Abstract
Objective: Diabetic retinopathy (DR) is one severe complication of diabetes, and involves reactive oxygen species (ROS) produced under oxidative stress (OS) conditions. Keap1-Nrf2-ARE is one important endogenous anti-OS signal pathway. This study generated a type 2 DR rat model, on which expression of Nrf2/ARE pathway related proteins were measured to investigate functional role and mechanism of Keap1-Nrf2-ARE signal pathway in DR. Patients and Methods: Using DR model rats, blood samples were collected for measuring FBG, TC, TG, HDL-C, and LDL-C for evaluating blood glucose and lipid. Retinas were collected for measuring ROS content using DCFH-DA staining, and caspase-3 activity was measured by colorimetry. Cell apoptosis was measured by flow cytometry. Keap1 and Nrf2 proteins were quantified by Western blot. Aqueous humor was collected for measuring MDA, SOD, GSH-Px, and T-AOC. Results: Model rats had significantly elevated blood FBG, TG, TC, and LDL-C, plus decreased HDL-C. Model rats also had higher retinal ROS content, enhanced caspase-3 activity, and potentiated apoptosis. Compared to the control group, model rats had elevated MDA and lower activity of SOD, GSH-Px, and T-AOC in aqueous humor, plus lower Keap1 and higher Nrf2 expression in retina. Conclusion: DR rats showed significantly elevated retinal apoptosis, with prominent OS. Under diabetic conditions, activation of Keap1-Nrf2-ARE pathway may play a role in alleviating OS damage and protecting retina.
Keywords: Diabetic retinopathy, Keap1-Nrf2-ARE, apoptosis, reactive oxygen species
Introduction
Diabetic retinopathy (DR) is a retinal disease caused by microvascular disorder frequently occurred in diabetic patients. At early stages, visual function is intact. When reaching the terminal stage, de novo angiogenesis on the retina or posterior wall of vitreous body can lead to retinal folding and detaching, eventually leading to vision loss, forming one of major reasons for blinding in working age population [1,2]. Among confirmed diabetic patients, the incidence of DR is 10%~15%, and is even higher in developing countries [3,4].
Reactive oxygen species (ROS) refers to a class of oxidative damaged substances produced under oxidative stress (OS) condition and can lead to modification of large intracellular molecules such as proteins, lipids, and nucleic acids, and plays crucial roles in onset and progression of various diseases. Under DR condition, large amounts of ROS can cause alternation and damage of retinal micro-vessel, retinal cells and retinal ganglion cells. The body has a complicated system of OS response system to reduce oxidant production or to enhance anti-oxidative potency, thus alleviating ROS damage on cells [5,6]. Nuclear factor (NF)-E2 related factor 2 (Nrf2)/antioxidant response element (ARE) is an important endogenous anti-OS signal pathway. Under OS status, Nrf2 can translocate into the nucleus for binding with ARE to initiate transcription and expression of various downstream related detoxification enzymes and anti-oxidases, to alleviate body OS damage, thus protecting tissue damage [7-9]. This study generated a type 2 DR rat model in parallel with control rats, and measured expression of Nrf2/ARE pathway related proteins in rat retinal tissues, and measured the content of lipid peroxidase and multiple anti-oxidative factors in aqueous humor, thus investigating the role of Keap1-Nrf2-ARE signal pathway in DR from the perspective of OS, along with related mechanisms.
Materials and methods
Major reagent and materials
Healthy male SD rats (6 weeks old, body weight 220~240 g) were purchased from Weifang Medical Laboratory Animal Center. Trizol was purchased from Invitrogen (US). PrimeScript RT reagent kit and SYBR Green were purchased from Takara (China). Rabbit anti-keap1 and Nrf2 antibody was purchased from Abcam (US). Mouse anti-β-actin primary antibody was purchased from Sangon Bio (China). Streptozotocin (STZ)-citric acid buffer and DCFH-DA primer were purchased from Sigma (US). Caspase 3 activity assay kit (colorimetry method) was purchased from Jimei Biotech (China). Annexin V-FITC/PI cell apoptosis kit, assay kits for MDA, superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and total antioxidant capacity (T-AOC, ABTS fast method) were purchased from Beyotime Biotech (China).
