells and provides a costimulatory signal for T cell activation. Proc Natl Acad Sci U S A. 2005;102:17113–17118. doi: 10.1073/pnas.0508643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sizing ID, Bailly V, McCoon P, Chang W, Rao S, Pablo L, Rennard R, Walsh M, Li Z, Zafari M, Dobles M, Tarilonte L, Miklasz S, Majeau G, Godbout K, Scott ML, Rennert PD. Epitopedependent effect of anti-murine TIM-1 monoclonal antibodies on T cell activity and lung immune responses. J Immunol. 2007;178:2249–2261. doi: 10.4049/jimmunol.178.4.2249. [DOI] [PubMed] [Google Scholar]


