Abstract
Objective: To examine the association of coexistence of adenomyosis and endometrial carcinoma on tumor characteristics and survival outcome of patients. Methods: Clinical and pathological data were retrospectively reviewed from 1584 patients who underwent surgical treatment of endometrial carcinoma. Statistical analyses were performed to evaluate associations of the presence or absence of adenomyosis with demographics, clinical parameters, histopathological factors, and survival outcomes. Results: Adenomyosis was found in 150/1584 patients, and was significantly associated with premenopausal status (46% vs. 35.15%, P = 0.008), younger age (60.67% vs. 41.92% < 55 years old, P < 0.001), lower positive p53 expression (53.36% vs. 63.32%, P = 0.034), earlier disease stage (I-II) (92.67% vs. 85.56%, P = 0.016), lower grade of the tumors (1-2) (91.33% vs. 84.52%, P = 0.025), lower likelihood of outer-half myometrial invasion (10% vs. 22.25%, P < 0.001), and absence of pelvic lymph node metastasis (97.04% vs. 92.09%, P = 0.037). The presence of adenomyosis was also associated with better survival outcomes, with a higher 5-year survival rate (92.1% vs. 84.1%, P = 0.045). In multivariate analysis, age, BMI, stage/grade of tumors, and myometrial invasion were independent prognostic factors associated with survival outcomes. Conclusion: The presence of adenomyosis was associated with less aggressive behavior of endometrial cancer and is a protective factor associated with better outcomes of patients.
Keywords: Endometrial carcinoma, adenomyosis, prognostic factors, survival outcome
Introduction
Endometrial carcinoma is the most common gynecological cancer among women in developed countries. In the USA an estimated 49,560 women were diagnosed with endometrial cancer in 2013 [1]. Some of the risk factors of endometrial carcinoma include obesity, diabetes, and cumulative exposure to estrogen. The majority of patients with endometrial cancer are diagnosed in an early stage, in which the disease is curable with surgical treatment. Adenomyosis is a condition of the uterus, where the endometrial tissue in the uterine myometrial layers also grows outside the endometrial lining [2]. Typical symptoms include dysmenorrhea, chronic pelvic pain, and menorrhagia. It has been reported that adenomyosis coexists with endometrial carcinoma in 16-34% of hysterectomy specimens obtained from the surgical treatment of endometrial carcinoma [3-8]. The role of adenomyosis in pathogenesis and clinical behavior of endometrial carcinoma remains unclear, despite evidence of a frequent association between adenomyosis and endometrial carcinoma.
Studies have been performed to investigate whether the presence of adenomyosis is associated with tumor progression of endometrial cancer, and the results appeared controversial. While there are studies that observed a relationship between adenomyosis and deep myometrial invasion [4,9-11], other studies suggested the presence of adenomyosis may be considered as an enabling factor that allows malignant cells to invade the myometrium by increasing the contact area [5,8,12]. Some of these studies are based on case reports or relatively small sample size cohort studies.
The present study retrospectively reviewed a relatively large cohort and evaluated the association of adenomyosis with tumor and clinical characteristics of patients, as well as the survival outcome of patients in a large-scale comprehensive analysis.
Patients and methods
Patients
Clinical data were retrospectively reviewed from patients who were admitted to the hospital during the period from January 2008 to December 2014. These patients were diagnosed with endometrial carcinoma and no other primary malignancies were found. The patients received surgical treatment. Among the endometrial cancer cohort, adenomyosis was found in 150/1584 cases.
Clinical information
Clinical information was collected, including patient demographics (e.g. age, body mass index), menopausal status, histopathologic characteristics (e.g. histologic subtype, tumor grade, invasion, lymph node metastasis), and the immunohistochemistry findings on protein expression in cancer specimens (e.g. estrogen-receptor, progesterone-receptor, p53, Ki-67, survivin, and CA125). The surgical treatment information and treatment outcome on survival time were also reviewed.
Statistical analysis
Statistical analyses were performed using SPSS 16.0. Survival curves were constructed with the Kaplan-Meier method. Significance of adenomyosis on survival outcome was examined with the log-rank test in univariate analysis. Cox regression was used to identify the independent prognostic factors of survival. Nonparametric test was used for data not following normal distribution. Categorical variables were evaluated with Chi-square test. p values of less than 0.05 were considered statistically significant.
Results
Clinical and pathological data from 1584 patients with endometrial carcinoma were reviewed. Among them, 1434 patients had no adenomyosis and 150 patients had adenomyosis. The incidence rate of adenomyosis co-existence was 9.47%. The endometrial carcinoma patients were divided into two groups: 1) with adenomyosis; and 2) without adenomyosis.