Generation of DR rat model
Type 2 diabetic rat model was prepared by high-fat and high-glucose diet feeding plus intraperitoneal injection of small dosage of STZ. In brief, SD rats were acclimated for 1-week normal feeding first, followed by model preparation. Model rats received high fat and high glucose diet for 2 months. After 12 h fasting, 1% STZ-citrate buffer was injected into the peritoneal cavity at 40 mg/kg dosage. After 7-day model preparation, fasting plasma glucose (FPG) was measured from tail vein blood. Those FPG levels higher than 16.7 mmol/L were assigned as successful diabetic models (59.2% successful rate). High-fat and high-glucose diet was then resumed until the endpoint. Control rats received standard diet, and received citrate buffer via intraperitoneal injection after 2 months feeding, and received standard diet until the endpoint.
Sample collection
At 8 weeks and 12 weeks after model preparation, rats were anesthetized by intraperitoneal injection of 2% pentobarbital. Sterile insulin needle (0.5 mL) was used for making a puncture at 1 mm of anterior chamber within cornea edge. Aqueous humor was collected from central region for measuring lipid oxidation and anti-oxidant indexes. After drawing aqueous humor, the eyeball was removed, and anterior part was removed along the circular edge to detach the retina. Residual vitreous body was cleared, and the retinal tissue was rinsed by PBS. Part of the retina was used for extracting RNA, and part was homogenized in RIPA lysis buffer for extracting protein. Some retina tissues were used for measuring caspase-3 activity and cell apoptosis, and for ROS content. Whole blood samples were collected from the heart for quantifying blood glucose and lipid level.
Blood glucose and lipid assay
Model 7180 automatic biochemical analyzer (Hitachi, Japan) was used to measure rat FPG, total cholesterol (TC), triglyceride (TG), high density lipoprotein cholesterol (HDL-C), and low density lipoprotein cholesterol (LDL-C) contents.
Cell apoptosis assay in retina
Rats were sacrificed and the eyeball was removed to separate retina, which was digested within 0.125% trypsin for 20 min at 37°C, followed by PBS rinsing. Binding buffer (500 μL) was added to re-suspend cells, after which 5 μL Annexin V-FITC was added and the mixture was incubated in dark for 15 min at room temperature. PI was added at 5 μL for 5 min staining, and cell apoptosis was measured by Gallios flow cytometry (Beckman-Coulter, US).
ROS content assay in retina
Rats were sacrificed for removing eyeballs, and retina was detached and was incubated in DCFH-DA (1:1000 dilution in serum-free 1640 culture medium) at 37°C for 30 min in dark. Retinas were then digested in 0.125% trypsin for 20 min at 37°C, followed by PBS rinsing. Cells were re-suspended in 500 μL PBS, and were measured for intracellular ROS contents using Gallios flow cytometry (Beckman-Coulter, US).
Caspase-3 enzyme activity in retinal tissues
The assay for caspase-3 activity was performed following the manual instruction of test kit. In brief, pNA standard samples were diluted in gradient, and 100 μL standards at 200 μM, 100 μM, 50 μM, 25 μM, 12.5 μM, 6.25 μM and 0 μM were added into 96-well plate or 100 μL colorimetry cubes. Absorbance values at 405 nm (A405) were measured for preparing the standard curve. 100 μL Caspase Lyssi buffer was mixed with 3~10 mg tissues, and was homogenized on ice for 5~10 min. The lysate was centrifuged at 4°C for 12000 g for 10~15 min. The supernatant was saved for measuring caspase 3 activity immediately. 65 μL assay buffer was added into 96-well plate, along with 25 μL supernatant form lysed samples, plus 10 μL Ac-DEVD-pNA (2 mM). The plate was then incubated at 37°C for 60~120 min. A405 was measured when visible color change was observed. The incubation time can be extended if there was no significant color change. A405 of test samples minus A405 of blank control group resulted in pNA absorbance values produced by casapse-3 hydrolysis products. Based on standard curve, enzyme content can be obtained, and relative enzyme activity unit was defined as A405 of experimental group divides A405 of control group times 100%.
