Abstract
Objective: To compare the expression of imprinted genes PHLDA2 and IGF2 in the peripheral blood, placenta and fetal umbilical cord blood from normal single chorionic twins (MCDA) and single chorionic twins combined with sIUGR, in order to explore the pathogenesis relationship between the expression of PHLDA2 and IGF2 with single chorionic twins combined with sIUGR. Methods: Immunohistochemical method was applied to detect the expression of PHLDA2 and IGF2 in maternal placenta of MCDA and normal MCDA. ELISA method was applied to detect the expression of PHLDA2 and IGF2 in two groups of umbilical cord blood. Results: There was a significant difference in two groups of maternal age and neonatal birth weight (P<0.05). Immunohistochemical results: IGF2 in the normal MCDA maternal placenta was high expression. The number of IGF2-positive cells in MCDA-sIUGR group A1 decreased slightly and the staining decreased slightly. The number of IGF2-positive cells in A2 group was significantly decreased, and the staining was weakened. In normal MCDA placenta, PHLDA2 cells were colorless, or occasionally in pale yellow; the number of PHLDA2 positive cells in MCDA sIUGR A1 group was slightly increased, the staining was light yellow or colorless. In A2 group, the number of positive cells increased significantly, the staining was brown or brownish-yellow. ELISA results showed that there were significant differences in the expression of IGF2 and PHLDA2 between cord blood of MCDA sIUGR and neonatal cord blood (P<0.05). The expression of IGF2 in MCDA sIUGR and normal MCDA twins were also significantly different (P<0.01). Conclusion: The differential expression of PHLDA2 and IGF2 may be one of the causes of selective intrauterine growth restriction.
Keywords: MCDA, selective intrauterine growth restriction, sIUGR, placenta, PHLDA2, IGF2
Introduction
Monochorionic diamniotic, (MCDA) selective intrauterine growth restriction, (sIUGR) refers to: If we performed ultra sound on monozygotic twins to measure the body mass to either fetus or new born, if the body mass less than 10% of local population reference range in corresponding gestational period. About 12% of MCDA sIUGR may occur [1]. sIUGR fetal growth restriction would cause 15% of fetal death (IUFD) [2]. MCDA sIUGR is one of the most common complications in monozygotic twins’ pregnancy, it not only affects the fetal growth and outcome, but also had a long term effect on new born cardiovascular system and metabolism system [3].
The cause of MCDA sIUGR is still complicated and unknown. Most of etiology studies focus on “Placenta” researches, for example: the greater the mass differences between the two placentas the greater the new born weight difference will be. The abnormal cord insertion often to be the most probably cause of sIUGR [4]. However, there is less study on the molecular level, and some previous studies show that maternal imprinted gene (PHLDA2) and paternally imprinted gene (IGF2) may be the cause of intrauterine growth retardation infant (IUGR) [5,6]. But the single fetal IUGR and normal single birth are divided by new born baby’s weights. It does not exclude the heterogeneity between two groups, like environmental or genetic. In this study, in order to sequence differences and minimize the environmental impact or genetic factors, the aim of this study was to adapt the monozygotic twins to find out the PHLDA2 and IGF2 gene expression levels in normal placenta and growth restriction placenta.
Materials and methods
Subjects
The specimen were selected from the lying-in women from the Obstetrics and Gynecology Department of Nanfang Hospital of Southern Medical University during Jan 2014 to Jun 2015. There were 20 cases whom had been diagnosed as monozygotic twins with sIUGR.
The diagnostic criteria of sIUGR were as follows: monozygotic twins, ultrasound measured its body mass, if any, less than 10% of the local population reference. During the same period, 20 cases of normal monozygotic twins were taken in as control group. The control group was similar with the experimental group, except without any complication.
