Abstract
Aims: This study aimed to examine the heat shock protein Hsp90 family protein (GRP94) expression in breast cancer tissues and its correlation with clinicopathologic features, including the survival of patients with breast cancer. Methods: GRP94 mRNA expression was examined in normal breast and breast cancer tissues using real-time PCR. We also analyzed GRP94 protein expression with immunohistochemistry in 139 breast cancer patients whose ages ranged from 29 to 83 years (median =53 years). On evaluation of cytoplasmic GRP94 immunostaining, cases with a score of ≥ or ≤ six were regarded as having high or low GRP94 expression, respectively. The relationship between GRP94 expression levels and the clinical features of breast cancer were also analyzed. Results: GRP94 mRNA expression was markedly greater in breast cancer tissues than that in normal breast tissues (P=0.0027). Immunohistochemical analysis revealed increased GRP94 protein expression in the cytoplasm of breast cancer cells, which did not positively correlate with age, tumor size classification, lymph node metastasis classification, clinical stage, or estrogen receptor expression in breast cancer patients, but did negatively correlate with progesterone receptor expression (P=0.032). Furthermore, patients with breast cancer tissue that expressed high GRP94 had a significantly shorter survival time than did patients with a low GRP94 expression (P<0.001). A multivariate analysis suggested that the level of GRP94 expression was an independent prognostic indicator (P<0.001) for the survival of patients with breast cancer. Conclusion: High GRP94 expression levels were found to be an independent and unfavorable prognostic indicator of breast cancer survival.
Keywords: GRP94, overexpression, breast cancer
Introduction
Breast cancer is the leading cause of cancer death in women worldwide [1-4]. The incidence of breast cancer among Chinese women is rapidly rising. Alterations in the expression of a few important genes and signaling molecules, such as PTEN, the IL-6/STAT3 signaling pathway, and miR-221/222 are often responsible for the occurrence and development of breast cancer [5-7]. GRP94 encodes a member of the adenosine triphosphate (ATP)-metabolizing molecular chaperone family with roles in stabilizing and folding other proteins. In cells, GRP94 is localized to melanosomes and the endoplasmic reticulum (ER) and was initially recognized as a protein strongly upreglated in cells upon glucose starvation [8], as a major calcium-binding factor in the ER [9], and as the most abundant resident ER protein [10]. In addition, the absence of GRP94 was reported to be lethal to embryos [11], which could be attributed to its responsibility in chaperoning multiple essential proteins, including Toll-like receptors (TLRs), and the low-density lipoprotein receptor-related protein 6 (LRP6) that is a co-receptor for Wnt/β-catenin signaling [11-14]. GRP94 regulates the processing of LRP6, which leads to the activation of the pro-proliferative and pro-survival Wnt-β-catenin signaling pathway [13]. In the past few years, many studies have demonstrated that the expression of this protein is correlated with various pathogenic states that include tumor formation. GRP94 regulates the maturation and secretion of insulin-like growth factors (IGFs), which are important mitogenic factors [11]. Specifically, the binding of IGF-1 or IGF-2 to IGF-1R leads to PI3K/AKT signaling pathway activation. In breast cancer cells that can proliferate under chronic exposure to reactive oxygen species (ROS), in vitro, GRP94 expression increased, but not HSP90 or GRP78 [15]. The studies mentioned above hinted at the importance of GRP94 in the pathogenesis of tumors, including breast cancer. However, the correlation between GRP94 expression and breast cancer survival in patients has never been elucidated.
In this study, GRP94 mRNA expression levels were compared between breast cancer tissue and normal tissues. Additionally, the correlation between GRP74 protein expression and the clinicopathologic features of breast cancer patients was investigated. We found that GRP94 mRNA and protein expression were higher in breast cancer tissues than in normal breast tissues. Furthermore, higher GRP94 protein expression was associated with breast cancer progression and a worse outcome. These data suggest that overepressed GRP94 is an unfavorable prognostic factor for the survival of breast cancer patients.
Materials and methods
Sample collection
A total of 41 paired breast cancer and normal tissue samples were collected from patients at the Maternal and Child Health Care Hospital of Guiyang. These patients had not undergone radiotherapy, chemotherapy, or hormone therapy before surgery. This study was approved by the by the Zhejiang Taizhou Hospital Ethics Committee and informed consent was obtained from all patients. The breast cancer tissue chip was purchased from Shanghai Chao Xin Biotechnology Co., Ltd. (Shanghai, China). Tissue specimens were obtained from patients who underwent surgery at the Zhejiang Taizhou Hospital between January 2001 and August 2004 and were followed up for 108 to 150 months. The patients ranged in age from 29 to 83 years (mean age 53 years). Clinical and pathologic data of the patients included age, pathological grade, tumor size, distant lymph node metastasis, and lymph node invasion. The inclusion and exclusion criteria followed the guidelines of the clinical pathology of breast cancer staging (2009 American Joint Committee on Cancer, seventh edition).