Assay for lipid peroxidation and anti-oxidant indexes in aqueous humor
As the final product of lipid peroxidation, malondialdehyde (MDA) can be used to evaluate the level of lipid peroxidation. The assay follows manual instruction of MDA test kit. Further assays were used to measure indexes of anti-oxidant including SOD, GSH-Px and T-AOC in aqueous humor, in order to evaluate OS condition.
qRT-PCR for gene expression assay
RNA was extracted by Trizol, and was used to produce cDNA by reverse transcription using PrimeScript RT reagent kit. Using cDNA as the template, PCR amplification was performed under TaqDNA polymerase. In a total of 10 μL reaction system, one added 5.0 μL 2XSYBR Green Mixture, 0.5 μL forward/reverse primer (5 μm/L), 1 μL cDNA and ddH2O. Reverse transcription was performed at 50°C for 15 min, followed by 85°C for 5 min. PCR was performed starting from 95°C denature for 5 min, followed by 40 cycles consisting of 95°C for 15 sec and 60°C for 1 min. PCR was performed on an ABI ViiA7 fluorescent quantitative PCR cycler for data processing.
Western Blot for protein expression assay
Retinal tissues were lysed by RIPA buffer for extracting proteins. Samples (40 μg/lane) were loaded and separated in SDS-PAGE gel electrophoresis, and were transferred to PVDF membrane. The membrane was blocked in 5% defatted milk powder for 60 min at room temperature, and was incubated in primary antibody (DJ-1 at 1:300, PTEN at 1:300, p-AKT at 1:200, Bcl-2 at 1:300 and β-actin at 1:500) at 4°C overnight. Excess antibody was washed out, and secondary antibody (1:5000) was added for 60 min incubation at room temperature. ECL approach was used to expose the membrane, which was fixed and scanned for image analysis.
Statistical analysis
SPSS 18.0 was used for data analysis. All measurement data are presented as mean ± standard deviation. Comparison of measurement data between two groups was performed by 2-sample independent t-test or Mann-Whitney U test. Comparison of multiple groups was performed by one-way ANOVA, followed by Bonferroni post-hoc comparison between any two groups. A statistical significance was defined when P<0.05.
Results
Abnormal blood glucose and lipid in diabetic model rats
Among 10 model rats, two of them were removed due to unqualified blood glucose levels, obtaining eight rats with diabetic model (80% successful rate). During the experimental process, one rat died due to severe infection. The remaining seven rats accomplished the whole experiment and assays. Blood glucose level (FBG) in model rats was significantly higher than control group (P<0.001). Blood lipid assay showed significantly elevated TC, TG and LDL-C contents in model rats, plus remarkably lower HDL-C level (P<0.05, Table 1).
Table 1.