We compared various clinical parameters and demographics between the two groups. There were no statistically significant differences between the two groups in terms of the types of hysterectomy, whether or not adnexectomy was performed, and excision of pelvic lymph node or aortic lymph node (Table S1). Patients with adenomyosis were younger in age (higher percentage of patients with age < 55 years old, P < 0.001) (Table 1). In addition, a higher percentage of patients was in a premenopausal state (46% in the adenomyosis group vs. 35.15% in the group without adenomyosis, P = 0.008). There were no significant differences in BMI between the two groups.
Table 1.
Associations of clinical characteristics with the incidence of adenomyosis co-existence in patients with endometrial carcinoma
| Total | With Adenomyosis | Without Adenomyosis | χ2 | P | |
|---|---|---|---|---|---|
| Menopausal Status | |||||
| Premenopausal | 573 | 69 (46%) | 504 (35.15%) | ||
| Postmenopausal | 1011 | 81 (54%) | 930 (64.86%) | 6.928 | 0.008 |
| Age, years | |||||
| Median | 55 | 53 | 55 | ||
| < 55 | 745 | 91 (60.67%) | 654 (41.92%) | ||
| ≥ 55 | 839 | 59 (39.33%) | 780 (58.08%) | 12.363 | < 0.001 |
| BMI | |||||
| Median | 26.07 | 27 | 26.65 | ||
| < 25 | 488 | 42 (28.38%) | 446 (31.23%) | ||
| ≥ 25 | 1088 | 106 (71.62%) | 982 (68.77%) | 0.511 | 0.475 |
Pathological characteristics of endometrial cancer were also examined (Table 2). The majority of the patients (> 90%) in this study had endometrioid histology. The presence of adenomyosis was significantly associated with a higher likelihood of early stage (I-II) disease (92.67% vs. 85.56%, P = 0.016) and Grade 1-2 tumors (91.33% vs. 84.52%, P = 0.025), when compared with non-adenomyosis cases. When the tumor details were compared, the presence of adenomyosis was significantly associated with a lower likelihood of invasion of ≥ 1/2 of the thickness of the myometrium (10% vs. 22.25%, P < 0.001). Also, the adenomyosis group was more likely to have an absence of pelvic lymph node metastasis (97.04% vs. 92.09%, P = 0.037). Other tumor characteristics, including cervical invasion, adnexa invasion, had no significantly difference between the two groups. All of the patients in the adenomyosis group had the absence of aortic lymph node metastasis (100% vs. 95.64%).
Table 2.
Association of histopathological characteristics with the incidence of adenomyosis co-existence in patients with endometrial carcinoma
| With Adenomyosis n = 150 | Without Adenomyosis n = 1434 | χ2 | P | |
|---|---|---|---|---|
| Histology | ||||
| Endometrioid cancer | 140 (93.33%) | 1294 (90.23%) | ||
| Non-endometrioid cancer | 10 (6.67%) | 140 (9.76%) | 1.519 | 0.218 |
| Stage | ||||
| I-II | 139 (92.67%) | 1227 (85.56%) | ||
| III-IV | 11 (7.33%) | 207 (14.44%) | 5.771 | 0.016 |
| Grade | ||||
| 1-2 | 137 (91.33%) | 1212 (84.52%) | ||
| 3 | 13 (8.67%) | 222 (15.48%) | 4.991 | 0.025 |
| Myometrial invasion | ||||
| < 1/2 | 135 (90%) | 1115 (77.75%) | ||
| ≥ 1/2 | 15 (10%) | 319 (22.25%) | 12.237 | < 0.001 |
| LVSI | ||||
| No | 144 (96%) | 1341 (93.51%) | ||
| Yes | 6 (4%) | 93 (6.49%) | 1.513 | 0.219 |
| Cervical invasion | ||||
| No | 124 (82.67%) | 1105 (77.06%) | ||
| Yes | 26 (17.33%) | 329 (22.94%) | 2.457 | 0.117 |
| Adnexa invasion | ||||
| No | 144 (96.64%) | 1330 (93.46%) | ||
| Yes | 5 (3.36%) | 93 (6.54%) | 2.333 | 0.127 |
| Pelvic LN metastasis | ||||
| No | 131 (97.04%) | 1176 (92.09%) | ||
| Yes | 4 (2.96%) | 101 (7.91%) | 4.339 | 0.037 |
| Aortic LN metastasis | ||||
| No | 96 (100%) | 767 (95.64%) | ||
| Yes | 0 | 35 (4.36%) | - | - |
LVSI: Lymphovascular space invasion.
