Abstract
Inflammatory response and apoptosis play an important role in progression of spinal cord injury (SCI). Recently, aberrant microRNAs (miRNAs) have emerged as a key regulator in SCI. However, it remains unknown whether and how miRNAs mediated the inflammatory response after SCI. The aim of this study was to evaluate the potential role of miRNAs in SCI and elucidate underlying molecular mechanisms. First, we analyzed the microRNA expression profile in spinal cords from rats following SCI, using miRNA microarray. Interestingly, miR-182-5p was one of miRNAs most significantly downregulated in SCI. It has been reported as an inflammation suppressor in different organ injury models. Here, we used a cell model to verify the regulatory function and mechanism of miR-182-5p on inflammatory response in SCI. Overexpression of miR-182-5p attenuated H2O2-induced inflammation as reflected by reduction in proinflammatory cytokines in C8-D1A cells. Meanwhile, enhanced miR-182-5p expression significantly suppressed H2O2-induced apoptosis. Toll-like receptor 4 (TLR4), an important regulator of nuclear factor kappa-B (NF-κB) signaling pathway, was identified as a novel target of miR-182-5p in C8-D1A cells. Furthermore, overexpression of TLR4 reversed inhibitory effects of miR-182-5p overexpression on inflammation and apoptosis. More importantly, we found that miR-182-5p blocked phosphorylation of nuclear p65 and promoted phosphorylation of IκB-α in H2O2-treated C8-D1A cells. Our results confirm that miR-182-5p alleviates inflammation and apoptosis via inactivation of TLR4/NF-κB pathway in an H2O2-induced cell model. Our findings suggest that miR-182-5p may be a potential therapeutic target of SCI in the future.
Keywords: Spinal cord injury, inflammation, apoptosis, microRNA-182-5p, TLR4/NF-κB pathway
Introduction
Spinal cord injury (SCI) is a damaging condition leading to the loss of sensory and motor functions [1,2]. In the USA, approximately 12,000 new cases with SCI per year have been reported with nearly $40.5 billion per year in costs. [3]. Despite improvements in medical care, treatment of SCI has remained a serious challenge. Given the key role of inflammatory response in secondary injury, targeting the reduction of secondary inflammation holds promising potential for SCI patients. Several studies have proven that prevention of inflammation could promote neuronal recovery after SCI in rats [4-6]. However, detailed mechanisms of such inflammatory response in SCI are not fully understood.
microRNAs (miRNAs) are a class of 21-23 nucleotides long small non-coding RNA molecules that function as regulatory molecules in many biological processes such as cell growth, apoptosis, cell-cycle, and even the pathogenesis of several diseases by specifically binding to complementary sequences at 3’-UTR of their target genes [7-9]. An increasing number of studies have been carried out to characterize miRNA expression and function in SCI. For example, Hu et al. identified changes in miRNA profiles in spinal tissue of SCI rats and found that miR-21 was downregulated and played an important role in limiting secondary cell death following SCI [10]. Zhu et al. showed that miR-449a inhibited expression of neuronal markers nestin, NeuN, and CGRP and promoted cellular apoptosis and inflammation in SCI rats [11]. However, studies on miRNA after SCI are limited, especially concerning their effect on inflammation.
In the present study, we performed microarray analysis to investigate expression profiles of miRNAs in SCI rats. We selected miR-182-5p to further explore its function and mechanism in regulating production of inflammatory factors and apoptosis using an SCI cell model. This study should improve awareness of miR-182-5p regulation after SCI and inform future direction of treatments for patients with SCI.
Materials and methods
Experimental animals
A total of 12 female Sprague-Dawley (SD) rats (220-250 g) were purchased from the Animal Center of the Chinese Academy of Sciences (Shanghai, China). All animal procedures were conducted in accordance with guidelines and were reviewed and approved by the Institutional Animal Care and Use Committee of the Sixth People’s Hospital Affiliated to Shanghai Jiaotong University. All rats were randomly divided into 2 groups: Sham group (n = 6) and SCI group (n = 6). All animals were housed in individual cages in a temperature- and light cycle-controlled environment with free access to food and water.
Establishment of contusion SCI model
Rats were intraperitoneally injected with 10% chloral hydrate (400 mg/kg). A rat model of contusion was established by heavy impact according to the method established by Zhang et al. [12]. Briefly, the skin was incised along the midline of the back after sterilization and the vertebral column was exposed. A laminectomy was performed at the T9 level. A 10 g 2 cm-diameter metal rod was vertically dropped from a 25 mm height to impact the exposed spinal cord and to cause SCI. Sham groups received the same surgical procedure but sustained no impact injury.
Microarray analysis
Total RNA was harvested from spinal cord after SCI using miRNeasy mini kit (Qiagen), following manufacturer protocol. RNA quality and quantity were determined using Nanodrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). Total RNA was labeled using miRCURY™ Hy3™/Hy5™ power labeling kit (Exiqon Inc., Woburn, MA, USA) and hybridized on miRCURY™ LNA Array (v18.0) (Exiqon, Copenhagen, Denmark). After washing and staining, the arrays were scanned in an Agilent G2565BA Microarray Scanner System (Agilent Technologies, Santa Clara, CA, USA). Scanned images were then imported into GenePix Pro 6.0 software (Axon) for grid alignment and data extraction. Three numbers of replicates were conducted in each group. Observations with adjusted p-values ≥ 0.05 were removed and excluded from further analysis. A heat map of the 55 microRNAs most obvious differences was created using a method of hierarchical clustering by GeneSpring GX, version 7.3 (Agilent Technologies, California, United Stages).