Blood glucose and lipid level in two groups of rats
| Index | Control (n=6) | DM group (n=7) | t value | P value |
|---|---|---|---|---|
| FBG (mmol/L) | 4.71±0.61 | 22.35±2.81 | 16.170 | <0.001 |
| TC (mmol/L) | 1.13±0.12 | 2.65±0.23 | 14.521 | <0.001 |
| TG (mmol/L) | 1.21±0.16 | 2.87±0.31 | 11.789 | <0.001 |
| HDL-C (mmol/L) | 0.99±0.08 | 0.86±0.06 | 3.347 | 0.003 |
| LDL-C (mmol/L) | 0.85±0.08 | 1.09±0.14 | 3.699 | 0.002 |
Enhanced cell apoptosis and ROS content in rat retinal tissues
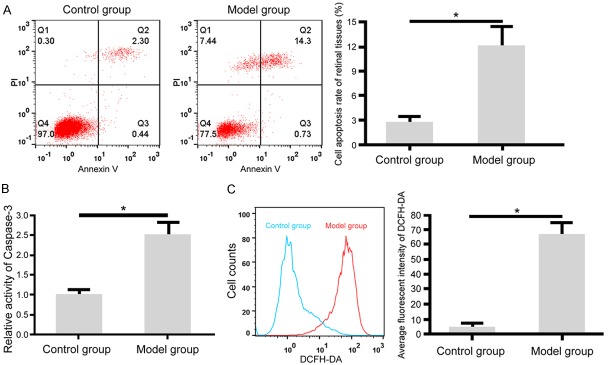
Colorimetric assay showed that compared to the control group, model rats had significantly elevated caspase-3 activity in retinal tissues (Figure 1B). Flow cytometry showed elevated cell apoptosis in retinal tissues of model rats compared to control group (Figure 1A). DCFH-DA staining showed significantly higher ROS contents in model rat retinal tissues than control rats (Figure 1C).
Figure 1.
Enhanced cell apoptosis and ROS content in diabetic rat retinal tissues. A: Flow cytometry for retinal cell apoptosis; B: Colorimetric assay for measuring caspase-3 activity in rat retinal tissues; C: Flow cytometry for rat retinal tissue ROS content. *, P<0.05 comparing between two groups.
Enhanced OS and suppressed anti-oxidant potency in diabetic rat aqueous humor
Lipid peroxidation assay showed that MDA content in model rat aqueous humor was increased by 4.54-fold (P<0.001 compared to the control group). Anti-oxidant indexes showed remarkably lower SOD, GSH-Px and anti-oxidant potency in aqueous humor of model rats (P<0.05 comparing to control rats, Table 2).
Table 2.
MDA and anti-oxidant assay in rat aqueous humor
| Index | Control (n=6) | DM group (n=7) | t value | P value |
|---|---|---|---|---|
| MDA (μM) | 9.67±1.23 | 43.86±3.69 | 23.065 | <0.001 |
| SOD (mU/mL) | 69.22±7.13 | 43.59±5.81 | 7.149 | <0.001 |
| GSH-Px (mU/mL) | 21.66±3.12 | 15.91±2.86 | 3.418 | 0.003 |
| T-AOC (mM) | 2.43±0.29 | 1.52±0.19 | 6.797 | <0.001 |
Significantly enhanced Nrf2/ARE signal pathway in diabetic rat retinal tissues
qRT-PCR assay showed that, compared to control rats, the model group showed significantly decreased expression of Keap1 mRNA in retinal tissues, plus enhanced Nrf2 mRNA expression (Figure 2A). Western blot showed that, compared to control rats, Keap1 protein expression was remarkably decreased, while Nrf2 protein expression was elevated (Figure 2B).
Figure 2.
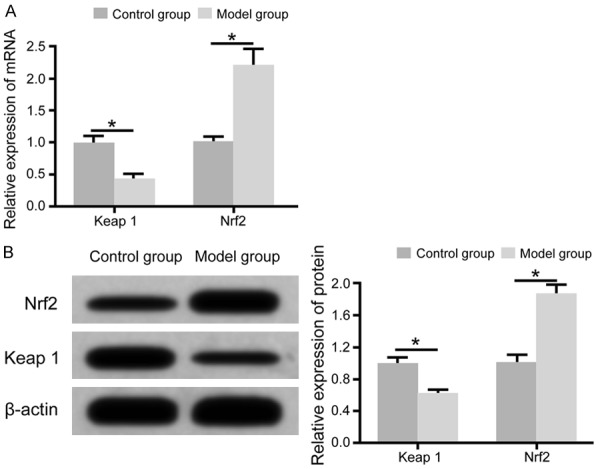
Significantly enhanced Nrf2/ARE pathway activity in diabetic rat retinal tissues. A: qRT-PCR for Keap1 and Nrf2 mRNA expression in retina; B: Western blot for Keap1 and Nrf2 protein expression in retina. *, P<0.05 comparing between two groups.