At the molecular level, we examined expression of several proteins using immunohistochemistry on available tissue samples. The percentage of positive staining of p53 was lower in the adenomyosis group (53.39% vs. 63.32%, P = 0.034) (Figure 1). Other tumor markers including ER, PR, Ki-67, survivin, and CA125 had no significant differences between the two groups (Table 3).
Figure 1.
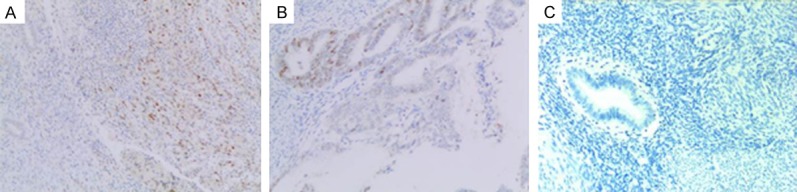
Representative IHC images of p53 in tissues. A: With adenomyois group; B: Without adnomyosis group; C: Negative control.
Table 3.
Immunohistochemistry results of endometrial cancer
| Total | With Adenomyosis | Without Adenomyosis | χ2 | P | |
|---|---|---|---|---|---|
| ER | |||||
| Positive | 1174 | 112 (94.87%) | 1062 (90.14%) | ||
| Negative | 123 | 6 (5.13%) | 117 (9.86%) | 2.926 | 0.087 |
| PR | |||||
| Positive | 1111 | 101 (86.32%) | 1010 (85.88%) | ||
| Negative | 182 | 16 (13.67%) | 166 (14.12%) | 0.117 | 0.896 |
| P53 | |||||
| Positive | 807 | 63 (53.39%) | 744 (63.32%) | ||
| Negative | 486 | 55 (46.61%) | 431 (36.68%) | 4.507 | 0.034 |
| Ki-67 | |||||
| Positive | 1230 | 111 (94.87%) | 1119 (96.97%) | ||
| Negative | 41 | 6 (5.13%) | 35 (3.03%) | 1.494 | 0.222 |
| Survivn | |||||
| Positive | 687 | 58 (59.79%) | 629 (62.59%) | ||
| Negative | 415 | 39 (40.21%) | 376 (37.41%) | 0.294 | 0.588 |
| CA125 | |||||
| Positive | 794 | 72 (65.45%) | 722 (66.54%) | ||
| Negative | 401 | 38 (34.55%) | 363 (33.46%) | 0.053 | 0.818 |
ER: Estrogen-receptor; PR: progesterone-receptor.
Survival analysis was also performed. Endometrial cancer cases with adenomyosis had a significantly higher 5-year survival rate (92.1% vs. 84.1%, P = 0.045) (Table 4 and Figure 2). However, the presence of adenomyosis was not an independent prognostic factor. The risk factors for survival (in the univariate analysis) listed in Table 4, include: age, BMI, menopausal status, histology, stage of disease, grade of tumor, myometrial invasion, cervical invasion, adnexa invasion, pelvic lymph node metastasis, aortic lymph node metastasis, and LVSI. On multivariate analysis, after considering all the significant variables from univariate analysis, the age, BMI, disease stage, tumor grade, and myometrial invasion remained independent significant factors associated with survival, but not adenomyosis.
Table 4.
