Abstract
Gliomas are fast growing and usually manifest in an aggressive infiltrative model. MMP2 overexpression is associated with brain tumor malignancy and metastasis formation. The aim of this study was to investigate the influence of MMP2 on glioma formation and clinical outcomes by performing analysis at the DNA, RNA, and protein levels. Methylation status and mRNA level were evaluated in 162 samples; the MMP2 protein level was analyzed in 28 patient preoperative and postoperative blood samples using protein microarray analysis and conventional ELISA. The MMP2 MSP analysis revealed a gradually increasing gene promoter demethylation frequency, and the Kaplan-Meier analysis showed that the methylated gene promoter is related to longer overall survival (Log-rank test X 2 = 12.508, df = 1, P < 0.0001). Relative mRNA expression was significantly downregulated when the promoter was methylated. Pairwise comparison analysis showed statistically significant (Mann-Whitney test, P < 0.05) differences in the MMP2 expression median when comparing different glioma grades. The Kaplan-Meier analysis revealed that low MMP2 expression was associated with better survival (Log-rank test X 2 = 7.732, df = 1, P = 0.005). At the protein level, MMP2 expression in patient sera showed no differences between malignancy grades and patient preoperative and postoperative states, while the ELISA assay showed the tendency of accumulating MMP2 protein in higher malignancy patient sera samples. The Kaplan-Meier analysis showed the tendency of having a shorter survival time with a higher MMP2 protein level in patient sera. MMP2 has a significant role in glioma pathogenesis and could be used as a potential molecular marker for tumor progression.
Keywords: Matrix metallopeptidase 2, gene expression, DNA methylation, ELISA, protein array, glioma
Introduction
Gliomas are the most common among primary brain tumors, with a frequency of approximately 7/100.000 new cases per year [1]. Tumor grading is based on the 2007 WHO classification system that distinguishes gliomas according to histological features [2]. Astrocytomas are the most common and account for 2/3 of all gliomas [3]. Astrocytic tumors are divided into 4 grades: I-pilocytic astrocytoma, II-diffuse astrocytoma, and III and IV are known as anaplastic astrocytoma and glioblastoma multiforme (GMB) respectively. Gliomas, especially assigned as highly malignant (grade III and IV), are fast growing and usually manifest in an aggressive infiltrative model. This is due to a high morbidity and mortality rate: overall survival with GMB diagnosis is less than 2 years, despite all known treatment strategies [4,5]. Treatment effectiveness is also aggravated by gliomas’ heterogeneity-even morphologically identical tumors sometimes are characterized by various reactions to treatment and show different clinical outcomes. Consequently, it is important to identify predictive molecular markers that are critical to the process of glioma formation and malignancy.
Several studies have shown the MMP2 gene as an important marker in glioma genesis. [6-9]. Protein coded by the MMP2 gene is known as matrix metallopeptidase 2 and belongs to the large family of zinc-dependent endopeptidases [6]. A protein with the property of degradation of IV collagen (structural component of extracellular matrix) is involved in physiological processes such as X chromosome inactivation, tissue specific gene expression, and morphogenesis [10]. However, it is also believed to be involved in various pathological processes during glioma formation [9]. MMP2 overexpression is associated with brain tumor malignancy and metastasis formation [11]. Mice models have also shown a significant correlation between the MMP2 protein and survival time, where GBM-bearing mice had considerably better survival prognosis in the absence of the MMP2 protein [9]. MMP2 is also associated with neo-angiogenesis and tumor vascularization [12]. Studies in mice showed a correlation between the MMP2 protein concentration and the increasing formation of new blood vessels with a variable vascular phenotype. Nevertheless, newly formed vasculature was characterized by an irregular structure and shape, possibly causing glioma cells to be more prone to apoptosis [9]. Therefore, the MMP2 gene could be expected to have a dual role in tumor growth, and further studies are crucial for a better understanding of the origin of glioma. The aim of this study was to investigate the influence of MMP2 on glioma formation and clinical outcomes by performing analysis at the DNA, RNA, and protein levels.
Materials and methods
Patients and sample collection
This study was approved by the Kaunas Regional Biomedical Research Ethics Committee. All participants provided written informed consent in accordance with the Helsinki Declaration. Patient glioma tumor tissues were obtained after resection at the National Centre of Neurosurgery at the Hospital of Lithuanian University of Health Sciences Kauno Klinikos between 2004 and 2016. Database closure was in April 2017, and patients who were still alive at this date were censored during further analysis. All tumors were histologically diagnosed and assigned to a malignancy grade at the Department of Pathology of the Hospital of Lithuanian University of Health Sciences Kauno Klinikos according to the 2007 World Health Organization (WHO) classification [2]: Tissue specimens were snap-frozen in liquid nitrogen immediately post resection and stored until further analysis.