Discussion
Diabetic mellitus (DM) is one group of metabolic disorders of proteins, lipids, and electrolytes caused by insulin secretion deficient or decreased insulin sensitivity of target tissues, and is mainly featured as abnormally elevated blood glucose [10,11]. With life style transition and diet habits, the incidence of DM is progressively increasing. About 95.2% of DM patients belong to type 2 diabetic mellitus (T2DM), which is the most popular metabolic disorder with severe healthy burdens [12,13]. DR is one retinal disease caused by retinal micro-vessel lesion, and frequently occurs in DM patients. In adolescence, about 80%~90% blinded cases are caused by DR, and this figure is still about 30%~40% in adults [14]. DR can cause a class of symptoms including eye vascular aneurysm, bleeding plaque, sclerosis exfiltration, venous string, intra-retinal microvascular abnormality (IRAM) and macular edema, severely affecting patient visual function and life quality [15].
OS is one major pathogenic mechanism of DR, and is the common initiating pathway under various patho-physiological processes of DR. Hyperglycemia or DM can potentiate body OS via multiple mechanism to produce large amounts of ROS. Under OS conditions, the body will initiate and activate endogenous Keap1-Nrf2-ARE signal pathway. Keap-1-Nrf2-ARE signal pathway can modulate various downstream proteasome and type II detoxifying enzymes to antagonize body OS damage, thus relieving inflammatory damage and cell apoptosis, thus exerting the role for protecting cells from OS injury [16,17].
Nrf2 is one family member of cap-n-collar (CNC) lysine transcription activators, and is one newly identified transcription factor very sensitive to OS. As the critical and central regulatory protein in cellular OS response, Nrf2 has seven Neh structural domain (Nrf2-ECH homology), among which Neh1 domain plays a role in binding and recognizing ARE for facilitating target gene transcription. Neh2 mainly binds with Kelch-lie ECH-associated protein-1 (Keap1) [18,19], which locates on 19p13.2 site, and can negatively regulate Nrf2 transcriptional activity via binding onto DGR domain for anchoring into cytoplasm with actin [20-22]. ARE is one DNA fragment with specific sequence, and locates on the 5’-promoter region of various type II detoxifying enzyme and anti-oxidase gene. After such promoting DNA fragment, there are SOD, GST, hemoglobin oxidase (HO)-1, NADPH, quinone oxidoreductase 1 (NQO1), glutamine cystine ligase (GCL), and catalase (CAT), all of which can be activated by various oxidative and electrophilic compounds [22-24]. Under a non-OS state, Keap1 anchors Nrf2 into cytoplasm to suppress its expression and trans-regulatory activity. When under an OS state, ROS can change the conformation of Keap1, depriving its potency for coupling Nrf2. Such de-coupled Nrf2 can bind with nuclear ARE, and up-regulate the expression of various genes including phase II detoxifying enzymes, anti-oxidant proteinase and ubiquitinating enzymes [24-27]. This study generated a T2DM DR rat model, and used normal rats as the control group to measure the expression of Nrf2/ARE pathway in rat retinal tissues. We also measured the content of lipid peroxidation produce and multiple anti-oxidant factors in rat aqueous humor, thus investigating the role and mechanism of Keap1-Nrf2-ARE signal pathway in DR from the perspective of OS regulation.
