Abstract
Objective: To investigate the effect of silencing information regulator 6 (SIRT6) on HIF1α expression of cell line A549 in non-small cell lung cancer and on tumor angiogenesis in lung cancer. Methods: Cell line A549 in the logarithmic growth phase was transfected with Ad-SIRT6 and Ad-null respectively. According to the study design, the cells were divided into control group, Ad-null group and Ad-SIRT6 group. The HIF1α and HIF2α mRNA expression in each group were detected by real-time quantitative PCR (qPCR). The level of prolyl hydroxylase (PHD) 1-3 after 48 h of Ad-SIRT6-transfected cell line A549 and the levels of VEGF-C, VEGF-D, VEGFR-2 and VEGFR-3 in the supernatants were determined by ELISA. The nude mice were injected subcutaneously with Ad-null or Ad-SIRT6 transfected cell line A549. The tumor volume was observed at 6, 12, 18, 24 and 30 d after inoculation, and the tumor mass was weighed at 30 d. Also, microvessel density (MVD) and the number of positive HIF1α and VEGF cells were detected by immunohistochemistry. The VEGF and HIF1α levels in tumor tissue were detected by ELISA and qPCR respectively. Results: qPCR showed that the levels of HIF-1α mRNA, VEGF-C, VEGF-D, VEGFR-2 and VEGFR-3 in the supernatant were decreased, the level of PHD2 was increased (P<0.05), and the levels of HIF-2α mRNA, PHD1 and PHD3 did not change much (P>0.05) in the Ad-SIRT6 group as compared with those in the control group and Ad-null group. The tumor growth rate was decreased, and the tumor volume at 12-30 d after inoculation was less in the Ad-SIRT6 group than in the control group and Ad-null group (P<0.05); the tumor mass was also lower than that of control and Ad-null groups (P<0.05). Immunohistochemistry showed that MVD and the number of HIF-1α and VEGF positive cells were less in the Ad-SIRT6 group than in control and Ad-null groups (P<0.05); and HIF-1α and VEGF levels in tumor tissue were decreased in the Ad-SIRT6 group compared to the control and Ad-null groups (P<0.05). There were no significant differences in the above measurements between the control group and Ad-null group (P>0.05). Conclusion: SIRT6 overexpression can inhibit HIF1α and VEGF expression, promoting PHD2 expression, which can inhibit angiogenesis and xenograft growth and may play a role in reducing HIF1α and VEGF expression.
Keywords: Non-small cell lung cancer, SIRT6, HIF1α expression, tumor angiogenesis
Introduction
Metabolic reprogramming is one of the basic characteristics of cancer cells, many of which generate energy by increasing aerobic glycolysis rather than oxidative metabolism [1]. Histone deacetylases (HDAC) may play an important role in this process [2]. Sirtuin, a highly conservative protein family, is a nicotinamide adenine dinucleotide (NAD+) -dependent HDAC and/or mono-ADP-ribosyltransferase. Mammals have seven subcellular localization sirtuins (SIRT1-7), among which SIRT1, SIRT6 and SIRT7 are located in the nucleus, SIRT2 in the cytoplasm, and SIRT4, SIRT5 in the mitochondria. SIRT6 has been confirmed to be involved in various cellular processes, including metabolism and aging [2-4]. In addition, the SIRT6 gene, a tumor suppressor gene, regulates glycolysis to inhibit the transcriptional activity of the Myc gene which is the biosynthesis suppressor gene of ribosomes [3,4]. Protein degradation of HIF1α is inhibited during hypoxia, thereby up-regulating VEGF expression and promoting angiogenesis [5,6]. However, the effect of the regulation of energy metabolism by SIRT6 on HIF1α and VEGF expression and angiogenesis has still been unknown. In this study, we aimed to explore the effect of SIRT6 on HIF1α expression and angiogenesis in lung cancer through transfecting cell line A549 in non-small cell lung cancer with lentivirus-mediated SIRT6 overexpression and constructing a xenograft model. The study was sponsored by the Shanghai Natural Science Foundation (15ZR1434300).