RNA extraction and real-time PCR
RNA was extracted from breast cancer tissues and normal breast tissues using the Trizol reagent (Takara, Shiga, Japan). RNA was transcribed into cDNA and amplified with the specific GRP94 sense primer: 5’-GGATGGTCTGGCAACATGGA-3’, and the antisense primer: 5’CCGAAGCGTTGCTGTTTCAA-3’. The GAPDH gene was used as an internal control using the sense primer 5’-CGGAGTCAACGGATTTGGTCGTAT-3’ and the antisense primer 5’-AGCCTTCTCCATGGTGGTGAAGAC-3’. The assays were performed in accordance with the manufacturer’s instructions (Takara). Cycling conditions were 95°C for 10 min to activate DNA polymerase, followed by 45 cycles at 95°C for 12 s, 56°C for 12 s, and 72°C for 12 s. PCR reactions for each gene were performed in triplicate. Independent experiments were also performed in triplicate.
Immunohistochemistry
According to standard protocols, the paraffin-embedded breast cancer sections (3 μm in thickness) were deparaffinized in 100% xylene and rehydrated in a descending ethanol series (100, 90, 80, and 70% ethanol). Heat-induced antigen retrieval was performed in 10 mM citrate buffer for 2 min at 100°C. A peroxidase blocking reagent containing 3% hydrogen peroxide and serum was used to block endogenous peroxidase activity and non-specific antigens, followed by incubation overnight with a rabbit anti-human GRP94 polyclonal antibody (Proteintech Group, Inc., Chicago, IL, USA) at a 1:50 dilution, at 4°C. The sections were visualized with 3,3N-Diaminobenzidine (DAB) tetrahydrochloride (Beyotime® Biotechnology, Shang Hai, China) and counterstained with hematoxylin, mounted in a neutral gum, and analyzed using a bright field microscope.
Evaluation of staining
Two pathologists who were blinded to the clinical parameters evaluated the stained tissue sections separately. The score for each section was recorded according to the sum of the cytoplasmic staining intensity and the percentage of the positive staining areas of the cells. The staining intensity was scored as follows: negative expression, 1; weak expression, 2; positive expression, 3; and strong expression, 4. The percentage of positive staining cells was defined by a scale of 0-3 (0: <10%, 1: 10-25%, 2: 26-75%, and 3: >76%). For statistical analysis, a final staining score of 0-5 and 6-7 was considered low or high GRP94 expression, respectively.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 software (Jhttp://www.graphpadGraphpad.com/company/Inc., La Jolla, CA, USA) and SPSS 20.0 software (SPSS, Inc., Chicago, IL, USA). The two-tailed Student’s ttest was used for the comparisons between the two groups. A Chi-Square (χ2) test was used to examine the correlation between GRP94 mRNA and protein expression and the clinicopathologic features of breast cancer. The correlation between GRP94 protein expression and patient survival was performed by KaplanMeier analysis with the log-rank test. A P value <0.05 was considered statistically significant.
Results
Elevated GRP94 mRNA in breast cancer
To assess the role of GRP94 in breast cancer, real-time PCR analyses were conducted to measure GRP94 mRNA expression levels in 41 freshly collected breast cancer tissues and matched normal breast tissues. Breast cancer tissues exhibited higher GRP94 mRNA expression levels than normal breast tissues (P=0.0027) (Figure 1).
Figure 1.
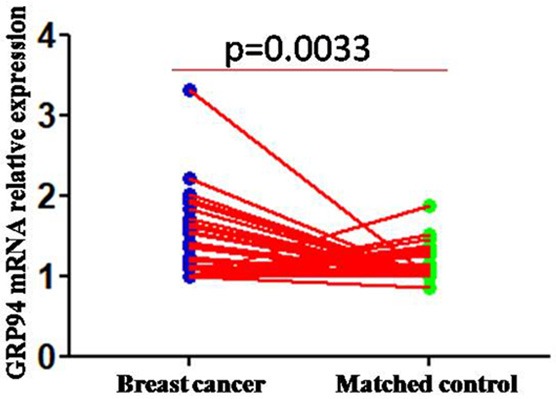
Upregulated GRP94 mRNA was displayed in breast cancer tissues compared to normal breast tissues.