Insulin resistance and insulin secretion dysfunction are major patho-physiological mechanism of T2DM pathogenesis. This study used high-fat and high-glucose diet to induce insulin resistance in rats, and injected STZ to damage islet B cells, leading to compensatory insulin secretion insufficiency to generate T2DM model [28]. Test results showed abnormally elevated blood glucose and blood lipid levels in model rats after 12-week, indicating successful generation of T2DM. Test results on separated rat retina showed significantly elevated retinal apoptosis in model rats, plus higher ROS content, indicating major OS in model rat retina, and possible role of ROS in retinal cell apoptosis. Cao et al. [29] showed that under high-glucose stress condition, ROS content in retinal ganglion cells was remarkably elevated, with major enhancement of cell apoptosis. Sun et al. [30] showed that under DM condition, rat retinal cells showed remarkably elevated cell apoptosis and ROS level, indicating important role of ROS and cell apoptosis in DR. All these studies supported our observation showing elevated cell apoptosis and ROS content in DM rat retinal tissues. MDA is the most commonly used index evaluating body OS, and its level can reflect the status of lipid peroxidation of the body, and indirectly reflecting tissue oxidation injury condition. This study showed abnormally elevated MDA content in DM rat retinal tissues, plus remarkably decreased anti-oxidase index and anti-oxidation potency. Further assays found even lower Keap1 expression plus higher Nrf2 expression in DM model retinal tissues, indicating initiating of endogenous OS system in DM rat retina. Sun et al. [30] found that under DM condition, rat retinal tissues had remarkably elevated MDA content, while SOD and GSH-Px level were significantly decreased, and such diabetic status can further induce up-regulation of Nrf2 expression. Treatment using grape seed proanthocyanidin extract (GSPE) can further elevate Nrf2 expression, decrease retinal cell apoptosis and suppress ROS content. Cao et al. [29] found that under high glucose induction, retinal ganglion cells had elevated apoptosis and ROS content, accompanied by suppressed cellular anti-oxidation indexes including SOD, GSH-Px and T-AOC, whilst OS protein Nrf2 expression was enhanced, and the negative regulatory protein for Nrf2 called Keap1 was down-regulated. Xu et al. [31] showed that in DM model mouse retinal tissues, Nrf2 expression and nuclear translocation were remarkably enhanced, with potentiated peroxidation products. These results showed that although the body has initiated OS mechanism, the real anti-oxidation potency of the body is still lower, with abnormally higher ROS substances. All these observations are consistent with our results showing elevated Keap1-Nrf2-ARE signal pathway activity in mouse retinal tissues, while OS substances MDA content was still elevated along with lower levels of anti-oxidase and T-AOC. Xu et al. [31] further showed that after Nrf2 gene knockout, DM mouse had aggravated retina-blood barrier structure or function disruption, leading to release of inflammatory factors, and elevated ROS content and retinal cell apoptosis. These results further confirm the role of Keap1-Nrf2-ARE activation in alleviating OS damage and in protecting the retina.
Acknowledgements
This work was supported by the Medical and Health Science Technology Department of Shandong Province (No. 2017WS739) and weifang science and technology bureau (NO. 2016YX018).
Disclosure of conflict of interest
None.