Materials and methods
Cells and reagents
Cell line A549 were purchased from Shanghai Cell Bank of the Chinese Academy of Sciences, RPMI 1640 medium and fetal bovine serum (FBS) from Gibco, USA, Ad-null and Ad-SIRT6 from Suzhou GenePharma, Lipofectamine ™ 2000, Trizol from Invitrogen Corporation, penicillin/streptomycin from Hyclone USA, PrimeScript ™ RT-PCR kit, and SYBR® Premix ExTaq ™ II kit from Japan TaKaRa company, HIF-1α, HIF-2α primers from Shanghai Bioengineering Co., Ltd., VEGF-C, VEGF-D, VEGFR-2 and VEGFR-3 detection kits from Shenzhen Jingmei Biotech Co., Ltd., PHD 1-3 detection kits from R&D Systems, USA, Fluorescence labeled CD34 mAb from Miltenyi Biotech, Germany, HIF-1α and VEGF monoclonal antibodies from Santa Cruz Biotech, and immunohistochemical and DAB reagent from Beijing Zhongshan Company.
Cell transfection and grouping
The cells were cultured in RPMI 1640 medium containing 10% FBS, incubated at 37°C in a saturated humidity incubator with 5% CO2, subcultured to the required number for the experiments in trypsin/EDTA in a 1:2 ratio with cell fusion of 80%-90%, resuspended in RPMI-1640 culture medium, inoculated into six-well plates at a density of 4 × 105 cells/well, and transfected with 200 pfu/cell after cell attachment in the Ad-SIRT6 group or Ad-null group. Cell line A549 without transfection was used as the control group.
Detection of HIF-1α and HIF-2α mRNA levels by QPCR
Cell line A549 transfected with Ad-SIRT6 of different intensity for 24 h and 48 h were collected. The total RNA was extracted with Trizol after the lysate was added and its purity and concentration were determined by NanoDrop 2000. 500 ng RNA was taken from each sample, and cDNA was synthesized using the PrimeScript™ RT-PCR kit following the instructions. Also, 1 μl cDNA was taken from each sample as template for qPCR on ABI 7300 fluorescent quantitative PCR. A total of 20 μl was used in the reaction system, containing 1 μl of each upstream primer (10 μmol/L), 2 μl of 2 × dNTP mix and 10 μl of 2 × SYBR® Premix ExTaq ™, and ddH2O was used to make up to 20 μl for insufficiency. The reaction conditions: denaturation at 94°C for 5 min, denaturation at 94°C for 30 s, annealing at 60.4°C for 45 s, extension at 72°C for 1 min, 35 cycles, and final extension at 72°C for 10 min. The relative expression levels of HIF-1α and HIF-2α were calculated by 2-ΔΔCt method with GAPDH as an internal reference. HIF-1α upstream primer: 5’-CCTGGGGAGCGTGGGGAGCGTTAGAGAGA-3’; downstream primer: 5’-GGGTACCAGGGTGAGTCCAAGA-3’; HIF-2α upstream primers: 5’-CGCGTGTTAGGAGAGAGAGGGACT-3’; 5’-GGTAC GGTCGGCAAGAGAAGTC-3’; GAPDH upstream primer: 5’-AGGTCGGTGTGAACGGATTTG-3’; downstream primer: 5’-TGTAGACCATGTAGTTGAGGTCA-3’.
Detection of PHD1-3 level
The cells transfected for 48 h were collected from each group, fully lysed after the addition of lysate, and centrifugated at 12000 r/min for 10 min to collect the supernatant. The PHD1-3 level was detected by kit in strict accordance with the instructions.
Detection of VEGF-C, VEGF-D, VEGFR-2 and VEGFR-3 levels
The supernatants transfected for 48 h were collected from each group. The levels of VEGF-C, VEGF-D, VEGFR-2 and VEGFR-3 were detected by kit in strict accordance with the instructions.