The Methylation status and mRNA level were evaluated in 162 samples of different grades of glioma: 10 samples were diagnosed as I grade astrocytoma, 45 as II grade, 24-III and 83 had IV grade GMB diagnosis. A MMP2 protein analysis was performed on 28 patients’ blood samples before and after tumor resection (II-8, III-3, IV-17) using a protein microarray analysis, and 33 glioma blood samples before tumor resection (II-8, III-3, IV-22), and 17 healthy controls with ELISA analysis.
DNA extraction and bisulfite modification
DNA was extracted from ~100 mg frozen tissue sample using the desalting method with chloroform. DNA concentrations were measured with the NanoDropTM 2000 system. 400 ng of extracted DNA was used for the bisulfite treatment. The bisulfite modification was performed using an EZ DNA Methylation™ Kit (ZymoResearch).
Evaluation of MMP2 promoter methylation status
The MMP2 promoter methylation status was determined using methylation specific PCR (MSP). The reaction was performed in 15 µL total volume, consisting of: 7.5 µL Hot Start PCR Master Mix with Hot start Taq DNA polymerase (Thermo Fisher Scientific), 4.5 µL nuclease-free water (Thermo Fisher Scientific), 1 µL (10 pmol/µL) of each primer, specific to methylated/unmethylated promoter (Metabion International) and ~20 ng of bisulfite-treated DNA as a template. Primers sequences for methylated MMP2 sequence were 5’-GGACGTTAAGGGTTTAGAGC-3’ (sense), 5’-CAATACACGACCTCGTCAC-3’ (antisense), for unmethylated-5’-GGATGTTAAGGGTTTAGAGT-3’ (sense), 5’-CAATACACAACCTCATCAC-3’ (antisense). Also, three controls were performed: positive- “Bisulfite converted Universal Methylated Human DNA Standard & Control primer “(Zymo Research), negative-bisulfite treated human blood lymphocytes DNA and water control (no template control). MS-PCR performed in 38 cycles with following conditions: Taq Polymerase activation 95°C 5 min, denaturation 95°C 15 sec, annealing 59°C 30 sec, extension 72°C 15 sec and final extension 72°C 5 min. The products after amplification were visualized using the agarose gel electrophoresis method. Each sample methylation status was evaluated according to visible signals and documented using an 0 (unmethylated) and 1 (methylated) system.
RNA extraction, cDNA synthesis and gene expression analysis
Total RNA from the homogenized sample was purified using TRIzol reagent (Life Technologies) and ultrasound (ultrasonic processor, Cole Parmer). The extracted RNA concentration and purity were evaluated using NanoDropTM for spectophotometric analysis. The reverse transcription (RT) reaction with random hexamer primers (Thermo Fisher Scientific) and RevertAid H Minus M-MuLV Reverse Transcriptase (Thermo Fisher Scientific) was performed in a total volume of 20 µL according to the manufacturer’s protocol. 2 µg of purified RNA was used for synthesis. After synthesis, cDNA samples were diluted to 5 ng/µL and stored at -80°C until analysis. qRT-PCR performed with TaqMan probes on 7500 Fast Real-time PCR detection system (Applied Biosystems). Reactions were made in triplicate in a total volume of 12 µl, which included 15 ng of sample cDNA, TaqMan Universal Master Mix (Thermo Fisher Scientific), TaqMan Gene Expression Assay for MMP2 (Hs01548727_m1) (Thermo Fisher Scientific), and nuclease-free water (Thermo Fisher Scientific). GAPDH was used as a reference gene and “FirstChoice® Human Brain Reference Total RNA” (Ambion) after cDNA synthesis was applied as an endogenous control. The reaction was performed in 40 cycles at the following conditions: 95°C 10 min, 95°C 15 sec, 60°C 1 min. After reaction, all plates were normalized according to endogenous control and an average of CT (threshold cycle) for both target (MMP2) and reference (GAPDH) genes in each sample were calculated. Data were analysed using the log2 (2-ΔΔCt) method where: CT1 = CT (MMP2)-CT (GAPDH); CT2 = CT (MMP2 endogenous control value)-CT (GAPDH endogenous control value); ΔΔCT = CT1-CT2. Finally, log2 (2-ΔΔCt) was calculated.