References
- 1.Rhee SY, Woo JT. Response: features of long-standing Korean type 2 diabetes mellitus patients with diabetic retinopathy: a study based on standardized clinical data. Diabetes Metab J. 2017;41:494–495. doi: 10.4093/dmj.2017.41.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shahsuvaryan ML. Diabetic retinopathy: in need of multidimensional pharmacotherapy. QJM. 2018;111:205–206. doi: 10.1093/qjmed/hcx247. [DOI] [PubMed] [Google Scholar]
- 3.Petrovski G, Kaarniranta K, Petrovič D. Oxidative stress, epigenetics, environment, and epidemiology of diabetic retinopathy. J Diabetes Res. 2017;2017:6419357. doi: 10.1155/2017/6419357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabanayagam C, Yip W, Ting DS, Tan G, Wong TY. Ten emerging trends in the epidemiology of diabetic retinopathy. Ophthalmic Epidemiol. 2016;23:209–222. doi: 10.1080/09286586.2016.1193618. [DOI] [PubMed] [Google Scholar]
- 5.Mustapha NM, Tarr JM, Kohner EM, Chibber R. NADPH oxidase versus mitochondria-derived ROS in glucose-induced apoptosis of pericytes in early diabetic retinopathy. J Ophthalmol. 2010;2010:746978. doi: 10.1155/2010/746978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Xu X, Sheng M, Zhang X, Gu Q, Zheng Z. PRMT-1 and DDAHs-induced ADMA upregulation is involved in ROS- and RAS-mediated diabetic retinopathy. Exp Eye Res. 2009;89:1028–1034. doi: 10.1016/j.exer.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Wang K, Chen Z, Huang L, Meng B, Zhou X, Wen X, Ren D. Naringenin reduces oxidative stress and improves mitochondrial dysfunction via activation of the Nrf2/ARE signaling pathway in neurons. Int J Mol Med. 2017;40:1582–1590. doi: 10.3892/ijmm.2017.3134. [DOI] [PubMed] [Google Scholar]
- 8.Tabei Y, Murotomi K, Umeno A, Horie M, Tsujino Y, Masutani B, Yoshida Y, Nakajima Y. Antioxidant properties of 5-hydroxy-4-phenyl-butenolide via activation of Nrf2/ARE signaling pathway. Food Chem Toxicol. 2017;107:129–137. doi: 10.1016/j.fct.2017.06.039. [DOI] [PubMed] [Google Scholar]
- 9.Luo C, Urgard E, Vooder T, Metspalu A. The role of COX-2 and Nrf2/ARE in anti-inflammation and antioxidative stress: aging and anti-aging. Med Hypotheses. 2011;77:174–178. doi: 10.1016/j.mehy.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Wang J, Zhang X, Zhu H. Inverse relationship between serum bilirubin levels and diabetic foot in Chinese patients with type 2 diabetes mellitus. Med Sci Monit. 2017;23:5916–5923. doi: 10.12659/MSM.907248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu T, Weng Z, Pei C, Yu S, Chen Y, Guo W, Wang X, Luo P, Sun J. The relationship between neutrophil-to-lymphocyte ratio and diabetic peripheral neuropathy in type 2 diabetes mellitus. Medicine (Baltimore) 2017;96:e8289. doi: 10.1097/MD.0000000000008289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bastawrous A, Mathenge W, Wing K, Bastawrous M, Rono H, Weiss HA, Macleod D, Foster A, Peto T, Blows P, Burton M, Kuper H. The incidence of diabetes mellitus and diabetic retinopathy in a population-based cohort study of people age 50 years and over in Nakuru, Kenya. BMC Endocr Disord. 2017;17:19. doi: 10.1186/s12902-017-0170-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strauss MB, Moon H, La S, Craig A, Ponce J, Miller S. The incidence of confounding factors in patients with diabetes mellitus hospitalized for diabetic foot ulcers. Wounds. 2016;28:287–294. [PubMed] [Google Scholar]