Xenograft model establishment and animal grouping
Thirty nude mice were randomly divided into 3 groups (10 in each group): control group, Ad-null group and Ad-SIRT6 group. A549 cells transfected with Ad-SIRT6 plasmid were inoculated in Ad-SIRT6 group, A549 cells transfected with Ad-null plasmid in Ad-null group, and A549 cells without transfection in control group. A549 cells were trypsinized by 0.25% trypsin, and single cell suspension was prepared by adding PBS at a concentration of 1 × 107 cells/ml for modeling. The skin of nude mice was disinfected with iodophor and inoculated subcutaneously at 0.1 ml each, and put back into cages to observe the tumor formation daily with the tumor formation standard of subcutaneous tumor diameter >0.5 cm.
Detection of xenograft volume and mass
The volume of tumor at 6, 12, 18, 24 and 30 d after inoculation was observed, and the long and short diameters of tumor were measured by vernier caliper. The volume of tumor = long diameter × short diameter 2 × 0.5 was calculated according to the formula and the growth curve was drawn. After 30 d of inoculation, the tumor tissue was taken, weighed to record the mass, and rapidly stored in liquid nitrogen for subsequent molecular experiments.
Evaluation of tumor angiogenesis
MVD and number of HIF1α and VEGF positive cells in tumor tissue were detected by immunohistochemistry. VEGF and α levels in tumor tissue were detected by ELISA and qPCR respectively.
Statistics analysis
All analyses were performed using SPSS 18.0 software. All data were expressed as X ± SD. Comparison was performed using ANOVA among multiple groups and using t-test between two groups. P<0.05 was regarded as statistically significant.
Results
HIF-1α and HIF-2α mRNA levels in Ad-SIRT6-transfected cell line A549 were detected by qPCR at 24 and 48 h after transfection. The results showed that compared with the control and Ad-null groups, HIF-1α mRNA level was decreased (P<0.05) and HIF-2α mRNA level did not change much in the Ad-SIRT6 group (P>0.05). There were no significant differences in HIF-1α and HIF-2α mRNA levels between the control group and Ad-null group (P>0.05). The PHD1-3 level in Ad-SIRT6-transfected cell line A549 was measured by ELISA at 48 h. The results showed that compared with the control and Ad-null groups, the PHD2 level was increased (P<0.05), while PHD1 and PHD3 level changed little in the Ad-SIRT6 group (P>0.05) (Figures 1 and 2).
Figure 1.
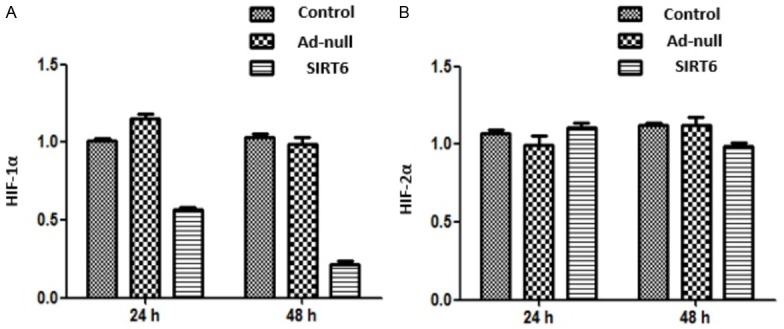
Effect of SIRT6 overexpression on HIF-1α and HIF-2α expression in cell line A549.
Figure 2.
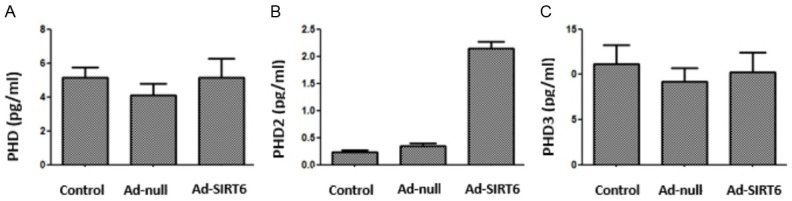
Effect of SIRT6 overexpression on PHD1-3 expression in cell line A549. 2 Effect of SIRT6 overexpression on VEGF-C, VEGF-D, VEGFR-2 and VEGFR-3 expression in lung cancer cell line A549.