Quantitative real-time PCR analysis
For miRNA, total RNA was isolated from spinal cord tissues and cells using miRNeasy mini kit (Qiagen), following manufacturer protocol. cDNA was synthesized using miRNA specific stem-loop primers (Applied Biosystems, Foster City, CA, USA). For mRNA, total RNA was isolated from cancer cells using TRIzol®. Reverse transcription was performed according to the instructions of PrimeScript RT Kit (Takara Biotechnology Ltd., Liaoning, China). qRT-PCR assays were carried out using SYBR Green Master Mixture (Roche) reagent on a 7500 Fast Real-Time PCR System (Applied Biosystems, USA). Primer sequences were as follows: miR-182-5p, forward 5’-GGCTCAAGAATCTACATTTCAACAG-3’ and reverse 5’-ACACACCCCCACTACAGGGCTCT-3’; TLR4, forward 5’-AGTTGATCTACCAAGCCTTGAGT-3’ and reverse 5’-GCTGGTTGTCCCAAAATCACTTT-3’; U6 forward: 5’-GCTTCGGCAGCACATATACTAAAAT-3’, reverse: 5’-CGCTTCACGAATTTGCGTGTCAT-3’. GAPDH, forward 5’-CTGGGCTACACTGAGCACC-3’, and reverse 5’-AAGTGGTCGTTGAGGGCAATG-3’. Expression of miR-182-5p and TLR4 was normalized to U6 and GAPDH, separately, and relative expression was calculated by comparative CT (2-ΔΔCT) method.
Cell culture and treatment
Murine astrocyte C8-D1A (Cat No. CRL-2541) was purchased from ATCC and cultured in DMEM (Abcam, Cambridge, MA) media supplemented with 10% fetal bovine serum (FBS, Gibco, Rockville, MD) and 1% pen-strep at 37°C in a humidified atmosphere with 5% CO2. For H2O2 intervention, a final concentration of 10 μM H2O2 (Sigma, St. Louis, MO, USA) was added into the cultured C8-D1A cells for 12 hours, according to a previous study.
Cell transfection
miR-182-5p mimics, miR-182-5p inhibitor, and negative control were obtained from Ribobio (Guangzhou, China). For enforced expression of TLR4 in C8-D1A cells, open reading frame region of human TLR4 gene was amplified and inserted into the pcDNA3.1 eukaryotic expression vector (Invitrogen, USA). Before transfection, C8-D1A cells were cultured in a 6-well culture plate at a density of 2×105 cells per well for 24 hours. miR-182-5p mimics, miR-182-5p inhibitor, and negative control or 2 μg/ml TLR4 plasmid was then transfected into C8-D1A cells with the use of Lipofectamine 2000 (Invitrogen), according to the manufacturer instructions, for 24 hours. Then, 200 μM H2O2 exposure was performed.
Cell apoptosis
After 48 hours of H2O2 treatment, cells were collected and then stained with 5 μL Annexin V-FITC and 5 μL PI (BD Biosciences, USA). Subsequently, cell apoptosis was analyzed by flow cytometry (Becton Dickinson, USA). Annexin V-positive cell population was considered as apoptotic cells.
Enzyme-linked immunosorbent assay
To determine the release of inflammatory cytokines, cell supernatants were removed and levels of tumor necrosis factor (TNF) α, interleukin (IL) 1β, and interleukin (IL) 6 were measured with use of the Valukine ELISA kit (R&D Systems), according to manufacturer instructions.
Luciferase reporter assay
The 3’-UTR sequence of TLR4, together with a corresponding mutated sequence, was synthesized. They were then embedded into the pmiR-GLO vector (Promega, Madison, WI, USA), named wt-TLR4 3’-UTR and mt-TLR4 3’-UTR, respectively. For luciferase reporter assay, HEK293T cells were transfected with wt-TLR4 3’-UTR or mt-TLR4 3’-UTR and miR-182-5p mimics, miR-182-5p inhibitor, or their corresponding negative controls. Cells were collected 48 hours after transfection and assayed with Luciferase Reporter Assay System (Promega Corporation, Madison, Wisconsin, USA). To correct efficiencies of transfection and gathering, Renilla luciferase expressed by pRL-TK was co-transfected as an internal control. Tests were repeated in triplicate.
Western blotting assay
Cells were harvested after intervention treatment and cellular proteins were extracted using RIPA lysis buffer. Protein concentration was determined using BCA Protein Assay Kit (Beyotime Biotechnology, China). 20-40 μg proteins of each sample were separated via 10% SDS-PAGE and then transferred to a PVDF membrane (Millipore Corporation, Billerica, MA, USA). The membrane was blocked with 10% skimmed milk (in PBS, PH 7.2, containing 0.1% Tween-20) for 1 hour at room temperature. Primary antibodies against cleaved caspase 3, total caspase 3, TLR4, nuclear p-p65, p-IκBα (1:2000, Cell Signaling Technology, Danvers, USA), and β-actin (1:2000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) were incubated with the membranes at 4°C overnight. Detection was by peroxidase-conjugated secondary antibodies (1:2000, Abcam, Cambridge, UK)) and chemiluminescence (Milipore Corporation). Densitometry analysis was performed using Image J software.