MMP2 protein level evaluation in patient blood serum
Patients’ protein levels in blood serum was measured using a protein microarray [13]. Preoperative venous blood from astrocytic glioma patients was collected before any invasive procedures, chemo- or radiotherapy. Postoperative blood was collected 7 days after tumour resection. For controls we invited healthy subjects with no indications of glioma and no signs of infection or inflammation at the day blood was collected. Blood from healthy individuals was collected using the same procedure as for astrocytic glioma patients. Blood samples were collected using 3 ml vacutainer system. Within one-hour blood was centrifuged for 10 min. at 1300 × g and after dividing into aliquots supernatant (blood serum) was stored at -80°C. The MMP2 in each patient’s blood serum was measured using the “Custom Human Cytokine Antibody Array” (10 targets) (Abcam). It’s an ELISA based analytical-type protein array: antibodies against 10 different proteins, including MMP2, are printed in duplex on a nitrocellulose membrane by the manufacturer. All procedures were carried out according to the manufacturer’s protocol [13]: 5 × diluted blood serum was added on the membrane and incubated overnight at 4°C. The next day, the array was washed and incubated with a biotin-conjugated secondary antibody cocktail against targets. After washing, the membrane was incubated with HRP-streptavidin, washed again and covered with a detection buffer mixture. Signals were detected with chemiluminescence using “BioSpectrum Imaging system” (UVP, Analytik Jena Company, UK). The software program ImageJ (National Institutes of Health, USA) was used to evaluate the results: each spot intensity was measured using equal squares. Background-same membrane Negative Control Spots (printed buffer with no antibodies) averaged signal-were eliminated from each result. For every target, including MMP2, duplex signal intensities were averaged. Each membrane was normalized too: one membrane was selected as the “reference membrane” and the other membranes’ results were calculated using the algorithm: X(Ny) = X(y) * P1/P(y), where: P1-mean signal density of positive control spots (controlled amount of biotinylated antibody printed onto the membrane) on reference membrane; P(y)-mean signal density of positive control spots on membrane “Y”; X(y)-” mean signal density on membrane “y”; X(Ny)-normalized signal intensity for spot “X” on membrane “Y”.
ELISA
The enzyme-linked immunosorbent assay (ELISA) was used for a quantitative analysis of the human glioma serum samples. The analysis was performed with the commercial “MMP2 Human ELISA kit” (Thermo Fisher Scientific) according to the manufacturer’s recommendations. The serum samples were diluted 15-fold for the determination of MMP-2. Optical density was measured at a wavelength of 450 nm with the absorbance reader “Sunrise” (Tecan Trading). The intra-assay coefficient of variation (CV%) of MMP-2 is reported by the manufacturer to be 3.2% at a mean concentration of 24 ng/mL, SD = 0.8. The cutoff point was 160 ng/mL for MMP-2. The positive results of MMP-2 are below the cutoff values. The samples were assayed in duplicate and the mean concentrations were used for statistical analysis.
Statistical analysis
The statistical analysis was carried out with the software of IBM SPSS Statistics 22 (IBM SPSS) and GraphPad Software Inc. “Prism 6”. All continuous variables were described as median. In order to evaluate methylation status differences between tumor grades, the mRNA levels, and the patients’ clinical data (gender, age, survival group), a Pearson’s chi-square test was used. Gene expression differences across the gene methylation groups were evaluated using a Mann-Whitney test and across grade groups using a Kruskal-Wallis test. To evaluate differences in protein levels in patients’ blood serum in two dependent groups, Wilcoxon test was used and for evaluation of the statistical differences between the two independent groups-Mann-Whitney test. The survival time of patients was calculated from the date of operation until the date of death, or the date of the last follow-up and analyzed with the Kaplan-Meier curves using a Log-rank test. To test the statistical hypothesis, the significance level of 0.05 was selected.
Results
MMP2 promoter methylation is an indicator of better prognosis in glioma
The MMP2 promoter methylation status was measured in 162 samples of different grades of glioma. As mentioned before, an active form of MMP2 coding protein is believed to be involved in a variety of pathological processes during glioma formation. As shown in Figure 1A this group consisted of 55 samples with variations in the percentages of determined unmethylated promoter status: 20% (2/10) of pilocytic astrocytoma, 17.8% (8/45) of diffuse astrocytoma, 29.2% (7/24) and 45.8% (38/83) of anaplastic astrocytoma and glioblastoma multiforme, respectively. The analysis revealed gradually increasing gene’s unmethylated promoter frequency with significant increment in GMB. As the results have shown a possible tendency that the promoter methylation status could be inversely proportional to the glioma prognosis, we decided to evaluate the interface between this modification and patients’ survival. The Kaplan-Meier analysis has shown (Figure 1B) that the methylated gene‘s promoter is related to longer overall survival (Log-rank test X 2 = 12.508, df = 1, P < 0.001). Also in order to evaluate correlations between promoter methylation and the patients’ clinical data, the methylation statuses were analysed in different age groups (≤ 60, > 60), gender (male, female), and survival time (≤ 24, > 24 months). As shown in Table 1, the analysis failed to show any significant correlation between the promoter methylation and the patient‘s gender and age (χ 2 = 8.835, df = 1, P > 0.05). However, analysis revealed that 2-year survival was more likely in the case of the methylated gene promoter (χ 2 = 11.540, df = 1, P = 0.003). Therefore, results indicate that the lack of promoter methylation in the case of the MMP2 gene is associated with glioma malignancy and aggressiveness.