The levels of VEGF-C, VEGF-D, VEGFR-2 and VEGFR-3 in supernatants of Ad-SIRT6-transfected cell line A549 were detected by ELISA. The results showed that compared with control and Ad-null groups, the levels of VEGF-C, VEGF-D, VEGFR-2 and VEGFR-3 were decreased in the SIRT6 group (P<0.05). There were no statistically significant differences in the levels of VEGF-C, VEGF-D, VEGFR-2 and VEGFR-3 between the control and Ad-null groups. (P>0.05) (Figure 3).
Figure 3.

Effect of SIRT6 overexpression on VEGF expression in lung cancer cell line A549.
SIRT6 overexpression inhibits xenograft growth in A549 nude mice
The tumor growth rate was decreased in Ad-SIRT6 group and the tumor volume at 12-30 d after inoculation was less than that in the control and Ad-null groups (P<0.05). Moreover, the tumor mass was less in the Ad-SIRT6 group than in the control and Ad-null groups (P<0.05). There were no statistically significant differences in tumor volume and mass between the control and Ad-null groups. See Figure 4.
Figure 4.
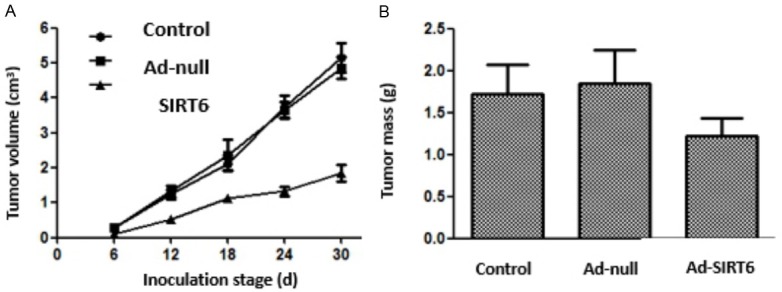
Effect of SIRT6 overexpression on the growth of xenografts in nude mice.
SIRT6 overexpression inhibits xenograft angiogenesis in A549 nude mice
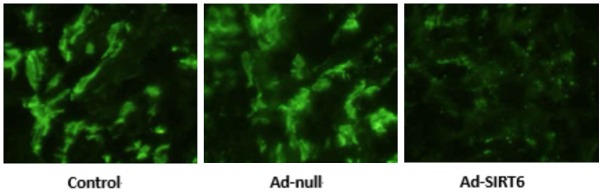
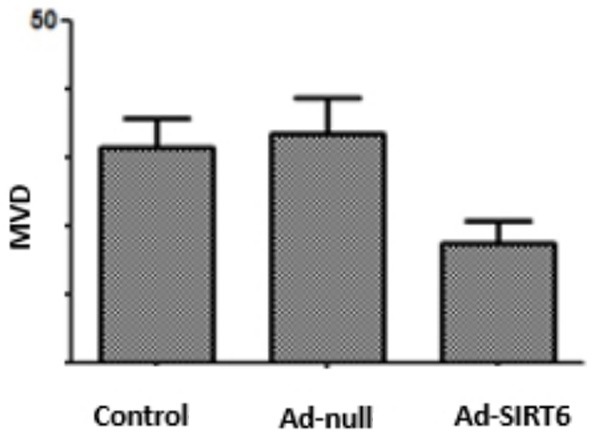
Immunohistochemistry showed that MVD level was lower in the Ad-SIRT6 group than in the control and Ad-null groups (P<0.05). There were no statistically significant differences in MVD level between the control and Ad-null groups (P>0.05). See Figure 5.
Figure 5.
Effect of SIRT6 overexpression on MVD level of xenografts in nude mice.