Statistical analysis
Statistical analysis was performed using SPSS 15.0 (SPSS, Chicago, IL, USA). Quantitative data are presented as mean ± standard deviation (SD). Comparison between data was calculated using Student’s t-test and one-way variance analysis. Differences were considered statistically significant when P values < 0.05.
Results
microRNA-182-5p was downregulated in spinal cord in rats after SCI
To profile expression of miRNAs after SCI, we first established the animal model of SCI, in accordance with previously described methods, and then performed a microarray in spinal cord after SCI [10]. Our data show that 24 miRNAs were downregulated and 31 miRNAs were upregulated compared with the sham group (Figure 1A). Among them, miR-182-5p was one of the most downregulated miRNAs. It has previously been shown to reduce inflammatory response in various injury models [13,14], therefore, we focused on miR-182-5p in SCI for further study. Subsequently, we measured expression of miR-182-5p in spinal cord tissue of 6 SCI rats by qRT-PCR. Our results showed that miR-182-5p was significantly reduced in SCI group compared with sham group (Figure 1B). In an SCI model, activation of the NF-κB pathway has been demonstrated to mediate inflammatory cells and apoptosis [15]. Thus, we tested protein expression levels of phosphorylated p65 (p-p65), an important mediator of NF-κB activation, using Western blot. Consistent with a previous study [4], we observed that p-p65 protein levels were remarkably elevated in injured spinal cords of two SCI rats (Figure 1C). These results indicate that miR-182-5p might be involved in development of SCI through NF-κB pathway.
Figure 1.
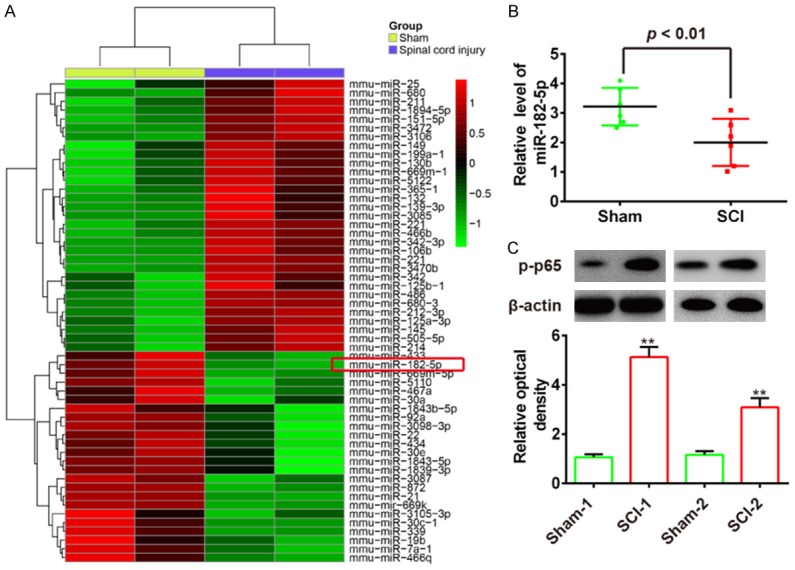
miR-182-5p is downregulated in rats after spinal cord injury (SCI). A. Heat map of miRNA profiles represented the significantly regulated miRNAs. The color code in the heat maps is linear with green as the lowest and red as the highest. miRNAs that were upregulated are shown in green to red, whereas miRNAs that were downregulated are shown from red to green. B. miR-182-5p expression was validated by qRT-PCR in rats after SCI (n = 6). P < 0.01 vs. Sham group. C. Expression of p-p65 in SCI rats was measured by Western Blot. (n = 2). Data represents the mean ± SD of three independent experiments. **P < 0.01 vs. Sham group.
Elevation of miR-182-5p attenuated hydrogen peroxide induced apoptosis in C8-D1A cells
To explore the roles of miR-182-5p in SCI, C8-D1A cells, the most abundant cells in the central nervous system (CNS) [16], were applied for construction of a cell model of SCI under H2O2 simulation [5]. First, we detected expression of miR-182-5p in C8-D1A cells under different concentrations of H2O2 stimulation. Results showed that expression of miR-182-5p was significantly reduced in a dose dependent manner (Figure 2A). We found that a concentration of 200 μM significantly changed miR-182-5p levels and caused mild cell damage after treatment. Therefore, we chose this concentration for further study.
Figure 2.
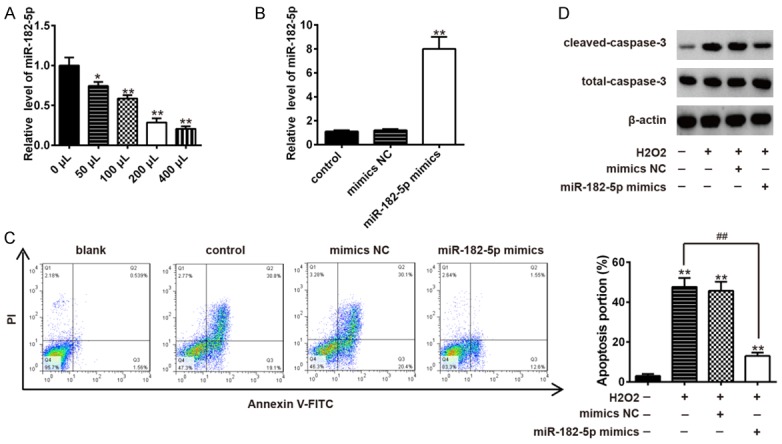
Overexpression of miR-182-5p suppressed apoptosis in H2O2 treated C8-D1A cells. A. Expression level of miR-182-5p in C8-D1A cells was elevated by transfection of miR-182-5p mimics in H2O2 treated C8-D1A cells as measured by qRT-PCR. Data represents the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01 vs. untreated group. B. Transfection of miR-182-5p mimics significantly increased expression level of miR-182-5p in C8-D1A cells. Data represent the mean ± SD of three independent experiments. **P < 0.01 vs. control group. C. Apoptosis was detected by flow cytometry in H2O2 treated C8-D1A cells after transfection with miR-182-5p mimics. Data represent the mean ± SD of three independent experiments. **P < 0.01 vs. control group. ##P < 0.01 vs. H2O2 alone group. D. Protein expression levels of cleaved-caspase 3 and total caspase 3 were detected by Western blot in H2O2 treated C8-D1A cells after transfection with miR-182-5p mimics.