Figure 1.
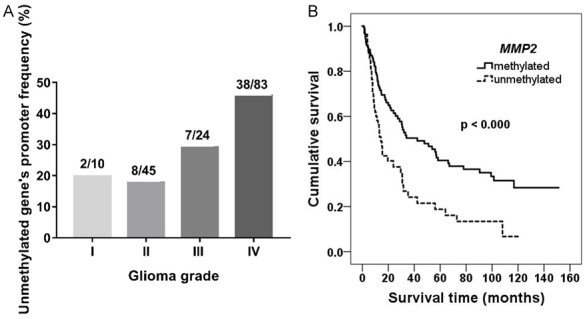
Analysis of unmethylated MMP2 promoter effect on patient survival prognosis. A. Unmethylated genes’ promoter frequency (%) in different grades of glioma. B. Kaplan-Meier survival curves, indicating survival (months) dependency from MMP2 promoter methylation status in all glioma patients; Log-rank test χ 2 = 12.508, df = 1, P < 0.000.
Table 1.
Relationship between MMP2 promoter methylation status and clinical patients’ data
| N | Methylated (%) | Unmethylated (%) | p value | ||
|---|---|---|---|---|---|
| Cases | 162 | 107 (66.0) | 55 (34.0) | ||
| Tumor grade | I | 10 | 8 (80.0) | 2 (20.0) | 0.009 |
| II | 45 | 37 (82.2) | 8 (17.8) | ||
| III | 24 | 17 (70.8) | 7 (29.2) | ||
| IV | 83 | 45 (54.2) | 38 (45.8) | ||
| Low grade glioma | 55 | 45 (81.8) | 10 (18.2) | 0.002 | |
| High grade glioma | 107 | 62 (57.9) | 45 (42.1) | ||
| Gender | Male | 73 | 50 (68.5) | 23 (31.5) | 0.552 |
| Female | 89 | 57 (64.0) | 32 (36.0) | ||
| Age, yr | > 60 | 51 | 31 (60.8) | 20 (39.2) | 0.337 |
| ≤ 60 | 111 | 76 (68.5) | 35 (31.5) | ||
| Survival (months) | > 24 | 67 | 53 (79.1) | 14 (20.9) | 0.003 |
| ≤ 24 | 95 | 54 (56.8) | 41 (43.2) |
Promoter methylation plays an important role in MMP2 activity
As mentioned in the previous section, the methylation analysis showed a gradually increasing unmethylated gene promoter frequency, with the highest rate in the GMB. Since methylation is thought to be one of the main mechanisms controlling gene expression, we decided to check whether the activity of a gene is dependent on this modification. MMP2 mRNA levels were measured using qRT-PCR with TaqMan probes in the same set that was used in DNA methylation analysis. mRNA levels were calculated using 2-ΔΔCt method: all samples were normalized to the values of the housekeeping gene GAPDH mRNA level. All results were calculated as relative expression values in log2 of fold change, including comparison with determined MMP2 mRNA levels in normal brain control. The study revealed (Figure 2A) a significant (Mann-Whitney test P = 0.024), inversely proportional correlation between MMP2 activity and promoter methylation status, indicating methylation’s importance in gene functional activity. In order to evaluate whether the interface between methylation status and the mRNA level values are grade dependent, further analysis was performed. Expression levels in tumors with methylated MMP2 promoter were compared with those with an unmethylated gene promoter in each group of glioma grade. However, the Mann-Whitney analysis (Figure 2B) did not show any significant correlations (P > 0.050). Higher expression in anaplastic tumors in cases when the promoter was methylated (P = 0.048) could be associated with an insufficient number of samples.
Figure 2.