SIRT6 overexpression inhibits the xenograft angiogenesis-related factors in A549 nude mice
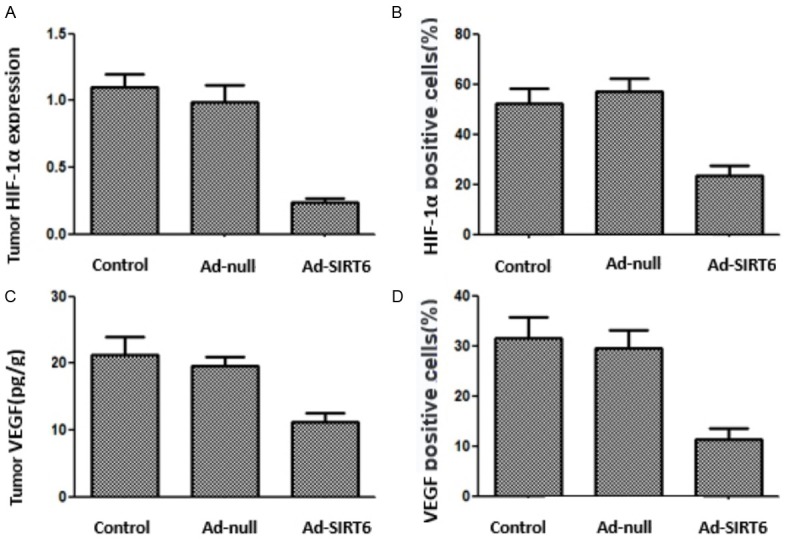
qPCR showed that the HIF-1α mRNA level was lower in the Ad-SIRT6 group than in the control and Ad-null groups (P<0.05). ELISA disclosed that the VEGF level was lower in the Ad-SIRT6 group than in the control and Ad-null groups (P<0.05). Immunohistochemistry showed that the number of HIF-1α and VEGF-positive cells was less in Ad-SIRT6 group than in the control and Ad-null groups (P<0.05). There were no statistically significant differences in above indicators between the control and Ad-null groups (P>0.05) (Figures 6 and 7).
Figure 6.
SIRT6 overexpression inhibits xenograft angiogenesis-related factors in A549 nude mice.
Figure 7.
Immunohistochemical staining of xenograft HIF-1α and VEGF in nude mice with SIRT6 overexpression.
Discussion
Cell proliferation is uncontrolled in cancer. Cells become malignant by acquiring a range of abilities that allow them to avoid the strict regulatory loop controlling cell proliferation and homeostasis [7]. Genomic instability promotes cell carcinogenesis by generating a genomic environment that facilitates mutation. Metabolic reprogramming is an important feature of cancer cells that meets the needs of cancer cells for new energy [8,9]. Under normal conditions, cells rely on mitochondrial oxidative phosphorylation to generate glucose energy, and cancer cells show enhanced glycolysis. Pyruvate produced from glucose generates lactate in the cytoplasm rather than being involved in the TCA cycle of the mitochondria. Since oxygen is confined to mitochondria during pyruvate oxidation, anaerobic glycolysis of normal cells is usually activated under hypoxic conditions. Even under normoxic conditions, cancer cells are still obviously active in glycolysis metabolism. In addition, due to the unique hypoxic environment of the tumor, this metabolic change may also bring survival advantages to the tumor cells [10]. The pressure on cells in living organisms that are subject to nutrient fluctuations and genotoxic damage can affect the integrity of the genomes. SIRT6 regulates many cancer-related pathways, including ensuring genomic stability by regulating DNA double-strand breaks and base excision repair (BER), and inhibiting inflammation and maintaining telomere stability via NFκB [11-13]. Moreover, SIRT6, a highly conserved NAD+-dependent HDAC, is involved in processes such as metabolism, resistance, and longevity. SIRT6 as a histone H3K9 acetyl regulates the expression of proglycolytic genes. Hypoxia-inducible factor (HIF), an important transcription factor of cells in response to hypoxia, enables cells to adapt to a hypoxic microenvironment, maintain energy me-tabolism, and promote angiogenesis by regulating the expression of target genes, which are closely related to the tumor [14,15]. This study aimed to explore whether HIF-1α is involved in the regulation of energy metabolism by SIRT6 during tumor progression.