The chorionic of experimental group was examined in the early stage of pregnancy via ultrasound. Pregnant women during the first 6-9 weeks of pregnancy could be examined by the numbers of the gestational sacs; 10-14 weeks could be examined by the edge between amnion and placenta. The segment between amnion and placenta showed a “T” shape feature in monozygotic twins. On the other hand, a double chorionic twin showed a “λ” sign. Examine again before the delivery, if there was only one placenta, then defined as monochorionic twin amniotic sac or single amniotic sac placenta. If there showed an individual amniotic, an individual cord then defined as double amniotic sac. If we could not decide its condition, then run the pathological examination to clarify.
The palcenta tissues, cord blood and peripheral blood of investigated subjects were collected. The experimental group based new born baby’s weight were divided into large baby (group 1) and small baby (group 2) collecting research entity’s postpartum placenta, cord blood and peripheral blood. The control group based on the birth sequence were divided into large baby (group 1) and small baby (group 2). All specimens were all informed and consent. All the documents were reviewed and consented by the ethic committee of Nanfang Hospital of Southern Medical University.
Immunohistochemistry
Immunohistochemistry was applied to test the differential expression of PHLDA2 and IGF2 in the placenta. It was applied with IGF-II (bioworld Co., Ltd), dilution ratio 1:200; Envision anti-rabbit/mouse-HRP (DAKO Co., Ltd).
Five high-power fields (HPFs) were randomly selected according to their negative cell count: 0%-25%, 26%-50%, 51%-75% and ≥76%. The definition of point scale was 0-3 point, by and by its staining intensity. No colour, yellowish, brown yellow and brown were defined as 0 to 3 points, the product of two definitions if (≥3) then Significant Expression; (<3) then Low Expression, which included negative expression.
Enzyme-linked immunosorbent assay test (ELISA)
The ELISA was taken to test the differential expressions of PHLDA2 and IGF2 in two sets of maternal peripheral blood and two sets of fetal cord blood.
The evaluation of outcome was considered as follows: this curve is drafted according to the serum samples, with y-axis as OD value and x-axis as stander object’s density (Figure 1).
Figure 1.
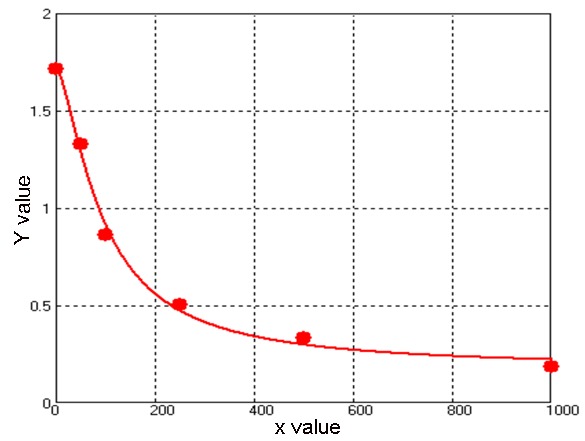
Curve for outcome evaluation; Formulation: y =(A - D)/[1 + (x/C)^B] + D; four parameters.
Statistic analysis
Statistical data are represented with (x̅±SD) or t-distribution. Comparison between groups was performed by One-way ANOVO and Welch test. LSD and Dunnett’s T3 are also adopted in the multiple comparisons. Two tailed and P<0.05 was considered as statistical significance.
Results
General information of patients with MDCA sIUGR and controls are shown in Table 1. There were significant differences between two groups of maternal age, gestational age and birth weight (P<0.05), the differences of birth weight in MDCA sIUGR group were also significant (P<0.05); However, no significant difference was observed in the birth weight in the controls (P<0.05) (Figure 2A).
Table 1.
General information of patients with MDCA sIUGR and controls
| Group | Age, years | Delivery weeks | Infant birthweight, kg |
|---|---|---|---|
| Patients with MDCA sIUG | 28.36±4.79* | 33.3±2.1** | |
| Group 1 | 2.3±0.3* | ||
| Group 2 | 1.3±0.6 | ||
| Controls | 31.04±5.13 | 35.6±1.1 | |
| Group 1 | 2.6±0.5** | ||
| Group 2 | 2.4±0.4 |
Patients with MDCA sIUG were compared with controls;
P<0.05;
Group 1 compared with group 2;
P>0.05.