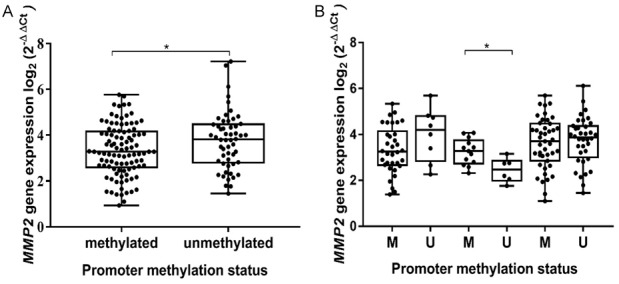
MMP2 mRNA level dependency on promoter methylation status. A. Each box represents distribution of MMP2 gene expression values according to promoter methylation status (M vs U). B. Boxes represent samples with methylated (M) and unmethylated (U) gene promoter in different grades of glioma. Middle lines mark median expression; lines outside the boxes represent maximum and minimum values; asterisk indicates significant difference (*P < 0.05). The relative mRNA expression was significantly downregulated when the promoter was methylated; however, this tendency was not dependent on grade.
High MMP2 activity is associated with worse clinical outcomes
Since it was demonstrated that MMP2 activity is dependent on promoter methylation status, the next step was to determine the impact of an active gene form on the prognosis of glioma. As shown in Figure 3A, the highest median of mRNA values was detected in glioblastoma multiforme, and the lowest-was detected in grade I pilocytic astrocytomas. These findings suggest that increased expression of MMP2 could be one of the causative factors during glioma formation. Also, we performed a pairwise comparison analysis, and it also shown to have statistically significant (Mann-Whitney test, P < 0.05) differences in MMP2 expression median when comparing different grades of glioma (Figure 3A). Also it was assessed whether mRNA levels are related to the clinical patient’s data, unfortunately no significant correlations were detected (age: P = 0.052; gender: P = 0.151; 2-years survival: P = 0.1484).
Figure 3.
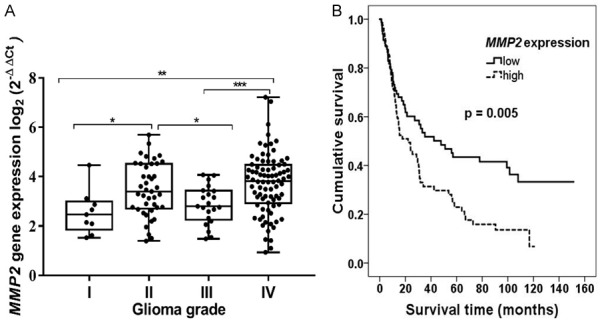
Connection between expression of MMP2, glioma grade and prognosis. A. Relative MMP2 mRNA expression in different grade glioma. Box plots represent sample distribution among different grades of glioma; asterisks indicate significance levels: *P < 0.05, **P < 0.01, ***P < 0.001. B. Kaplan-Meier survival curves, indicating survival (months) dependency on expression level of MMP2 gene; Log-rank test χ 2 = 7.732, df = 1, P = 0.005.
To better understand an active gene form’s influence on glioma prognosis, the determined expression values were categorised into two groups: “low” and “high”. Groups were divided according the median of the determined MMP2 expression values. The estimated median of the set was 3.327; therefore, the “low” group consisted of samples with an expression value 3.327 and less (n = 81) and the “high” group,-with expression values of 3.327 and higher (n = 81). None of the estimated mRNA values were equal to the determined median. Groups were compared in order to evaluate whether a patient’s survival is connected to the MMP2 expression level. The Kaplan-Meier analysis revealed that patients whose samples were categorised as “low” expression had a better survival prognosis when compared with those with a “high” expression level (Figure 3B, Log-rank test χ 2 = 7.732 df = 1, P = 0.005). Results reaffirmed previous observations, indicating that MMP2 could be an informative predictive marker in glioma diagnosis.
MMP2 protein accumulates in patient serum of higher malignancy gliomas
After completing the gene expression analysis in glioma tumor tissue, we evaluated the MMP2 expression at the protein level in the same patients’ blood serum. Relative protein expression was evaluated by an ELISA-based protein array on nitrocellulose membrane, and the results were further validated using conventional ELISA.
The MMP2 protein expression level on the protein array was measured in 33 glioma patient’s blood serum and 17 controls. A low level of protein expression was observed as compared to the control serum (Figure 4A). There were no differences observed between the MMP2 level in the glioma patients’ preoperative (S1) and postoperative (S2) serum, but the median was higher in the postoperative samples. Further, there were no differences observed among the different malignancy grades (II, III and IV) (Figure 4B), and between the glioma patient and control groups.
Figure 4.