Apoptosis is a prominent characteristic in the spinal cord after SCI [17]. Thus, we investigated the effects of miR-182-5p on cell apoptosis. miR-182-5p mimics were transfected into C8-D1A cells and expression levels of miR-182-5p in C8-D1A cells was significantly increased after transfection (Figure 2B). Then, we examined the alteration of apoptosis of miR-182-5p mimics transfected C8-D1A cells after H2O2 stimulation. Results of flow cytometry showed that H2O2 treatment obviously promoted apoptosis of C8-D1A cells. However, overexpression of miR-182-5p significantly suppressed H2O2 and induced apoptosis in C8-D1A cells (Figure 2C). Expression of cleaved-caspase 3, an executioner caspase that indicates the level of cellular apoptosis [18], was examined using Western blotting analysis. Markedly increased expression of cleaved-caspase 3 was found in H2O2 treated C8-D1A cells. In line with results of flow cytometry, overexpression of miR-182-5p attenuated H2O2 and induced expression of cleaved-caspase 3 and exhibited effective anti-apoptotic effects (Figure 2D). These results suggest that overexpression of miR-182-5p inhibits apoptosis in H2O2-treated C8-D1A cells.
Overexpression of miR-182-5p attenuated H2O2-induced inflammatory responses in C8-D1A cells
Among all mechanisms of secondary injury of SCI, inflammation is the most important because it can directly or indirectly control the sequelae [5,6]. Previous studies have demonstrated that miR-182-5p has an anti-inflammation property in many types of injury models [13,19]. Thus, we assumed that miR-182-5p has a similar mechanism in SCI. Secretion of pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6 were determined using ELISA. As shown in Figure 3A-C, we found that H2O2 enhanced secretion of TNF-α, IL-1β and IL-6, whereas overexpression of miR-182-5p significantly reduced production of TNF-α, IL-1β, and IL-6. These data suggest that overexpression of miR-182-5p attenuates inflammatory responses in H2O2-treated C8-D1A cells.
Figure 3.
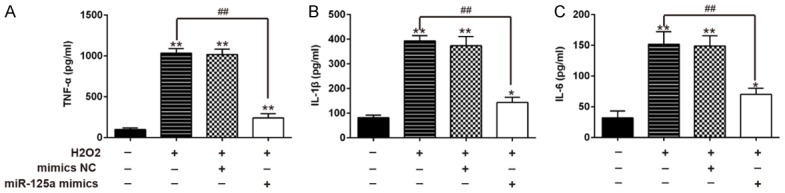
Overexpression of miR-182-5p attenuated H2O2-induced inflammation response. C8-D1A cells were transfected with miR-182-5p mimics for 48 hours and then exposed to 200 μM H2O2 for 10 h. TNF-α (A), IL-6 (B), and IL-1β (C) levels were measured by ELISA assay. Data represents the mean ± SD of three independent experiments. **P < 0.01 vs. control group. ##P < 0.01 vs. H2O2 alone group.
TLR4 was a direct target of miR-182-5p
To further elucidate underlying molecular mechanisms involved in the anti-inflammation role of miR-182-5p in SCI, we searched miRanda and TargetScan 7.1 databases for proposed target genes of miR-182-5p. We also retrieved previous reports of target genes of miR-182-5p. Toll-Like Receptor 4 (TLR4) was validated as a target of miR-182-5p that is related to inflammatory responses [13]. As suggested in Figure 4A, 4B, the complementary sequence of miR-182-5p was found in 3’-UTR of TLR4 mRNA. To confirm that miR-182-5p could interact with 3’-UTR of TLR4 through the complementary sequence, luciferase reporter assay was performed. These results showed that overexpression of miR-182-5p significantly decreased, while knockdown of miR-182-5p increased the luciferase activity of TLR4 with wt 3’-UTR, it did not influence that of TLR4 with mutant 3’-UTR (Figure 4C). Then, we performed qRT-PCR and Western blotting assays to examine whether miR-182-5p could modulate expression of TLR4 in C8-D1A cells. Results showed that overexpression of miR-182-5p in C8-D1A cells significantly reduced expression of TLR4, whereas knockdown of miR-182-5p increased its expression at mRNA and protein levels (Figure 4D, 4E). Furthermore, we also investigated whether miR-182-5p regulates expression of TLR4 in an SCI cell model. As expected, H2O2 enhanced expression of TLR4 while overexpression of miR-182-5p reduced the inhibitory effect of H2O2 on TLR4 expression. Knockdown of miR-182-5p enhanced the promoting effect of H2O2 on TLR4 expression. All data indicate that miR-182-5p may exert anti-inflammatory effects through suppressing expression of TLR4.