Risk factors for survival in endometrial cancer patients
| 5-year survival proportion (%) | Univariate | Multivariate | |||
|---|---|---|---|---|---|
|
| |||||
| HR (95% CI) | P | HR (95% CI) | P | ||
| Adenomyosis | |||||
| No | 84.1 | 1 | |||
| Yes | 92.1 | 0.375 (0.13-1.01) | 0.045 | ||
| Age, years | |||||
| < 55 | 90.3 | 1 | |||
| ≥ 55 | 80.1 | 2.15 (1.43-3.22) | < 0.001 | 2.65 (1.24-5.64) | 0.011 |
| BMI | |||||
| < 25 | 86.2 | 1 | |||
| ≥ 25 | 87.3 | 0.642 (0.44-0.93) | 0.019 | 0.392 (0.20-0.75) | 0.005 |
| Menopausal Status | |||||
| Premenopausal | 92.6 | 1 | |||
| Postmenopausal | 81.7 | 3.14 (1.90-5.22) | < 0.001 | ||
| Laparoscopic | |||||
| No | 83.1 | 1 | |||
| Yes | 86.4 | 0.68 (0.43-1.06) | 0.088 | ||
| Histology | |||||
| Endometrioid cancer | 88 | 1 | |||
| Non-endometrioid cancer | 59.8 | 5.451 (3.71-8.00) | < 0.001 | ||
| Stage | |||||
| I-II | 91.8 | 1 | |||
| III-IV | 47.7 | 12.01 (8.2-17.60) | < 0.001 | 7.26 (3.51-15.00) | < 0.001 |
| Grade | |||||
| 1-2 | 89.8 | 1 | |||
| 3 | 58.3 | 4.44 (3.057-10.967) | < 0.001 | 2.51 (1.28-4.92) | 0.007 |
| Myometrial invasion | |||||
| < 1/2 | 92.4 | 1 | |||
| ≥ 1/2 | 62.3 | 7.45 (5.06-10.96) | < 0.001 | 2.77 (1.30-5.89) | 0.008 |
| Cervical invasion | |||||
| No | 87.9 | 1 | |||
| Yes | 74.8 | 2.93 (2.01-4.23) | < 0.001 | ||
| Adnexa invasion | |||||
| No | 87.5 | 1 | |||
| Yes | 50.1 | 7.79 (5.24-11.58) | < 0.001 | ||
| Pelvic lymph nodemetastasis | |||||
| No | 87.9 | 1 | |||
| Yes | 55.2 | 10.37 (5.50-19.56) | < 0.001 | ||
| Aortic lymph nodemetastasis | |||||
| No | 86.6 | 1 | |||
| Yes | 40.5 | 13.09 (6.59-25.99) | < 0.001 | ||
| LVSI | |||||
| No | 87.5 | 1 | |||
| Yes | 50.1 | 6.19 (4.09-9.38) | < 0.001 | ||
Figure 2.
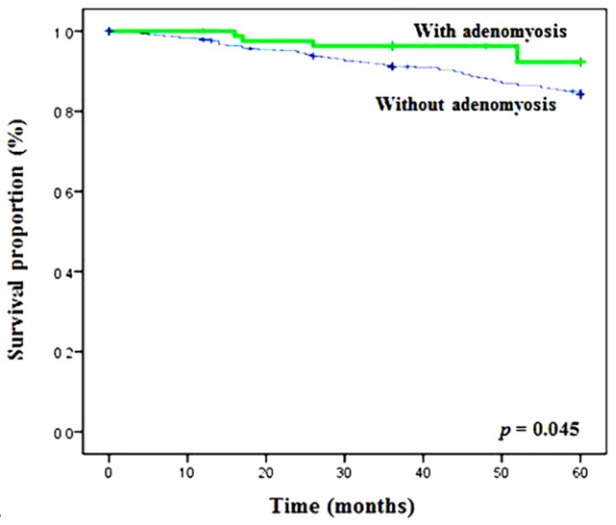
Survival outcomes of endometrial cancer patients, with or without adenomyosis. The p value was calculated based on log-rank test.
Discussion
The key findings of our study include that the presence of adenomyosis in patients with endometrial carcinoma was associated with 1) the less aggressive properties of the tumors and 2) better prognosis of patients.
Several characteristics of the tumors were investigated in this study, such as cancer invasion, lymph node metastasis, stage and grade of the tumors. We found decreased myometrial invasion (≥ 1/2) in the adenomyosis group, which is consistent with another study [13]. It was reported that the increased thickness of endometrial stroma in adenomyosis is associated with rapid cell proliferation under stimulation of estrogen or inflammatory cytokines [14], and the thickened endometrial stroma may contribute to a mechanical blockage of the endometrial cancer invasion in the myometrium. Both the current study and other finding support this hypothesis [13].
In the present study, the presence of adenomyosis was also associated with reduced incidence of aortic/pelvic lymph node metastasis, as well as lower grade and stage of endometrial carcinoma. Indeed, the lower incidences of aortic/pelvic lymph node metastasis in patients with adenomyosis were also observed in other studies [13,15]. Among the 150 patients who had adenomyosis in our study, none of them had aortic lymph node metastasis. This was much lower than the incidence observed in the patients without adenomyosis (4.36%), although statistical analysis could not be performed.