Figure 2.
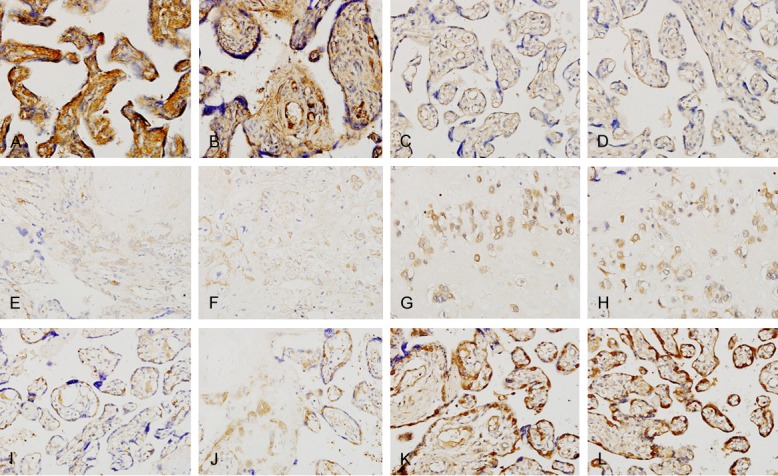
*Localization of IGF2 in placent of normal MCAD twin. A. (×400); B. (×400). *Localization of IGF2 in placent of MCAD Siugr. C. (×400); D. (×400). *Localization of IGF2 in placent of MCAD sIUGR. E. (×400); F. (×400). *Localization of PHLDA2 in placent of normal MCAD twin. G. (×400); H. (×400). *Localization of PHLDA2 in placenta of MCAD sIUGR. I. (×400); J. (×400). *Localization of PHLDA2 in placenta of MCAD sIUGR A2. K. (×400); L. (×400).
The locations of IGF-2 and sIUGR in the placenta were shown as follows: positive cells were found in a normal monochorionic placenta with IGF-2, and they mainly were the syncytiotrophoblast or trophocyte in cotyledons. It could also be found in the amnio-chorionic membrane. IGF-2 had a bigger granule and distributed diffusively in the cell, most common seen in the cytoplasm and matrix. The cell showed a brighter yellowish colour (Figure 2A, 2B). On the contrast, placenta with sIUGR showed less positive cell. The colour was comparatively dim to the IGF-2 in placenta (Figure 2C, 2D). In fetal groups, IGF-2 showed less positive cells significantly than that in normal pregnancy groups (Figure 2E, 2F).
PHLDA2 was located in the cytoplasm, mainly in trophoyte as yellow or brown granule. Cytoplasmic and membrance showed no colour in a normal placenta (Group B), or sometime appeared in light or pale yellow (Figure 2G, 2H). Large fetus group with sIUGR found slightly higher level of PHLDA2 positive cell than normal pregnancy, and cytoplasm and membrane showed light yellow or no colour change. Small fetus group showed significantly increased of PHLDA2 positive cells than those in normal pregnancy. The dyeing was significantly obvious, showed brown or yellow brown colour (Figure 2K, 2L).
There was significant difference in the expression of IGF2 and PHLDA2 between umbilical blood of two fetuses with single chorionic amniotic sacs and sIUGR. The expression level of IGF2 in cord blood of small fetus (A2) was significantly lower than that of large fetus (A1). The expression level of PHLDA2 in cord blood of small fetus (A2) was significantly higher than that of large fetus (A1). The difference between the two groups was significant (P<0.01) (Table 2). However, there was no significant difference in the expression level of IGF2 and PHLDA2 between umbilical blood of the two fetuses with normal single chorionic dual amniotic sac (P>0.05).
Table 2.