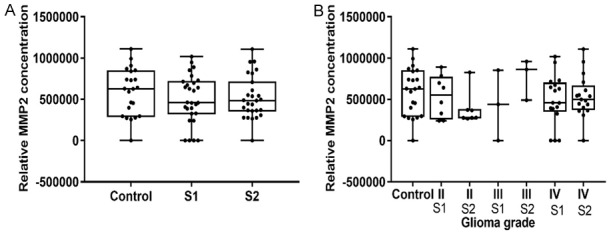
MMP2 protein level in glioma patients’ blood serum, measured using an ELISA-based protein array. A. Relative MMP2 protein concentration in blood serum in healthy controls and glioma patients in preoperative (S1) and postoperative serum (S2). B. Relative MMP2 protein level in blood serum of healthy controls, glioma patients in preoperative (S1) and postoperative serum (S2) in different grades of glioma.
Next, we measured the preoperative serum MMP2 protein concentration using conventional ELISA. The median MMP2 concentration in glioma patient serum was 37.1 ng/ml and ranged from 23.7 ng/ml to 54.3 ng/ml, in the control serum-42.4 ng/ml, and ranged from 29.4 ng/ml to 61.6 ng/ml, in grade II, -31.3 ng/ml, ranged from 23.7 ng/ml to 46.3 ng/ml, in grade III-28.1 ng/ml and ranged from 27.6 ng/ml to 42.9 ng/ml, and in grade IV-40.7 ng/ml and ranged from 31.4 ng/ml to 54.3 ng/ml. Grade II gliomas showed a statistically significant difference compared to the healthy control group (P = 0.015), and there was a statistically significant lower protein concentration compared to the glioblastoma patient serum (P = 0.007), (Figure 5A). The MMP2 protein expression values were categorized into two groups: the “low” protein expression group and the “high” trying to elucidate its influence on glioma prognosis. Groups were divided according to the MMP2 protein expression median value, 37.05 ng/ml, and all values bellow it were considered as “low” (n = 17), while all values above it were considered as “high” (n = 16). The Kaplan-Meier analysis showed that patients, with “low” serum MMP2 protein concentration had a tendency for better survival prognosis compared to patients whose MMP2 level was categorized as “high” (Figure 5B, Log-rank test, χ 2 = 2.261, df = 1, P = 0.133). The MMP2 protein concentration increase in the higher glioma grade shows protein involvement in malignant progression.
Figure 5.
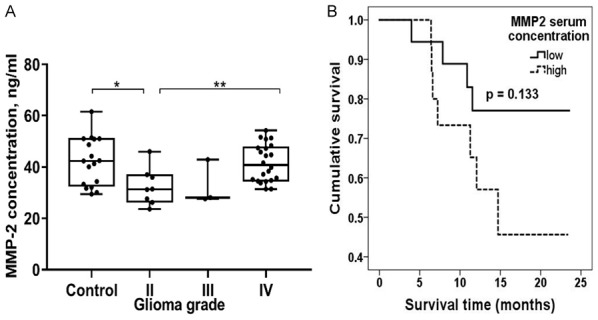
MMP2 protein expression measured using ELISA and glioma prognosis. A. MMP2 protein level in different grade glioma patient’s blood serum; Middle lines mark median. Asterisks indicate significance levels: *P < 0.05, **P < 0.01. B. Kaplan-Meier survival curves, indicating survival (months) dependency on MMP2 protein expression level; Log-rank test χ 2 = 2.261, df = 1; P = 0.133.
Discussion
Glioma is a combined result of a variety of different alterations in a cell occurring at the genetic, epigenetic and protein levels. Due to its complexity, these tumors, especially GBM, are often fatal. [4,5]. Therefore, it is crucial to evaluate the different factors that could have a direct impact on glioma formation and progression. In this study, we uses a variety of approaches to evaluate MMP2’s significance to glioma diagnosis in the DNA, RNA and protein levels. As already mentioned, one of the main MMP2 function is ECM remodelling, leading to cell invasion and metastasis. Chernov et al. [14] in their study using highly migratory glioblastoma and low migratory breast carcinoma cells, showed that MMP2 methylation is the key factor, regulating a gene’s functional activity. Thus, we started our study with the evaluation of methylation’s importance in glioma pathogenesis. In another study, [15] it was also shown that a gene’s methylation was determined in 20% of GBM samples compared to the control group, suggesting that unbalanced epigenetic regulation is an important genetic activity factor. Our analysis revealed that methylation frequency could be grade dependent. A number of studies have also shown that MMP2 activity correlates with worse clinical outcomes and grade of disease in cervical, non-small cell lung, pancreatic and bladder cancer, as well as malignant brain tumors, indicating MMP2’s importance in cancer biology [11,14,16-20]. These findings suggest that loss of methylation could be frequent event during glioma formation. What is more, the results correspond to those from survival analysis where a gene’s promoter methylation was associated with longer overall survival (P < 0.0001).