Figure 4.
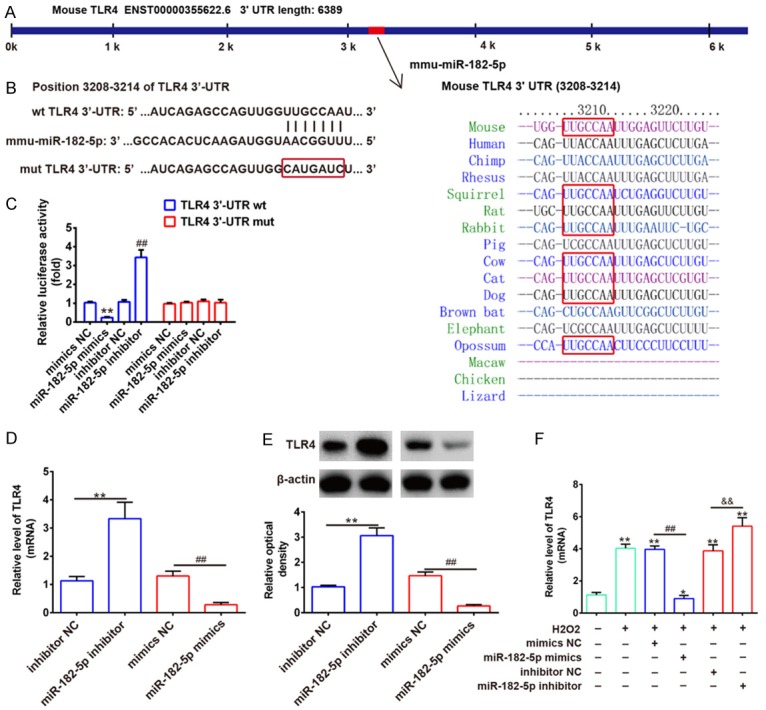
TLR4 is a direct target of miR-182-5p. A, B. The putative binding site of miR-182-5p and TLR4 is shown. C. Luciferase assay of HEK293 cells co-transfected with firefly luciferase constructs containing the TLR4 wild-type or mutated 3’-UTRs and miR-182-5p mimics, mimics NC, miR-182-5p inhibitor or inhibitor NC, as indicated (n = 3). Data represents the mean ± SD of three independent experiments. **P < 0.01 vs. mimics NC, ##P < 0.01 vs. inhibitor NC. D, E. Expression of TLR4 mRNA and protein after transfection with miR-182-5p mimic or miR-182-5p inhibitor was measured by qRT-PCR and Western blot. Data represents the mean ± SD of three independent experiments. **P < 0.01 vs. inhibitor NC, ##P < 0.01 vs. mimics NC. F. C8-D1A cells were transfected with miR-182-5p mimics or miR-182-5p inhibitor for 48 hours and then exposed to 200 μM H2O2 for 10 h. TLR4 expression was assessed by qRT-PCR. Data represents the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01 vs. control group. ##P < 0.01 vs. H2O2 + mimics NC group. &&P < 0.01 vs. H2O2 + inhibitor NC group.
Overexpression of miR-182-5p attenuated H2O2-induced inflammatory response and apoptosis by targeting TLR4
To further clarify whether TLR4 mediates the functional effects of miR-182-5p on H2O2-treated C8-D1A cells, TLR4 expression vector and miR-182-5p mimics were co-transfected into C8-D1A cells followed by H2O2 treatment. Then, the condition of apoptosis and degree of inflammatory response were assessed again. As shown in Figure 5A, we found that apoptosis suppressed by overexpression of miR-182-5p in H2O2-treated C8-D1A cells was reversed by elevation of TLR4. Similarly, secretion of pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 was suppressed by elevation of miR-182-5p while inhibitory effect was rescued by TLR4 overexpression (Figure 5B-D). All data strongly confirms that effects of miR-182-5p on inflammatory response and apoptosis are achieved through the target TLR4.
Figure 5.
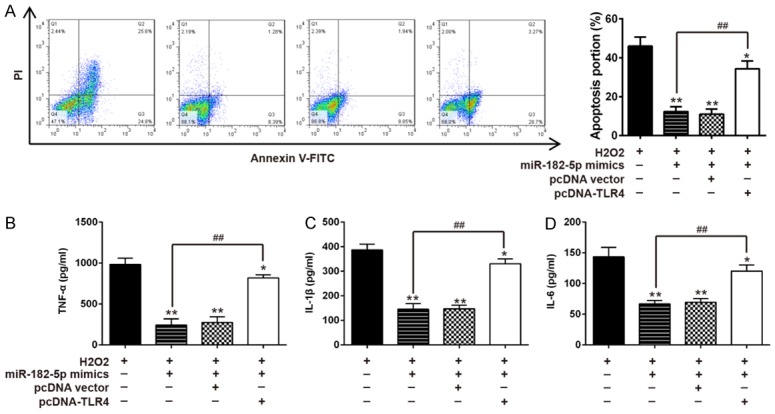
TLR4 overexpression abrogates the inhibitory effects of miR-182-5p mimics on H2O2 treated C8-D1A cells. C8-D1A cells were co-transfected with miR-182-5p mimics and pcDNA-TLR4 for 48 h and then exposed to 200 μM H2O2 for 10 h. A. Apoptosis was detected by flow cytometry. B-D. TNF-α, IL-6, and IL-1β levels in were measured by ELISA assay. Data represents the mean ± SD of three independent experiments. **P < 0.01 vs. H2O2 alone group, ##P < 0.01 vs. H2O2 + miR-182-5p mimics group.