IGF-2 and PHLDA2 levels between group1 and group 2 in the two investigated groups
| Variables | MCDA sIUGR patients | Z value | P value | Controls | Z value | P value | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Group 1 (n=10) | Group 2 (n=10) | Group 1 (n=10) | Group 2 (n=10) | |||||
| IGF2, ng/ml | 6.51±0.80 | 2.14±0.37 | -5.39 | <0.01 | 6.18±0.70 | 5.95±0.82 | 2.633 | >0.05 |
| PHLDA2, ng/ml | 0.32±0.13 | 0.56±0.31 | -5.38 | <0.01 | 0.42±0.32 | 0.51±0.11 | 0.012 | >0.05 |
Comparison of the expression of IGF2 and PHLDA2 in peripheral blood (serum) between MCDA sIUGR case group and control group were as follows: the expression level of IGF2 in MCDA sIUGR maternal serum was significantly lower than that in the control group, while the PHLDA2 concentration and the control group has no significant difference (Table 3).
Table 3.
IGF-2 and PHLDA2 levels between the two investigated groups
| Variables | MCDA sIUGR patients (n=10) | Controls (n=10) | Z value | P value |
|---|---|---|---|---|
| IGF2, ng/ml | 30.18±4.70 | 54.52±16.28 | -4.328 | <0.01 |
| PHLDA2, ng/ml | 1.06±0.31 | 0.95±0.63 | 0.727 | >0.05 |
Discussion
Because of monozygotic twins carry the same genetic information that it should have similar growth potential, thus the physical differences should not have too much differences. However, since the inner cell mass of fertilized eggs are formed in unequal blastomeres assignment when split from two sacks, it has led to inconsistent growth during early pregnancy of monozygotic twins. New born babies from this group also various, two new born body mass are differ over 25% [7]. However, compared with double chorionicity twins, monochorionic twins appear to have higher incident of serious sIUGR [4].
Monochorionic twins derived from a single fertilized oocytes, thus these twins should have identical gene. However, researches have found that many monochorionic twins show different representation, like selective intrauterine growth restriction [1,2]. Recent studies have revealed that the expression of epigenetic DNA methylation and imprinted genes may be involved in abnormal monochorionic twins discordant occurrence [3,5,8]. Chromosomal parental paternal or maternal origin of structural differences, only from one parent allele is selectively expressed, and the other allele is not expressed or weakly tabled. This phenomenon is known genomic imprinting. Gene has a gene imprinting phenomenon were called genes imprinted. Imprinted gene is associated with intrauterine fetal growth and placental function, imprinted gene expression disorders may lead to intrauterine fetal abnormalities.
IGF2 is a single-stranded weakly acidic protein composed by 67 amino acids. The main expression is in the embryonic period, as the major growth factor. The expression will rapidly shut down after the birth. The concentration of IGF2 in pregnant women’s blood would incline along the pregnancy progresses; it will reach its peak at the late stage of the pregnancy. IGF2 can promote its appendages embryo growth, development, cell proliferation, differentiation, cell metabolism, and embryo implantation. Also trophoblast are related to the process when cells invade into the decidua, placenta and also related to the fetal growth processes. Studies have found that, the over-expression of IGF2 would lead to fetal growth too fast and the huge incidence of placenta. Imprinting deletions, or downregulation would result in intrauterine growth restriction [9]. IGF2 plays an important role in embryonic and fetal growth.
IGF2 can promote cell metabolism and mitosis, it affects placenta’s formation and function in many ways [10]. Thomsen [11] et al. found that the IGF2 comes from the placenta mainly generate from placental trophoblast cells. In addition it is also expressed in cells witin the amniotic fluff layer. Irwin’s [12] in vitro cell migration assay shows: IGF2 is capable of enhancing trophoblast cell migration, and promoting their Matrigel invasion. IGF2 binds with IGF-2R via receptors, introduced trophoblast invasion to the combination of IGFBP1 and IGF2, which allows IGF2 to suppress trophoblast cells intrusion, in advance the generation of IUGR [13]. Studies have showed that the IGF2 mRNA in the newborn cord blood reveals a low expression of fetal growth restriction(FGR) babie [14]. The number of IGF2 positive cells in small fetus with MCDA sIUGR in the placenta compared with normal pregnancy was significantly reduced, and staining was also significantly reduced. IGF2 was lower in umbilical cord blood and maternal peripheral serum.