Analysis at the mRNA level revealed that gene methylation could also possibly affect MMP2 mRNA levels. Shukeir et al. [21] in their study also showed that hypomethylation increases a gene‘s expression and tumor invasiveness in late stage prostate cancer. However, our analysis failed to show any significant difference between promoter methylation status and mRNA levels. In different glioma grades, we found that a higher genetic expression is inversely proportional to methylation status.
We also showed that MMP2 expression at mRNA levels in glioblastoma was strongly increased compared to pilocytic astrocytomas. One other study [6] found a significant increase in MMP2 expression corresponding to glioma malignancy grade with the highest peak in GMB, where 100% of samples had been identified as MMP2 overexpressed. A Kaplan-Meier analysis also revealed that low MMP2 expression is related to better clinical outcomes and longer overall survival. With the consent of other studies, it was shown that methylation is an important mechanism during gliomagenesis that directly affects genetic activity. What is more, an increase in genetic activity seems to be related to glioma grade and clinical outcomes. Taken together, results from our study indicate that MMP2 has a significant role in tumor progression and could be used as a potential molecular marker for early diagnostics.
Next, we showed that the MMP2 protein accumulates in glioma patients’ blood serum samples. In our study MMP2 protein levels were assessed using protein chip and conventional ELISA. Measuring relative MMP2 protein concentration with ELISA-based assay in healthy controls and glioma patient’s serum, we noticed no differences among any of the groups. Xu et al. found that MMP2 in plasma accurately distinguished high-grade glioma patients from the controls [21]. On the other hand, they measured protein levels in the patients’ plasma samples, and not in their blood serum. Moreover, relative concentrations do not always properly reflect proportions with real concentrations.
The ELISA assay showed that the MMP2 concentration’s median value was 42.4 ng/ml and ranged from 29.4 ng/ml to 61.6 ng/ml in healthy control samples, and in glioma patients’ blood samples, the median value was 37.1 ng/ml and the concentration ranged from 23.7 ng/ml to 54.3 ng/ml, and it is around 10 times lower than observed in serum [22] and measured in plasma [23,24] in other studies. However, the MMP2 protein level measured by conventional ELISA showed the tendency that longer overall patient survival is related to lower MMP2 concentrations, indicating that MMP2 is involved in glioma progression.
In conclusion, MMP2 has a significant role in glioma pathogenesis and could be used as a potential molecular marker for tumor progression.
Acknowledgements
This research was funded by a grant (No. MIP-052/2015) from the Research Council of Lithuania.
Disclosure of conflict of interest
None.
References
- 1.Jiang Y, Uhrbom L. On the origin of glioma. Upsala J Med Sci. 2012;117:113–121. doi: 10.3109/03009734.2012.658976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathologica. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker C, Baborie A, Crooks D, Wilkins S, Jenkinson MD. Biology, genetics and imaging of glial cell tumours. Br J Radiol. 2011;84:90–106. doi: 10.1259/bjr/23430927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips T. The role of methylation in gene expression. Nat Educ. 2008;1:138. [Google Scholar]
- 5.Dreyfuss JM, Johnson MD, Park PJ. Meta-analysis of glioblastoma multiforme versus anaplastic astrocytoma identifies robust gene markers. Mol Cancer. 2009;8:71. doi: 10.1186/1476-4598-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagemann C, Anacker J, Ernestus RI, Vince GH. A complete compilation of matrix metalloproteinase expression in human malignant gliomas. World J Clin Oncol. 2012;3:67–79. doi: 10.5306/wjco.v3.i5.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramachandran RK, Sørensen MD, Aaberg-Jessen C, Hermansen SK, Kristensen BW. Expression and prognostic impact of matrix metalloproteinase-2 (MMP-2) in astrocytomas. PLoS One. 2017;12:e0172234. doi: 10.1371/journal.pone.0172234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu B, Guo P, Fang Q, Tao HQ, Wang D, Nagane M, Huang HJ, Gunji Y, Nishikawa R, Alitalo K, Cavenee WK, Cheng SY. Angiopoietin-2 induces human glioma invasion through the activation of matrix metalloprotease-2. Proc Nat Acad Sci U S A. 2003;100:8904–8909. doi: 10.1073/pnas.1533394100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du R, Petritsch C, Lu K. Matrix metalloproteinase-2 regulates vascular patterning and growth affecting tumor cell survival and invasion in GBM. Neuro Oncol. 2008;10:254–264. doi: 10.1215/15228517-2008-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 11.Wang M, Wang T, Liu S, Yoshida D, Teramoto A. The expression of matrix metalloproteinase-2 and -9 in human gliomas of different pathological grades. Brain Tumor Pathol. 2003;20:65–72. doi: 10.1007/BF02483449. [DOI] [PubMed] [Google Scholar]
- 12.Thorns V, Walter GF, Thorns C. Expression of MMP-2, MMP-7, MMP-9, MMP-10 and MMP-11 in human astrocytic and oligodendroglial gliomas. Anticancer Res. 2003;23:3937–3944. [PubMed] [Google Scholar]
- 13.Abcam website: human cytokine antibody array kit. 2017.