We found that SIRT6 overexpression in cell line A549 can reduce the HIF-1α level, suggesting that SIRT6 may regulate tumor energy metabolism via HIF-1α. Although HIF-2α plays an important role in the chronic hypoxic adaptation of tumor cells [16], this study found no effect on HIF-2α. SIRT6-deficient cells show not only increases in HIF1α activity and glycolytic glucose uptake, but also a decrease in mitochondrial respiration [17]. PHD, a key molecule regulating HIF-1α, mediates its degradation by catalyzing the hydroxylation of proline residues of HIF, which is the rate-limiting enzyme in the whole process [18]. To investigate the possible mechanism of SIRT6 inhibition of HIF-1α expression, this study further analyzed the effect of SIRT6 overexpression on the expression of PHD1-3, a major member of the PHD family. The results showed that only PHD2 level was elevated in PHD1-3 and PHD2 was the key enzyme of HIF-1α degradation [19]. Thus, the study further shows that SIRT6 reduces HIF-1α via down-regulating PHD2 expression. Furthermore, the present study found that SIRT6 overexpression can reduce the level of angiogenic factors. Given that HIF-1α plays an important role in promoting angiogenesis, this study speculated that SIRT6 can reduce VEGF-C, VEGF-D, VEGFR-2 and VEGFR-3 levels via inhibiting HIF-1α. The results of the research on xenografts showed that SIRT6 overexpression also inhibited HIF-1α expression and decreased VEGF level, which agrees with the results of the cell experiment that SIRT6 overexpression inhibited the angiogenesis of xenografts, suggesting that SIRT6 has anti-angiogenesis effects and is a potential target for the treatment of lung cancer.
In summary, SIRT6 overexpression can inhibit HIF1α and VEGF expression, promote PHD2 expression, and inhibit the growth of xenograft and angiogenesis by decreasing HIF1α and VEGF expression.
Acknowledgements
This study was funded by the Shanghai Natural Science Fund (No.15ZR1434300).
Disclosure of conflict of interest
None.
References
- 1.Li C, Zhang G, Zhao L, Ma Z, Chen H. Metabolic reprogramming in cancer cells: glycolysis, glutaminolysis, and Bcl-2 proteins as novel therapeutic targets for cancer. World J Surg Oncol. 2016;14:1–7. doi: 10.1186/s12957-016-0769-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, Teruya-Feldstein J, Moreira PI, Cardoso SM, Clish CB, Pandolfi PP, Haigis MC. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1α destabilization. Cancer Cell. 2011;19:416–28. doi: 10.1016/j.ccr.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kugel S, Sebastián C, Fitamant J, Ross KN, Saha SK, Jain E, Gladden A, Arora KS, Kato Y, Rivera MN, Ramaswamy S, Sadreyev RI, Goren A, Deshpande V, Bardeesy N, Mostoslavsky R. SIRT6 suppresses pancreatic cancer through control of Lin28b. Cell. 2016;165:1401. doi: 10.1016/j.cell.2016.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan H, Di G, Liu X, Li J, Wang L, Wu J, Zhou J, Zhang W, Ren R, Zhang W, Li Y, Yang J, Hao Y, Yuan T, Yuan G, Wang H, Ju Z, Mao Z, Li J, Qu J, Tang F, Liu GH. SIRT6 safeguards human mesenchymal stem cells from oxidative stress by coactivating NRF2. Cell Res. 2016;26:190. doi: 10.1038/cr.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang HW, Baxter LL, Loftus SK, Cronin JC, Trivedi NS, Borate B, Pavan WJ. Distinct microRNA expression signatures are associated with melanoma subtypes and are regulated by HIF1A. Pigment Cell Melanoma Res. 2015;27:777–787. doi: 10.1111/pcmr.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yalu R, Oyesiji AE, Eisenberg I, Imbar T, Meidan R. HIF1A-dependent increase in endothelin 2 levels in granulosa cells: role of hypoxia, LH/cAMP, and reactive oxygen species. Reproduction. 2015;149:11–20. doi: 10.1530/REP-14-0409. [DOI] [PubMed] [Google Scholar]