The PHLDA2 imprinted gene was located in the region of chromosome q11, which was a parental imprinted gene. Mainly expressed in the placenta, the expression level in the placenta increased, but decreased the expression level in the chorionic amniotic membrane, can regulate the differentiation of the placenta and embryonic growth and development. The expression level of PHLDA2 in the placenta may vary with the progression of pregnancy. All the way to 31 weeks of pregnancy, the expression level would be the minimal. Animal experiments found that: in the PHLDA2 gene excised mice, the placenta appeared overgrowth while the increased expression level of PHLDA2 gene may lead to placental and fetal development retardation.
Apostolidou S [15] and others have studied 200 normal pregnant born that the level of PHLDA2 in the placenta show a negative correlation with new born weights. McMinn J and Tycko B et al. [16] also have showed that the placenta in the condition of fetal growth restriction displayed a higher level of expression of PHLDA2.
This study investigated the expression of PHLDA2 in placentas and cord blood by both normal monochorionic twins and monochorionic twins merged with sIUGR. Monochorionic twins merged with sIUGR showed a higher expression of PHLDA2 in the placenta, which indicated PHLDA2 as a paternally imprinted may have a negative correlation with maternal imprinted gene expression onto the placenta and the fetal growth. These results were consistent with early researches. PHLDA2 might affect placenta’s functionality via reducing its volume which would result in reduce of new born weight. The expression of PHLDA2 might cause placental growth retardation, decreased function of the placenta and affect fetal growth and perinatal outcome.
Therefore, the placental increases PHLDA2 expression and decreases of the IGF2 expression, leading to abnormal placental function, fetal low birth weight. The results show that the abnormality of PHLDA2 and IGF2 in the placenta could partake the development of MCDA sIUGR.
Acknowledgements
This study was supported from the Special Funding Collaborative innovation and Platform construction of Guangdong Province in 2015 (2015B050501006).
Disclosure of conflict of interest
None.
References
- 1.Gratacos E, Carreras E, Becker J, Lewi L, Enriquez G, Perapoch J, Higueras T, Cabero L, Deprest J. Prevalence of neurological damage in monochorionic twins with selective intrauterine growth restriction and intermittent absent or reversed end-diastolic umbilical artery flow. Ultrasound Obstet Gynecol. 2004;24:159–163. doi: 10.1002/uog.1105. [DOI] [PubMed] [Google Scholar]
- 2.Ishii K, Murakoshi T, Hayashi S, Saito M, Sago H, Takahashi Y, Sumie M, Nakata M, Matsushita M, Shinno T, Naruse H, Torii Y. Ultrasound predictors of mortality in monochorionic twins with selective intrauterine growth restriction. Ultrasound Obstet Gynecol. 2011;37:22–26. doi: 10.1002/uog.8846. [DOI] [PubMed] [Google Scholar]
- 3.De Paepe ME, Shapiro S, Young L, Luks FI. Placental characteristics of selective birth weight discordance in diamniotic-monochorionic twin gestations. Placenta. 2010;31:380–386. doi: 10.1016/j.placenta.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Valsky DV, Eixarch E, Martinez JM, Crispi F, Gratacos E. Selective intrauterine growth restriction in monochorionic twins: pathophysiology, diagnostic approach and management dilemmas. Semin Fetal Neonatal Med. 2010;15:342–348. doi: 10.1016/j.siny.