- 14.Takagi H. Novel strategy for screening of diabetic retinopathy. J Diabetes Investig. 2018;9:726–727. doi: 10.1111/jdi.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiao X, Li G, Yin X. Study on clinical indicators of diabetic retinopathy. Minerva Endocrinol. 2017 doi: 10.23736/S0391-1977.17.02769-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Zhong Q, Mishra M, Kowluru RA. Transcription factor Nrf2-mediated antioxidant defense system in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013;54:3941–3948. doi: 10.1167/iovs.13-11598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batliwala S, Xavier C, Liu Y, Wu H, Pang IH. Involvement of Nrf2 in ocular diseases. Oxid Med Cell Longev. 2017;2017:1703810. doi: 10.1155/2017/1703810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandey P, Singh AK, Singh M, Tewari M, Shukla HS, Gambhir IS. The see-saw of Keap1-Nrf2 pathway in cancer. Crit Rev Oncol Hematol. 2017;116:89–98. doi: 10.1016/j.critrevonc.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Taguchi K, Yamamoto M. The KEAP1-NRF2 system in cancer. Front Oncol. 2017;7:85. doi: 10.3389/fonc.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmoll D, Engel CK, Glombik H. The Keap1-Nrf2 protein-protein interaction: a suitable target for small molecules. Drug Discov Today Technol. 2017;24:11–17. doi: 10.1016/j.ddtec.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Chen JY, Zhu GY, Su XH, Wang R, Liu J, Liao K, Ren R, Li T, Liu L. 7-deacetylgedunin suppresses inflammatory responses through activation of Keap1/Nrf2/HO-1 signaling. Oncotarget. 2017;8:55051–55063. doi: 10.18632/oncotarget.19017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng D, Wu R, Guo Y, Kong AN. Regulation of Keap1-Nrf2 signaling: the role of epigenetics. Curr Opin Toxicol. 2016;1:134–138. doi: 10.1016/j.cotox.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He L, Li P, Yu LH, Li L, Zhang Y, Guo Y, Long M, He JB, Yang SH. Protective effects of proanthocyanidins against cadmium-induced testicular injury through the modification of Nrf2-Keap1 signal path in rats. Environ Toxicol Pharmacol. 2017;57:1–8. doi: 10.1016/j.etap.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Zhang JC, Yao W, Dong C, Han M, Shirayama Y, Hashimoto K. Keap1-Nrf2 signaling pathway confers resilience versus susceptibility to inescapable electric stress. Eur Arch Psychiatry Clin Neurosci. 2017 doi: 10.1007/s00406-017-0848-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.David JA, Rifkin WJ, Rabbani PS, Ceradini DJ. The Nrf2/Keap1/ARE pathway and oxidative stress as a therapeutic target in type II diabetes mellitus. J Diabetes Res. 2017;2017:4826724. doi: 10.1155/2017/4826724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keleku-Lukwete N, Suzuki M, Yamamoto M. An overview of the advantages of KEAP1-NRF2 system activation during inflammatory disease treatment. Antioxid Redox Signal. 2017 doi: 10.1089/ars.2017.7358. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Zhu C, Dong Y, Liu H, Ren H, Cui Z. Hesperetin protects against H2O2-triggered oxidative damage via upregulation of the Keap1-Nrf2/HO-1 signal pathway in ARPE-19 cells. Biomed Pharmacother. 2017;88:124–133. doi: 10.1016/j.biopha.2016.11.089. [DOI] [PubMed] [Google Scholar]
- 28.Davidson EP, Holmes A, Coppey LJ, Yorek MA. Effect of combination therapy consisting of enalapril, α-lipoic acid, and menhaden oil on diabetic neuropathy in a high fat/low dose streptozotocin treated rat. Eur J Pharmacol. 2015;765:258–267. doi: 10.1016/j.ejphar.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Cao Y, Li X, Wang CJ, Li P, Yang B, Wang CB, Wang LX. Role of NF-E2-related factor 2 in neuroprotective effect of l-carnitine against high glucose-induced oxidative stress in the retinal ganglion cells. Biomed Pharmacother. 2015;69:345–348. doi: 10.1016/j.biopha.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Xiu C, Liu W, Tao Y, Wang J, Qu YI. Grape seed proanthocyanidin extract protects the retina against early diabetic injury by activating the Nrf2 pathway. Exp Ther Med. 2016;11:1253–1258. doi: 10.3892/etm.2016.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Z, Wei Y, Gong J, Cho H, Park JK, Sung ER, Huang H, Wu L, Eberhart C, Handa JT, Du Y, Kern TS, Thimmulappa R, Barber AJ, Biswal S, Duh EJ. NRF2 plays a protective role in diabetic retinopathy in mice. Diabetologia. 2014;57:204–213. doi: 10.1007/s00125-013-3093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]