GRP94 protein expression in breast cancer
We examined GRP94 protein expression in 140 archived paraffin-embedded breast cancer tissue samples using immunohistochemical staining. GRP94 was mainly expressed in the cytoplasm (Figure 2), and 52.1% of the patients (73/140) showed low, and 47.9% (67/140) showed high GRP94 expression, respectively.
Figure 2.
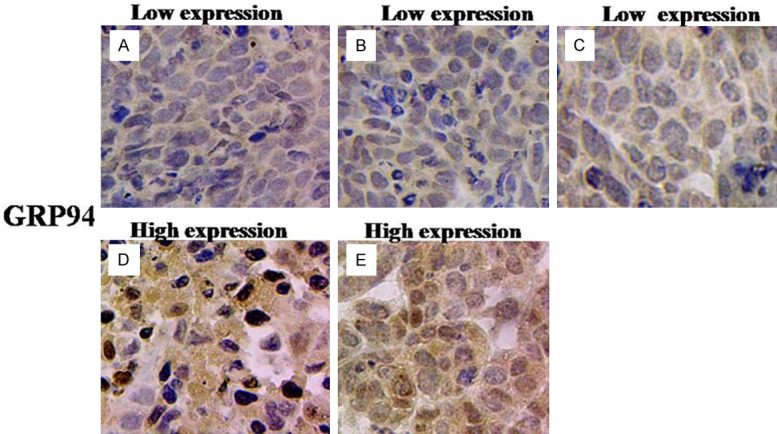
GRP94 protein expression in breast cancer (original magnification: ×400). A and B: Low expression of GRP94 protein in breast cancer tissues of I-II stage with the positive expression of PR and ER (High differentiation). C: Low expression of GRP94 protein in breast cancer tissues of I-II stage with the positive expression of PR and ER (Medium differentiation); D and E: High expression of GRP94 protein in breast cancer tissues of III stage with the negative expression of PR and ER (Low differentiation).
Correlation between GRP94 expression and the clinicopathologic parameters of breast cancer
The correlation between GRP94 expression and the clinicopathologic parameters of breast cancer patients are summarized in Table 1. In the 140 breast cancer tissue samples examined, no association between GRP94 expression and the patients’ ages, lymph node metastasis, tumor size, lymph node invasion, clinical stage, or the presence of ER expression were found. However, a positive and statistically significant correlation was observed between GRP94 expression and progesterone receptor (PR) expression (P=0.032) (Table 1). This result demonstrated the importance of GRP94 expression in PR-positive breast cancer.
Table 1.
Correlation between the clinicopathologic characteristics and expression of GRP94 in breast cancer
| Characteristics | n | GRP94 expression | |||
|---|---|---|---|---|---|
|
| |||||
| High | Low | r | P value | ||
| Age (y) | |||||
| ≤45 | 67 | 30 (44.8%) | 37 (55.2%) | 0.484 | |
| >45 | 73 | 37 (50.7%) | 36 (49.3%) | ||
| TNM classification | |||||
| I-II | 129 | 59 (45.7%) | 70 (54.3%) | 0.085 | |
| III-VI | 11 | 8 (72.7%) | 3 (27.3%) | ||
| T classification | |||||
| T1-T2 | 126 | 61 (48.4%) | 65 (51.6%) | 0.693 | |
| T3-T4 | 14 | 6 (42.9%) | 8 (57.1%) | ||
| N classification | |||||
| N0-N1 | 95 | 45 (47.4%) | 50 (52.6%) | 0.866 | |
| N2-N3 | 45 | 22 (48.9%) | 23 (51.1%) | ||
| ER | |||||
| Negative | 47 | 27 (57.4%) | 20 (42.6%) | -0.136 | 0.106 |
| Positive | 93 | 40 (43.0%) | 53 (57.0%) | ||
| PR | |||||
| Negative | 56 | 33 (58.9%) | 23 (41.1%) | -0.181 | 0.032* |
| Positive | 84 | 34 (40.5%) | 50 (59.5%) | ||
Statistically significant.
High GRP94 expression correlates with poor patient survival
We used the Kaplan-Meier analysis with the log-rank test to analyze the correlation between GRP94 expression levels in breast cancer tissues and patient survival. GRP94 expression levels were negatively associated with survival in breast cancer patients. Patients with higher GRP94 expression had a worse prognosis compared with patients with lower GRP94 expression (Figure 3; P<0.001).