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Shi X, He Z, Gao Y, Luo Y, Gou C, Fang Q. Placental expression of PHLDA2 in selective intrauterine growth restriction in monozygotic twins. Placenta. 2014;35:428–430. doi: 10.1016/j.placenta.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Janssen AB, Tunster SJ, Heazell AE, John RM. Placental PHLDA2 expression is increased in cases of fetal growth restriction following reduced fetal movements. BMC Med Genet. 2016;17:17. doi: 10.1186/s12881-016-0279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dashe JS, Fernandez CO, Twickler DM. Utility of Doppler velocimetry in predicting outcome in twin reversed-arterial perfusion sequence. Am J Obstet Gynecol. 2001;185:135–139. doi: 10.1067/mob.2001.113906. [DOI] [PubMed] [Google Scholar]
- 8.Schreiner F, Gohlke B, Stutte S, Bartmann P, Hecher K, Oldenburg J, El-Maarri O, Woelfle J. 11p15 DNA-methylation analysis in monozygotic twins with discordant intrauterine development due to severe twin-to-twin transfusion syndrome. Clin Epigenetics. 2014;6:6. doi: 10.1186/1868-7083-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakian S, Louie K, Wong EC, Havelock J, Kashyap S, Rowe T, Taylor B, Ma S. Altered gene expression of H19 and IGF2 in placentas from ART pregnancies. Placenta. 2015;36:1100–1105. doi: 10.1016/j.placenta.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Westwood M, Gibson JM, Sooranna SR, Ward S, Neilson JP, Bajoria R. Genes or placenta as modulator of fetal growth: evidence from the insulin-like growth factor axis in twins with discordant growth. Mol Hum Reprod. 2001;7:387–395. doi: 10.1093/molehr/7.4.387. [DOI] [PubMed] [Google Scholar]
- 11.Thomsen BM, Clausen HV, Larsen LG, Nurnberg L, Ottesen B, Thomsen HK. Patterns in expression of insulin-like growth factor-II and of proliferative activity in the normal human first and third trimester placenta demonstrated by non-isotopic in situ hybridization and immunohistochemical staining for MIB-1. Placenta. 1997;18:145–154. doi: 10.1016/s0143-4004(97)90086-2. [DOI] [PubMed] [Google Scholar]
- 12.Irwin JC, Suen LF, Faessen GH, Popovici RM, Giudice LC. Insulin-like growth factor (IGF)-II inhibition of endometrial stromal cell tissue inhibitor of metalloproteinase-3 and IGF-binding protein-1 suggests paracrine interactions at the decidua: trophoblast interface during human implantation. J Clin Endocrinol Metab. 2001;86:2060–2064. doi: 10.1210/jcem.86.5.7451. [DOI] [PubMed] [Google Scholar]
- 13.Fowden AL. The insulin-like growth factors and feto-placental growth. Placenta. 2003;24:803–812. doi: 10.1016/s0143-4004(03)00080-8. [DOI] [PubMed] [Google Scholar]
- 14.Whitehead CL, Walker SP, Mendis S, Lappas M, Tong S. Quantifying mRNA coding growth genes in the maternal circulation to detect fetal growth restriction. Am J Obstet Gynecol. 2013;209:133, e131–139. doi: 10.1016/j.ajog.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Apostolidou S, Abu-Amero S, O’Donoghue K, Frost J, Olafsdottir O, Chavele KM, Whittaker JC, Loughna P, Stanier P, Moore GE. Elevated placental expression of the imprinted PHLDA2 gene is associated with low birth weight. J Mol Med (Berl) 2007;85:379–387. doi: 10.1007/s00109-006-0131-8. [DOI] [PubMed] [Google Scholar]
- 16.McMinn J, Wei M, Schupf N, Cusmai J, Johnson EB, Smith AC, Weksberg R, Thaker HM, Tycko B. Unbalanced placental expression of imprinted genes in human intrauterine growth restriction. Placenta. 2006;27:540–549. doi: 10.1016/j.placenta.2005.07.004. [DOI] [PubMed] [Google Scholar]