At the molecular level, expression of mutated p53 in endometrial cancer was associated with patients lacking adenomyosis. The wild-type p53 usually has a shorter half-life when compared to the mutated p53; therefore, the positive expression of p53 detected in IHC of the present study represented predominately the mutated p53. It was reported that the high expression of p53 was associated with poor prognosis of endometrial carcinoma patients [16]. In the present cohort, positive expression of p53 was found in 53.39% of patients with adenomyosis (who had better prognosis), and 63.32% of patients without adenomyosis. The link between the presence of adenomyosis and the positive expression of p53 remains unclear and warrants further investigation.
The inflammatory signals were not investigated in this study, but other reports showed that the unique cytokine profile in patients with adenomyosis may alleviate the endometrial cancer progression. It is known that patients with adenomyosis have increased interferon (IFN)-α, IFN-γ, tumor necrosis factor (TNF)-α, and interleukin (IL)-10 [17,18]. The increase of these cytokines may amplify the host anti-tumor immune activities [19-22]. On the other hand, adenomyosis is known to have decreased secretions of cytokines, such as IL-8, IL-1β, and epidermal growth factor (EGF) that have an important role in tumor progression [18,23,24]. In summary, both the clinical and molecular studies from current and previous findings suggested the protective role of adenomyosis in the progression of endometrial carcinoma.
In this study, patients with adenomyosis had a significantly higher 5-year survival rate than patients without adenomyosis (92.1% vs. 84.1%), which is consistent with other reports [13,25]. This may be partly attributed to the lower expression of p53 in endometrial carcinoma. The presence of adenomyosis, however, was not an independent prognostic factor of patients’ survival. In addition to the expression of p53, patients with adenomyosis are generally younger, had earlier stage and lower grade of tumor, reduced myometrial invasion, and lymph node metastasis.
In conclusion, patients with adenomyosis were associated with less aggressive behavior of the tumors and the presence of adenomyosis may be a protective factor of endometrial carcinoma that is associated with better prognosis.
Acknowledgements
This study was funded by the International Cooperation Project of Department of Science and Technology in Hebei province (No. 14397703D).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Garcia L, Isaacson K. Adenomyosis: review of the literature. J Minim Invasive Gynecol. 2011;18:428–37. doi: 10.1016/j.jmig.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Benagiano G, Habiba M, Brosens I. The pathophysiology of uterine adenomyosis: an update. Fertil Steril. 2012;98:572–9. doi: 10.1016/j.fertnstert.2012.06.044. [DOI] [PubMed] [Google Scholar]
- 4.Kucera E, Hejda V, Dankovcik R, Valha P, Dudas M, Feyereisl J. Malignant changes in adenomyosis in patients with endometrioid adenocarcinoma. Eur J Gynaecol Oncol. 2011;32:182–4. [PubMed] [Google Scholar]
- 5.Ismiil N, Rasty G, Ghorab Z, Nofech-Mozes S, Bernardini M, Ackerman I, Thomas G, Covens A, Khalifa MA. Adenomyosis involved by endometrial adenocarcinoma is a significant risk factor for deep myometrial invasion. Ann Diagn Pathol. 2007;11:252–7. doi: 10.1016/j.anndiagpath.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Mittal KR, Barwick KW. Endometrial adenocarcinoma involving adenomyosis without true myometrial invasion is characterized by frequent preceding estrogen therapy, low histologic grades, and excellent prognosis. Gynecol Oncol. 1993;49:197–201. doi: 10.1006/gyno.1993.1107. [DOI] [PubMed] [Google Scholar]
- 7.Jacques SM, Lawrence WD. Endometrial adenocarcinoma with variable-level myometrial involvement limited to adenomyosis: a clinicopathologic study of 23 cases. Gynecol Oncol. 1990;37:401–7. doi: 10.1016/0090-8258(90)90376-v. [DOI] [PubMed] [Google Scholar]