Figure 3.
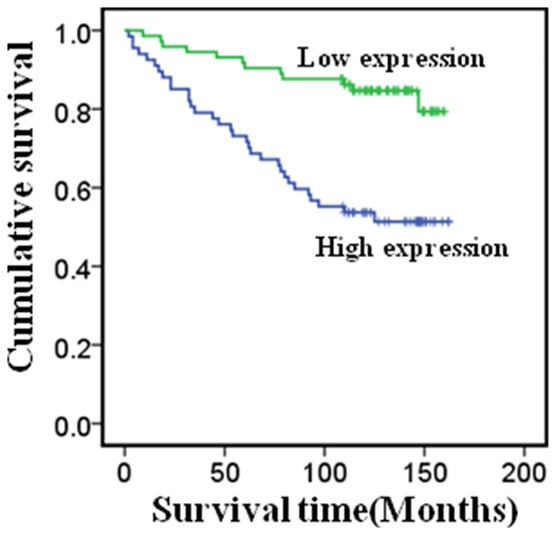
Overexpression of GRP94 protein is an unfavorable factor in breast cancer.
High GRP94 expression is an independent prognosis factor for breast cancer patients
To investigate if high GRP94 expression is an independent prognostic factor for breast cancer patients, we used the univariate and multivariate Cox proportional hazards model to analyze the significance of various survival variables. In the univariate analysis, the results showed that clinical stage (P=0.020), ER status (P=0.013), PR status (P=0.030) and GRP94 expression (P<0.001) were correlated with breast cancer patient prognosis. Multivariate analysis of these clinical parameters indicated that GRP94 expression (P<0.001) and the clinical stage (P=0.013) were independent prognostic factors for breast cancer patients (Table 2).
Table 2.
Summary of univariate and multivariate Cox regression analysis
| Parameter | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age (year) | 0.749 | 0.411-1.367 | 0.347 | 0.684 | 0.360-1.299 | 0.246 |
| <45 vs. ≥45 | ||||||
| TNM classification | 2.620 | 1.163-5.902 | 0.020* | 2.997 | 1.263-7.113 | 0.013* |
| ER | 2.114 | 1.169-3.824 | 0.013* | 1.782 | 0.795-3.994 | 0.160 |
| PR | 1.932 | 1.067-3.500 | 0.030* | 1.284 | 0.578-2.852 | 0.539 |
| T classification | 1.849 | 0.78-4.384 | 0.163 | 2.862 | 1.104-7.418 | 0.593 |
| N classification | 1.075 | 0.868-1.332 | 0.507 | 1.062 | 0.852-1.323 | 0.593 |
| GRP94 expression | 0.278 | 0.143-0.540 | <0.001* | 0.288 | 0.145-0.574 | <0.001* |
Statistically significant.
Discussion
GRP94 is a molecular chaperone that functions in the processing and transport of secreted proteins. GRP94 is a disulfide-linked homodimer, whose subcellular location is in the endoplasmic reticulum lumen and belongs to the heat shock protein 90 family. At present, there are many reports demonstrating that abnormal expression of GRP94 correlates with tumor pathogenesis, which indicates the importance of GRP94 in tumors.
GRP94 mRNA overexpression has been shown in some tumors. Huang et al. reported that GRP94 mRNA expression levels were increased in hepatocellular carcinoma compared to normal hepatocytes [16]. Moreover, GRP94 mRNA overexpression was also detected in glioma tissue compared with normal brain tissue, in lung cancer tissue compared with normal lung tissue, and in esophageal adenocarcinoma compared with normal esophageal tissue [17-19]. It is noteworthy that these data are similar to our breast cancer tissue results, where we found significantly increased GRP94 expression in breast cancer tissues compared with normal breast tissues.
To further investigate the importance of GRP94 in breast cancer pathogenesis, we examined the GRP94 protein expression in breast cancer tissues, which showed that the GRP94 protein was expressed in the cytoplasm of breast cancer cells. This result is analogous to previous reports in which GRP94 protein expression was shown in hepatocellular carcinoma, glioma, lung cancer, and esophageal adenocarcinoma. Additionally, our results are consistent with those of Nomura et al., who examined GRP4 expression in oral squamous cell carcinoma [20], Chen et al., who examined expression in esophageal adenocarcinoma [21], and Hodorova et al., who examined expression in breast cancer [22]. However, correlations of the GRP94 protein expression with the clinical features of breast cancer have seldom been investigated.