- 14.Chernov AV, Sounni NE, Remacle AG, Strongin AY. Epigenetic control of the invasion-promoting MT1-MMP/MMP-2/TIMP-2 axis in cancer cells. J Biol Chem. 2009;284:12727–12734. doi: 10.1074/jbc.M900273200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laffaire J, Everhard S, Idbaih A, Crinière E, Marie Y, de Reyniès A, Ducray F. Methylation profiling identifies 2 groups of gliomas according to their tumorigenesis. Neuro Oncol. 2011;13:84–98. doi: 10.1093/neuonc/noq110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rauvala M, Aglund K, Puistola U. Matrix metalloproteinases-2 and -9 in cervical cancer: different roles in tumor progression. Int J Gynecol Cancer. 2006;16:1297–1302. doi: 10.1111/j.1525-1438.2006.00448.x. [DOI] [PubMed] [Google Scholar]
- 17.Iniesta P, Morán A, De Juan C, Gómez A, Hernando F, García-Aranda C, Frías C, Díaz-López A, Rodríguez-Jiménez F, Balibrea J, Benito M. Biological and clinical significance of MMP-2, MMP-9, TIMP-1 and TIMP-2 in non-small cell lung cancer. Oncol Rep. 2007;17:217–223. [PubMed] [Google Scholar]
- 18.Yokoyama M, Ochi K, Ichimura M, Mizushima T, Shinji T, Koide N, Tsurumi T, Hasuoka H, Harada M. Matrix metalloproteinase-2 in pancreatic juice for diagnosis of pancreatic cancer. Pancreas. 2002;24:344–7. doi: 10.1097/00006676-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Gerhards S, Jung K, Koenig F, Daniltchenko D, Hauptmann S, Schnorr D, Loening S. Excretion of matrix metalloproteinases 2 and 9 in urine is associated with a high stage and grade of bladder carcinoma. Urology. 2001;57:675–9. doi: 10.1016/s0090-4295(00)01087-6. [DOI] [PubMed] [Google Scholar]
- 20.Forsyth PA, Wong H, Laing TD, Rewcastle NB, Morris DG, Muzik H, Edwards DR. Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas. Br J Cancer. 1999;79:1828–1835. doi: 10.1038/sj.bjc.6990291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shukeir N, Pakneshan P, Chen G, Szyf M, Rabbani SA. Alteration of the methylation status of tumor-promoting genes decreases prostate cancer cell invasiveness and tumorigenesis in vitro and in vivo. Cancer Res. 2006;18:9202–9210. doi: 10.1158/0008-5472.CAN-06-1954. [DOI] [PubMed] [Google Scholar]
- 22.Xu BJ, An QA, Srinivasa Gowda S, Yan W, Pierce LA, Abel TW, Rush SZ, Cooper MK, Ye F, Shyr Y, Weaver KD, Thompson RC. Identification of blood protein biomarkers that aid in the clinical assessment of patients with malignant glioma. Int J Oncol. 2012;40:1995–2003. doi: 10.3892/ijo.2012.1355. [DOI] [PubMed] [Google Scholar]
- 23.Kai H, Ikeda H, Yasukawa H. Peripheral blood levels of matrix metalloproteases-2 and -9 are elevated in patients with acute coronary syndromes. J Am Coll Cardiol. 1998;32:368–372. doi: 10.1016/s0735-1097(98)00250-2. [DOI] [PubMed] [Google Scholar]
- 24.Chau KY, Sivaprasad S, Patel N, Donaldson TA, Luthert PJ, Chong NV. Plasma levels of matrix metalloproteinase-2 and -9 (MMP-2 and MMP-9) in age-related macular degeneration. Eye (Lond) 2008;22:855–859. [PubMed] [Google Scholar]