Overexpression of miR-182-5p blocked TLR4/NF-κB pathway in H2O2-treated C8-D1A cells
As we know, NF-κB is a key regulator involved in the inflammatory process. Our results show that miR-182-5p inhibits H2O2-induced activation of TLR4, which can directly affect the NF-κB pathway [20,21]. Therefore, we hypothesize that miR-182-5p regulates NF-κB pathway in an SCI cell model. To further confirm that miR-182-5p restricts inflammatory responses by targeting the TLR4-mediated NF-κB signaling pathway, we measured protein expression levels of phosphorylated NF-κB p65 (p-p65) and phosphorylated IκBα (p-IκBα) by Western blotting. These results showed that there was a significant increase in phosphorylated p65 level and a decrease of p-IκBα in the H2O2 group compared to that in the control groups (Figure 6A, 6B). These values, however, were reversed by miR-182-5p in C8-D1A cells (Figure 6A, 6B). Moreover, the inhibitory effect of miR-182-5p was liberated when TLR4 was overexpressed in H2O2-treated C8-D1A cells (Figure 6A, 6B). These results indicate that miR-182-5p inhibits H2O2 induced inflammatory responses by attenuating release of pro-inflammatory cytokines through inhibition of TLR4/NF-κB activation.
Figure 6.
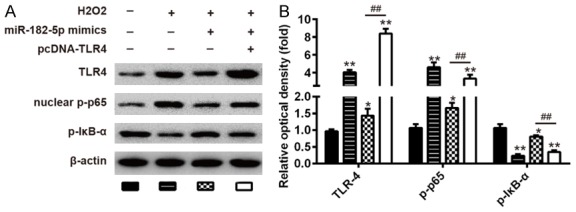
Overexpression of miR-182-5p blocked TLR4/NF-κB pathway in SCI cell model. C8-D1A cells were co-transfected with miR-182-5p mimics and pcDNA-TLR4 vector for 48 hours and then exposed to 200 μM H2O2 for 10 h. A. The levels of TLR4, nuclear p-p65, and p-IκB-α were measured by Western blot. B. The bands were semi-quantitatively analyzed by using Image J software, normalized to β-actin density. Data represents the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01 vs. H2O2 alone group. ##P < 0.01 vs. H2O2 + miR-182-5p mimics group.
Discussion
In our present study, we found that miR-182-5p was significantly downregulated in a rat SCI model. Notably, overexpression of miR-182-5p inhibited apoptosis and inflammation induced by H2O2 in C8-D1A cells. Moreover, TLR4 was proven to be a direct target of miR-182-5p and overexpressed TLR4 reversed inhibitory effects of miR-182-5p on apoptosis and inflammation in H2O2 treated C8-D1A cells. Furthermore, we demonstrated that miR-182-5p exerted its anti-inflammation role through modulation of TLR4/NF-κB pathway. Therefore, miR-182-5p may serve as a potential future therapeutic target for SCI.
Mounting evidence has confirmed that miRNAs are rich in the central nervous system (CNS) and play a major role in several pathological processes and pathological states such as brain tumors, neurodegenerative disease, and brain injury [22-24]. Recently, several studies have shown that miRNAs were altered after SCI and these alterations were associated with secondary injury responses including oxidative stress, inflammation, and apoptosis [25,26]. For example, Zhu et al. found that miR-494 was the most downregulated miRNA following SCI and upregulation of miR-494 by agomir improved functional recovery, reduced tissue damage, and inhibited apoptotic cells in a rat model of SCI [27]. Gao et al. demonstrated that miR-137 inhibited inflammatory response and apoptosis after spinal cord injury by targeting MK2 [5]. Therefore, clarification of the role of microRNA in SCI is very important and may generate a potential therapeutic strategy for SCI. In this study, we identified a large number of miRNAs that were significantly deregulated after SCI using miRNA microarray, suggesting that these miRNAs may play key roles in SCI.
For downregulated miRNAs, miR-182-5p was one of the most downregulated and has also been reported to play different roles in inflammatory diseases. For example, miR-182-5p ameliorated liver ischemia-reperfusion injury through inhibition of inflammatory reaction [13]. Another study from Blaya et al. showed that miR-182 was increased in biliary cells in mice fed with 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet and inhibition of miR-182 in DDC-fed mice reduced liver damage and inflammatory response [14]. Therefore, it is reasonable to speculate that miR-182-5p may play an important role in inflammatory processes of SCI. In the present study, we explored the function of miR-182-5p in SCI cell models on the basis of its impact on inflammatory reaction. We first validated that miR-182-5p expression was dramatically inhibited upon H2O2 stimulation in a dose-dependent manner. Sequentially, we found that overexpression of miR-182-5p not only inhibited apoptosis but also suppressed inflammation response induced by H2O2 in C8-D1A cells. These data indicate that miR-182-5p could inhibit inflammation response in an SCI cell model.