Previous reports showed that GRP94 overexpression was positively correlated with clinical progression in a few different tumor types [16-22]. And, consistent with these studies, we found that increased GRP94 expression was positively correlated with the T classification (tumor size), the N classification (lymph node metastasis), the clinical stages and ER expression in breast cancer patients. However, GRP94 was also negatively correlated with PR expression, which suggests that GRP94 overexpression may have an unfavorable role in PR-positive breast cancer patients. Moreover, the correlation between GRP94 expression and survival of breast cancer patients has never been reported.
In the past few years, more evidence has indicated that elevated GRP94 expression is an unfavorable prognostic indicator of tumors, including hepatocellular carcinoma, lung cancer, glioma, and oral squamous cell carcinomas [16-21]. In this study, we show evidence that GRP94 protein expression is inversely correlated with the survival of breast cancer patients. The patients with higher GRP94 protein expression had a shorter survival time. According to the univariate and multivariate analysis results, increased GRP94 protein expression significantly predicted a poor prognosis for breast cancer patients. These results are analogous to those reported in previous studies [16], in which high GRP94 expression was found to be an independent prognostic indicator for predicting poor survival in malignant tumors, and further highlight the critical role that GRP94 plays in promoting the pathogenesis of breast cancer.
Conclusion
Overall, this study showed that GRP94 mRNA and protein expression levels were significantly elevated in breast cancer tissues. In addition, increased GRP94 protein was correlated with poor clinical progression of breast cancer. Moreover, these results indicate that increased GRP94 protein is an independent and unfavorable prognostic indicator for breast cancer.
Acknowledgements
This study was supported by Nature Science Fund of Guangdong Province (No. 2017A030313540).
Disclosure of conflict of interest
None.
References
- 1.Redig AJ, McAllister SS. Breast cancer as a systemic disease: a view of metastasis. J Intern Med. 2013;274:113–126. doi: 10.1111/joim.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.M Braden A, V Stankowski R, M Engel J, A Onitilo A. Breast cancer biomarkers: risk assessment, diagnosis, prognosis, prediction of treatment efficacy and toxicity, and recurrence. Curr Pharm Des. 2014;20:4879–4898. doi: 10.2174/1381612819666131125145517. [DOI] [PubMed] [Google Scholar]
- 3.Zhao Z, Sun C, Li F, Han J, Li X, Song Z. Overexpression of histone demethylase JMJD5 promotes metastasis and indicates a poor prognosis in breast cancer. Int J Clin Exp Pathol. 2015;8:10325–10334. [PMC free article] [PubMed] [Google Scholar]
- 4.Yalcin B. Staging, risk assessment and screening of breast cancer. Exp Oncol. 2013;35:238–245. [PubMed] [Google Scholar]
- 5.Zhou XJ, Wu J, Shi L, Li XX, Zhu L, Sun X, Qian JY, Wang Y, Wei JF, Ding Q. PTEN expression is upregulated by a RNA-binding protein RBM38 via enhancing its mRNA stability in breast cancer. J Exp Clin Cancer Res. 2017;36:149. doi: 10.1186/s13046-017-0620-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aryappalli P, Al-Qubaisi SS, Attoub S, George JA, Arafat K, Ramadi KB, Mohamed YA, Al-Dhaheri MM, Al-Sbiei A, Fernandez-Cabezudo MJ, Al-Ramadi BK. The IL-6/STAT3 signaling pathway is an early target of manuka honeyinduced suppression of human breast cancer cells. Front Oncol. 2017;7:167. doi: 10.3389/fonc.2017.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li B, Lu Y, Yu L, Han X, Wang H, Mao J, Shen J, Wang B, Tang J, Li C, Song B. miR-221/222 promote cancer stem-like cell properties and tumor growth of breast cancer via targeting PTEN and sustained Akt/NF-kappaB/COX-2 activation. Chem Biol Interact. 2017;277:33–42. doi: 10.1016/j.cbi.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Lee AS, Bell J, Ting J. Biochemical characterization of the 94- and 78-kilodalton glucoseregulated proteins in hamster fibroblasts. J Biol Chem. 1984;259:4616–4621. [PubMed] [Google Scholar]