- 8.Seidman JD, Kjerulff KH. Pathologic findings from the Maryland Women’s health study: practice patterns in the diagnosis of adenomyosis. Int J Gynecol Pathol. 1996;15:217–21. doi: 10.1097/00004347-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Boes AS, Tousseyn T, Vandenput I, Timmerman D, Vergote I, Moerman P, Amant F. Pitfall in the diagnosis of endometrial cancer: case report of an endometrioid adenocarcinoma arising from uterine adenomyosis. Eur J Gynaecol Oncol. 2011;32:431–4. [PubMed] [Google Scholar]
- 10.Abushahin N, Zhang T, Chiang S, Zhang X, Hatch K, Zheng W. Serous endometrial intraepithelial carcinoma arising in adenomyosis: a report of 5 cases. Int J Gynecol Pathol. 2011;30:271–81. doi: 10.1097/PGP.0b013e318200868e. [DOI] [PubMed] [Google Scholar]
- 11.Quick CM, May T, Horowitz NS, Nucci MR. Low-grade, low-stage endometrioid endometrial adenocarcinoma: a clinicopathologic analysis of 324 cases focusing on frequency and pattern of myoinvasion. Int J Gynecol Pathol. 2012;31:337–43. doi: 10.1097/PGP.0b013e31823ff422. [DOI] [PubMed] [Google Scholar]
- 12.Ismiil ND, Rasty G, Ghorab Z, Nofech-Mozes S, Bernardini M, Thomas G, Ackerman I, Covens A, Khalifa MA. Adenomyosis is associated with myometrial invasion by FIGO 1 endometrial adenocarcinoma. Int J Gynecol Pathol. 2007;26:278–83. doi: 10.1097/01.pgp.0000235064.93182.ec. [DOI] [PubMed] [Google Scholar]
- 13.Matsuo K, Cahoon SS, Gualtieri M, Scannell CA, Jung CE, Takano T, Paulson RJ, Muderspach LI, Roman LD. Significance of adenomyosis on tumor progression and survival outcome of endometrial cancer. Ann Surg Oncol. 2014;21:4246–55. doi: 10.1245/s10434-014-3880-6. [DOI] [PubMed] [Google Scholar]
- 14.Mehasseb MK, Bell SC, Brown L, Pringle JH, Habiba M. Phenotypic characterisation of the inner and outer myometrium in normal and adenomyotic uteri. Gynecol Obstet Invest. 2011;71:217–24. doi: 10.1159/000318205. [DOI] [PubMed] [Google Scholar]
- 15.Musa F, Frey MK, Im HB, Chekmareva M, Ellenson LH, Holcomb K. Does the presence of adenomyosis and lymphovascular space invasion affect lymph node status in patients with endometrioid adenocarcinoma of the endometrium? Am J Obstet Gynecol. 2012;207:417, e1–6. doi: 10.1016/j.ajog.2012.06.051. [DOI] [PubMed] [Google Scholar]
- 16.Lax SF, Kendall B, Tashiro H, Slebos RJ, Hedrick L. The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer. 2000;88:814–24. [PubMed] [Google Scholar]
- 17.Wang F, Li H, Yang Z, Du X, Cui M, Wen Z. Expression of interleukin-10 in patients with adenomyosis. Fertil Steril. 2009;91:1681–5. doi: 10.1016/j.fertnstert.2008.02.164. [DOI] [PubMed] [Google Scholar]
- 18.Sotnikova N, Antsiferova I, Malyshkina A. Cytokine network of eutopic and ectopic endometrium in women with adenomyosis. Am J Reprod Immunol. 2002;47:251–5. doi: 10.1034/j.1600-0897.2002.01040.x. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 20.Ferrantini M, Capone I, Belardelli F. Interferon-alpha and cancer: mechanisms of action and new perspectives of clinical use. Biochimie. 2007;89:884–93. doi: 10.1016/j.biochi.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Tagashira Y, Taniguchi F, Harada T, Ikeda A, Watanabe A, Terakawa N. Interleukin-10 attenuates TNF-alpha-induced interleukin-6 production in endometriotic stromal cells. Fertil Steril. 2009;91(Suppl):2185–92. doi: 10.1016/j.fertnstert.2008.04.052. [DOI] [PubMed] [Google Scholar]
- 22.van Horssen R, Ten Hagen TL, Eggermont AM. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist. 2006;11:397–408. doi: 10.1634/theoncologist.11-4-397. [DOI] [PubMed] [Google Scholar]
- 23.Zitvogel L, Kepp O, Galluzzi L, Kroemer G. Inflammasomes in carcinogenesis and anticancer immune responses. Nat Immunol. 2012;13:343–51. doi: 10.1038/ni.2224. [DOI] [PubMed] [Google Scholar]
- 24.Palena C, Hamilton DH, Fernando RI. Influence of IL-8 on the epithelial-mesenchymal transition and the tumor microenvironment. Future Oncol. 2012;8:713–22. doi: 10.2217/fon.12.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gizzo S, Patrelli TS, Dall’asta A, DI Gangi S, Giordano G, Migliavacca C, Monica M, Merisio C, Nardelli GB, Quaranta M, Noventa M, Berretta R. Coexistence of adenomyosis and endometrioid endometrial cancer: role in surgical guidance and prognosis estimation. Oncol Lett. 2016;11:1213–1219. doi: 10.3892/ol.2015.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