Recently, TLR4 has been found in both CNS glia and neurons and its activation has attracted significant attention in studies regarding the pathophysiology of SCI. For example, genetic ablation of TLR4 significantly reduced histological damage to the spinal cord and improved motor function in a mouse model of SCI [28]. Interestingly, TLR4 has been found to be a direct target of miR-182-5p in liver ischemia-reperfusion injury [13], prompting us to investigate interaction between miR-182-5p and TLR4 in SCI. In this study, we demonstrated that TLR4 was a direct target of miR-182-5p in C8-D1A cells. More importantly, we found that overexpression of TLR4 reversed inhibitory effects of miR-182-5p on inflammation response and apoptosis in H2O2 treated C8-D1A cells. These results suggest that miR-182-5p may have a protection effect and anti-inflammatory property in SCI via suppressing TLR4 expression. However, these possible molecular mechanisms require further research to be fully understood.
It is well known that activation of TLR4 triggers nuclear translation of NF-κB, thus, initiating production of pro-inflammatory cytokines including TNF-α and IL-6 [29-31]. For example, He et al. found that L-3-n-butylphthalide (NBP) treatment improved tissue repair and attenuated inflammatory responses in an SCI rat model via inhibition of TLR4/NF-κB pathway [32]. To further explore the anti-inflammatory mechanism of miR-182-5p in an SCI cell model, expression levels of TLR4, p-p65, and IκB-α were determined. We found that treatment with H2O2 increased expression of TLR4 and p-p65 but decreased expression of IκB-α, indicating that H2O2 could promote activation of NF-κB signaling pathway in C8-D1A cells. Moreover, overexpression of miR-182-5p markedly attenuated NF-κB activation induced by H2O2. Still, TLR4 could partially rescue the inhibition of NF-κB signaling mediated by miR-182-5p. These results confirm that the anti-inflammatory activity of miR-182-5p in an SCI cell model is through inhibition of TLR4/NF-κB mediated pro-inflammatory signaling cascades.
In conclusion, our present study demonstrated that levels of miR-182-5p were significantly downregulated in SCI rats and overexpression of miR-182-5p protected against C8-D1A cell injury through a mechanism involving suppression of inflammation by modulating TLR4/NF-κB pathway. These data indicate that miR-182-5p/TLR4/NF-κB axis may be a promising strategy for targeted SCI therapies.
Acknowledgements
This work was supported by Scientific Research Project of Chinese Medicine of Shanghai Health and Family Planning Committee (No. 2014LP026B and No. ZJ2016008) and Plan of Shanghai Commision of Science and Technology (No. 16401933900).
Disclosure of conflict of interest
None.
References
- 1.Rahimi-Movaghar V, Yazdi A, Karimi M, Mohammadi M, Firouzi M, Zanjani LO, Nabian MH. Effect of decompression on complete spinal cord injury in rats. Int J Neurosci. 2008;118:1359–1373. doi: 10.1080/00207450701392340. [DOI] [PubMed] [Google Scholar]
- 2.Rahimi-Movaghar V, Saadat S, Vaccaro AR, Ghodsi SM, Samadian M, Sheykhmozaffari A, Safdari SM, Keshmirian B. The efficacy of surgical decompression before 24 hours versus 24 to 72 hours in patients with spinal cord injury from T1 to L1--with specific consideration on ethics: a randomized controlled trial. Trials. 2009;10:77. doi: 10.1186/1745-6215-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeVivo MJ, Go BK, Jackson AB. Overview of the national spinal cord injury statistical center database. J Spinal Cord Med. 2002;25:335–338. doi: 10.1080/10790268.2002.11753637. [DOI] [PubMed] [Google Scholar]
- 4.Zhou HJ, Wang LQ, Xu QS, Fan ZX, Zhu Y, Jiang H, Zheng XJ, Ma YH, Zhan RY. Downregulation of miR-199b promotes the acute spinal cord injury through IKKbeta-NF-kappaB signaling pathway activating microglial cells. Exp Cell Res. 2016;349:60–67. doi: 10.1016/j.yexcr.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Gao L, Dai C, Feng Z, Zhang L, Zhang Z. MiR-137 inhibited inflammatory response and apoptosis after spinal cord injury via targeting of MK2. J Cell Biochem. 2018;119:3280–3292. doi: 10.1002/jcb.26489. [DOI] [PubMed] [Google Scholar]
- 6.He J, Zhao J, Peng X, Shi X, Zong S, Zeng G. Molecular mechanism of MiR-136-5p targeting NF-kappaB/A20 in the IL-17-mediated inflammatory response after spinal cord injury. Cell Physiol Biochem. 2017;44:1224–1241. doi: 10.1159/000485452. [DOI] [PubMed] [Google Scholar]
- 7.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 8.Baranwal S, Alahari SK. miRNA control of tumor cell invasion and metastasis. Int J Cancer. 2010;126:1283–1290. doi: 10.1002/ijc.25014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11:441–450. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Hu JZ, Huang JH, Zeng L, Wang G, Cao M, Lu HB. Anti-apoptotic effect of microRNA-21 after contusion spinal cord injury in rats. J Neurotrauma. 2013;30:1349–1360. doi: 10.1089/neu.2012.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu Y, Wu Y, Zhang R. Electro-acupuncture promotes the proliferation of neural stem cells and the survival of neurons by downregulating miR-449a in rat with spinal cord injury. EXCLI J. 2017;16:363–374. doi: 10.17179/excli2017-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang HY, Zhang X, Wang ZG, Shi HX, Wu FZ, Lin BB, Xu XL, Wang XJ, Fu XB, Li ZY, Shen CJ, Li XK, Xiao J. Exogenous basic fibroblast growth factor inhibits ER stress-induced apoptosis and improves recovery from spinal cord injury. CNS Neurosci Ther. 2013;19:20–29. doi: 10.1111/cns.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang W, Liu G, Tang W. MicroRNA-182-5p ameliorates liver ischemia-reperfusion injury by suppressing toll-like receptor 4. Transplant Proc. 2016;48:2809–2814. doi: 10.1016/j.transproceed.2016.06.043. [DOI] [PubMed] [Google Scholar]