- 9.Koch G, Smith M, Macer D, Webster P, Mortara R. Endoplasmic reticulum contains a common, abundant calcium-binding glycoprotein, endoplasmin. J Cell Sci. 1986;86:217–232. doi: 10.1242/jcs.86.1.217. [DOI] [PubMed] [Google Scholar]
- 10.Lewis MJ, Mazzarella RA, Green M. Structure and assembly of the endoplasmic reticulum. The synthesis of three major endoplasmic reticulum proteins during lipopolysaccharideinduced differentiation of murine lymphocytes. J Biol Chem. 1985;260:3050–3057. [PubMed] [Google Scholar]
- 11.Wanderling S, Simen BB, Ostrovsky O, Ahmed NT, Vogen SM, Gidalevitz T, Argon Y. GRP94 is essential for mesoderm induction and muscle development because it regulates insulinlike growth factor secretion. Mol Biol Cell. 2007;18:3764–3775. doi: 10.1091/mbc.E07-03-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y, Liu B, Dai J, Srivastava PK, Zammit DJ, Lefrancois L, Li Z. Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity. 2007;26:215–226. doi: 10.1016/j.immuni.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu B, Staron M, Hong F, Wu BX, Sun S, Morales C, Crosson CE, Tomlinson S, Kim I, Wu D, Li Z. Essential roles of grp94 in gut homeostasis via chaperoning canonical Wnt pathway. Proc Natl Acad Sci U S A. 2013;110:6877–6882. doi: 10.1073/pnas.1302933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Wu BX, Metelli A, Thaxton JE, Hong F, Rachidi S, Ansa-Addo E, Sun S, Vasu C, Yang Y, Liu B, Li Z. GP96 is a GARP chaperone and controls regulatory T cell functions. J Clin Invest. 2015;125:859–869. doi: 10.1172/JCI79014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dejeans N, Glorieux C, Guenin S, Beck R, Sid B, Rousseau R, Bisig B, Delvenne P, Buc Calderon P, Verrax J. Overexpression of GRP94 in breast cancer cells resistant to oxidative stress promotes high levels of cancer cell proliferation and migration: implications for tumor recurrence. Free Radic Biol Med. 2012;52:993–1002. doi: 10.1016/j.freeradbiomed.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Huang CY, Batzorig U, Cheng WL, Huang MT, Chen W, Wei PL, Chang YJ. Glucose-regulated protein 94 mediates cancer progression via AKT and eNOS in hepatocellular carcinoma. Tumour Biol. 2016;37:4295–4304. doi: 10.1007/s13277-015-4254-9. [DOI] [PubMed] [Google Scholar]
- 17.Hu T, Xie N, Qin C, Wang J, You Y. Glucoseregulated protein 94 is a novel glioma biomarker and promotes the aggressiveness of glioma via Wnt/beta-catenin signaling pathway. Tumour Biol. 2015;36:9357–9364. doi: 10.1007/s13277-015-3635-4. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q, He Z, Zhang J, Wang Y, Wang T, Tong S, Wang L, Wang S, Chen Y. Overexpression of endoplasmic reticulum molecular chaperone GRP94 and GRP78 in human lung cancer tissues and its significance. Cancer Detect Prev. 2005;29:544–551. doi: 10.1016/j.cdp.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Langer R, Feith M, Siewert JR, Wester HJ, Hoefler H. Expression and clinical significance of glucose regulated proteins GRP78 (BiP) and GRP94 (GP96) in human adenocarcinomas of the esophagus. BMC Cancer. 2008;8:70. doi: 10.1186/1471-2407-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nomura H, Uzawa K, Yamano Y, Fushimi K, Ishigami T, Kato Y, Saito K, Nakashima D, Higo M, Kouzu Y, Ono K, Ogawara K, Shiiba M, Bukawa H, Yokoe H, Tanzawa H. Networkbased analysis of calcium-binding protein genes identifies Grp94 as a target in human oral carcinogenesis. Br J Cancer. 2007;97:792–801. doi: 10.1038/sj.bjc.6603948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Ding Y, Liu CG, Mikhail S, Yang CS. Overexpression of glucose-regulated protein 94 (Grp94) in esophageal adenocarcinomas of a rat surgical model and humans. Carcinogenesis. 2002;23:123–130. doi: 10.1093/carcin/23.1.123. [DOI] [PubMed] [Google Scholar]
- 22.Hodorova I, Rybarova S, Solar P, Vecanova J, Prokopcakova L, Bohus P, Solarova Z, Mellova Y, Schmidtova K. Gp96 and its different expression in breast carcinomas. Neoplasma. 2008;55:31–35. [PubMed] [Google Scholar]