- 7.Weinberg SE, Chandel NS. Targeting mitochondria metabolism for cancer therapy. Nat Chem Biol. 2015;11:9–15. doi: 10.1038/nchembio.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Zhang C, Hu W, Feng Z. Tumor suppressor p53 and its mutants in cancer metabolism. Cancer Lett. 2015;356:197–203. doi: 10.1016/j.canlet.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16:619. doi: 10.1038/nrc.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Courtnay R, Ngo DC, Malik N, Ververis K, Tortorella SM, Karagiannis TC. Cancer metabolism and the Warburg effect: the role of HIF-1 and PI3K. Mol Biol Rep. 2015;42:841–851. doi: 10.1007/s11033-015-3858-x. [DOI] [PubMed] [Google Scholar]
- 11.Xu Z, Zhang L, Zhang W, Meng D, Zhang H, Jiang Y, Xu X, Van Meter M, Seluanov A, Gorbunova V, Mao Z. SIRT6 rescues the age related decline in base excision repair in a PARP1-dependent manner. Cell Cycle. 2015;14:269–276. doi: 10.4161/15384101.2014.980641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park SJ, Huh JE, Shin J, Park DR, Ko R, Jin GR, Seo DH, Kim HS, Shin HI, Oh GT, Kim HS, Lee SY. Sirt6 cooperates with Blimp1 to positively regulate osteoclast differentiation. Sci Rep. 2016;6:26186. doi: 10.1038/srep26186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen W, Chen X, Sun Y. SIRT6 protects against lipopolysaccharide (LPS)-induced apoptosis in human dental pulp cells by deacetylation of Ku70. Biochem Biophys Res Commun. 2016 doi: 10.1016/j.bbrc.2016.10.137. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Park SY, Lee SW, Kim HY, Lee WS, Hong KW, Kim CD. HMGB1 induces angiogenesis in rheumatoid arthritis via HIF-1α activation. Eur J Immunol. 2015;45:1216–1227. doi: 10.1002/eji.201444908. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Zhu L, Fang J, Ge Z, Li X. LRG1 modulates epithelial-mesenchymal transition and angiogenesis in colorectal cancer via HIF-1α activation. J Exp Clin Cancer Res. 2016;35:1–11. doi: 10.1186/s13046-016-0306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J, Zhang X, Zhang Y, Zhu D, Zhang L, Li Y, Zhu Y, Li D, Zhou J. HIF-2α promotes epithelial-mesenchymal transition through regulating Twist2 binding to the promoter of E-cadherin in pancreatic cancer. J Exp Clin Cancer Res. 2016;35:26. doi: 10.1186/s13046-016-0298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong L, D’Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, Guimaraes A, Marinelli B, Wikstrom JD, Nir T, Clish CB, Vaitheesvaran B, Iliopoulos O, Kurland I, Dor Y, Weissleder R, Shirihai OS, Ellisen LW, Espinosa JM, Mostoslavsky R. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1α. Cell. 2010;140:280–93. doi: 10.1016/j.cell.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Zhang D, Wang X, Yao X, Ye C, Zhang S, Wang H, Chang C, Xia H, Wang YC, Fang J, Yan J, Ying H. Hypoxia-inducible miR-182 enhances HIF1α signaling via targeting PHD2 and FIH1 in prostate cancer. Sci Rep. 2015;5:12495. doi: 10.1038/srep12495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee G, Won HS, Lee YM, Choi JW, Oh TI, Jang JH, Choi DK, Lim BO, Kim YJ, Park JW, Puigserver P, Lim JH. Oxidative dimerization of PHD2 is responsible for its inactivation and contributes to metabolic reprogramming via HIF-1α activation. Sci Rep. 2016;6:18928. doi: 10.1038/srep18928. [DOI] [PMC free article] [PubMed] [Google Scholar]