- 14.Blaya D, Coll M, Rodrigo-Torres D, Vila-Casadesus M, Altamirano J, Llopis M, Graupera I, Perea L, Aguilar-Bravo B, Diaz A, Banales JM, Claria J, Lozano JJ, Bataller R, Caballeria J, Gines P, Sancho-Bru P. Integrative microRNA profiling in alcoholic hepatitis reveals a role for microRNA-182 in liver injury and inflammation. Gut. 2016;65:1535–1545. doi: 10.1136/gutjnl-2015-311314. [DOI] [PubMed] [Google Scholar]
- 15.Han X, Lu M, Wang S, Lv D, Liu H. Targeting IKK/NF-kappaB pathway reduces infiltration of inflammatory cells and apoptosis after spinal cord injury in rats. Neurosci Lett. 2012;511:28–32. doi: 10.1016/j.neulet.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 16.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradbury EJ, McMahon SB. Spinal cord repair strategies: why do they work? Nat Rev Neurosci. 2006;7:644–653. doi: 10.1038/nrn1964. [DOI] [PubMed] [Google Scholar]
- 18.Chakravarti A, Zhai G, Suzuki Y, Sarkesh S, Black PM, Muzikansky A, Loeffler JS. The prognostic significance of phosphatidylinositol 3-kinase pathway activation in human gliomas. J. Clin. Oncol. 2004;22:1926–1933. doi: 10.1200/JCO.2004.07.193. [DOI] [PubMed] [Google Scholar]
- 19.Wilflingseder J, Jelencsics K, Bergmeister H, Sunzenauer J, Regele H, Eskandary F, Reindl-Schwaighofer R, Kainz A, Oberbauer R. miR-182-5p Inhibition ameliorates ischemic acute kidney injury. Am J Pathol. 2017;187:70–79. doi: 10.1016/j.ajpath.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Fan HY, Qi D, Yu C, Zhao F, Liu T, Zhang ZK, Yang MY, Zhang LM, Chen DQ, Du Y. Paeonol protects endotoxin-induced acute kidney injury: potential mechanism of inhibiting TLR4-NF-kappaB signal pathway. Oncotarget. 2016;7:39497–39510. doi: 10.18632/oncotarget.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chunzhi G, Zunfeng L, Chengwei Q, Xiangmei B, Jingui Y. Hyperin protects against LPS-induced acute kidney injury by inhibiting TLR4 and NLRP3 signaling pathways. Oncotarget. 2016;7:82602–82608. doi: 10.18632/oncotarget.13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogata K, Sumida K, Miyata K, Kushida M, Kuwamura M, Yamate J. Circulating miR-9* and miR-384-5p as potential indicators for trimethyltin-induced neurotoxicity. Toxicol Pathol. 2015;43:198–208. doi: 10.1177/0192623314530533. [DOI] [PubMed] [Google Scholar]
- 23.Krichevsky AM. MicroRNA profiling: from dark matter to white matter, or identifying new players in neurobiology. ScientificWorldJournal. 2007;7:155–166. doi: 10.1100/tsw.2007.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kosik KS. The neuronal microRNA system. Nat Rev Neurosci. 2006;7:911–920. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- 25.Liu NK, Wang XF, Lu QB, Xu XM. Altered microRNA expression following traumatic spinal cord injury. Exp Neurol. 2009;219:424–429. doi: 10.1016/j.expneurol.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu G, Keeler BE, Zhukareva V, Houle JD. Cycling exercise affects the expression of apoptosis-associated microRNAs after spinal cord injury in rats. Exp Neurol. 2010;226:200–206. doi: 10.1016/j.expneurol.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu H, Xie R, Liu X, Shou J, Gu W, Gu S, Che X. MicroRNA-494 improves functional recovery and inhibits apoptosis by modulating PTEN/AKT/mTOR pathway in rats after spinal cord injury. Biomed Pharmacother. 2017;92:879–887. doi: 10.1016/j.biopha.2017.05.143. [DOI] [PubMed] [Google Scholar]
- 28.Impellizzeri D, Ahmad A, Di Paola R, Campolo M, Navarra M, Esposito E, Cuzzocrea S. Role of Toll like receptor 4 signaling pathway in the secondary damage induced by experimental spinal cord injury. Immunobiology. 2015;220:1039–1049. doi: 10.1016/j.imbio.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Froy O. Regulation of mammalian defensin expression by Toll-like receptor-dependent and independent signalling pathways. Cell Microbiol. 2005;7:1387–1397. doi: 10.1111/j.1462-5822.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- 31.Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, Birrer MJ, Szabo E, Zon LI, Kyriakis JM, Haimovitz-Friedman A, Fuks Z, Kolesnick RN. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 32.He Z, Zhou Y, Lin L, Wang Q, Khor S, Mao Y, Li J, Zhen Z, Chen J, Gao Z, Wu F, Zhang X, Zhang H, Xu HZ, Wang Z, Xiao J. Dl-3-n-butylphthalide attenuates acute inflammatory activation in rats with spinal cord injury by inhibiting microglial TLR4/NF-kappaB signalling. J Cell Mol Med. 2017;21:3010–3022. doi: 10.1111/jcmm.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]


