Abstract
Schwann cells (SCs) play a crucially supportive role in repair of injured peripheral nerve system (PNS). CXCL12 plays a significant role in migration of stem cells and embryonic developmental cells and CXCL12 is strongly chemotactic for a variety of cells. Our study was designed to determine the role of CXCL12 in Schwann cell proliferation and migration. Our study demonstrated that CXCL12 had no effect on Schwann cell proliferation while significantly promoting Schwann cell migration. CXCL12-induced Schwann cell migration was significantly attenuated by inhibition of its receptor CXCR4 and p38 MAPK through co-treatment with AMD3100 and SB203580, separately. Besides, Western blot, QRT-PCR, and ELISA indicated that treatment with CXCL12 enhanced expression of CXCL12 by Schwann cells. In conclusion, CXCL12-enhanced SCs migration is mediated by secreting CXCL12 and p38 MAPK via receptor CXCR4, suggesting that CXCL12 has potential application value for PNS regeneration and could serve as a new therapeutic strategy in peripheral nerve diseases.
Keywords: CXCL12, CXCR4, AMD3100, p38MAPK, migration
Introduction
Peripheral nerves are surrounded by Schwann cells which form myelinated nerve fibers [1]. Peripheral nerves easily become dysfunctional in trauma [2], autoimmune attacks [3], or neurotoxins [4]. There is a close relationship between these deficits and Schwann cells. It has been reported that CXCL12 is a powerful chemokine that can promote directional migration of various stem cells through interaction with its receptor CXCR4 and CXCR7 [5-7]. Furthermore, CXCL12 can promote regeneration of injured motor axon terminals [8]. CXCL12-CXCR4-CXCR7 chemokine axis are therapeutic targets for remyelination in demyelinating diseases [9]. It is still unclear, however, whether CXCL12 can promote migration of Schwann cells. In this study, we investigated the effect of CXCL12 on Schwann cells and its possible molecular mechanism. Our results show that CXCL12 promoted Schwann cell migration by acting on the receptor CXCR4 and p38MAPK and enhancing CXCL12 secretion of Schwann cells. Our research provides a new direction for the study of migration and repair of Schwann cells after peripheral nerve injuries.
Material and methods
Culturing SCs
Newborn 1-3 day SD rats used in this study were in accordance with protocol approved by the Institutional Animal Care and Use Committee of Shanghai Jiao Tong University, China. Newborn rats were killed under anesthesia with chloral hydrate and the bilateral sciatic nerves were taken under microscope and placed in DPBS containing 1% of penicillin-streptomycin. Then, ophthalmic forceps were used to remove epineurium and endoneurium, as soon as possible. After cleaning 3 times, scissors were used to cut nerve blocks into pieces. They were placed into clean 6-well plates and allowed to adhere for about 4 hours. DMEM/F-12 [containing 10% fetal bovine serum (FCS), 1% penicillin-streptomycin solution] was then added carefully to 6-well plates. The tissues were observed every day and culture media were changed every 3 days. Tissues were incubated for 1 week and passaged for 3 generations. This step was done to remove overgrown fibroblast cells and ensure pure and viable Schwann cells.
Immunofluorescence analysis
Immunofluorescent staining was used for characterization of the Schwann cells after 3 generations. After cell fixation with 4% paraformaldehyde (PFA; Sigma, USA) for 10 minutes at room temperature and permeabilization with 0.15% Triton-X 100 (Sigma, USA) for 15 minutes, the cells were blocked with 0.1% BSA (Sigma, USA) for 30 minutes and incubated at 37°C for 1 hour with rabbit monoclonal antibody against S-100 protein (1:1000; Abcam, USA) and rabbit polyclonal antibody against GFAP protein (1:500; Boster, China). After rinsing in PBS 3 times, goat anti-rabbit secondary antibody (1:1000; Jackson, USA) was applied in a dark place at room temperature for 1 hour. Cells were then stained with 4’,6’-diamidino-2-phenylindole hydro-chloride (DAPI; Sigma, USA). Images were photographed using a fluorescence microscope (Olympus BX51, Japan).
Determination of cell viability with CCK-8 assay
To investigate the viability of SCs in different concentrations of CXCL12, SCs were resuspended and seeded into a 96-well plate at an amount of 1000 cells per well. CXCL12 was directly added to the culture medium. After 24, 48, and 72 hours of culturing, cells were incubated with 10 ul of CCK-8 reagent (Dojindo, Kumamoto, Japan) for 2 hours at 37°C and color reaction was measured at 450 nm with an enzyme-labeled instrument (BioTek, USA).
Transwell assay
To test the migratory ability of SCs, SCs were resuspended in serum-free medium and then seeded in the upper chambers of Transwell system to a number of 40,000 cells per chamber, with or without AMD3100. The lower chambers contained medium with 10% fetal calf serum (FCS), with or without CXCL12. After 12 hours of incubation at 37°C, cells remaining on the upper surface were removed using cotton swabs whereas cells that migrated to the lower surface were fixed with 4% PFA for 15 minutes and stained with 0.1% crystal violet solution for 10 minutes. Cells were counted in at least 5 random fields under microscope. Assay was repeated three times for quantitative analysis. The amount of cells was quantified by Image J software (USA).
Western blotting
Cell samples were collected 24 hours after CXCL12 was added to SCs and then lysated in RIPA (Byetime, China). Protein concentrations were determined using bicinchoninic acid (BCA) protein assay kit (Byetime, China). Equal amounts of total protein (30 ug) were loaded onto 12% gradient gels for electrophoresis, followed by transfer to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked in 5% milk followed by incubation with anti-CXCL12 and p-p38 primary antibodies (1:1000; Abclonal).
Real-time reverse transcription-polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from SCs using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) and treated with RNase-free DNase I. Total RNA (1000 ng) was used as a template for reverse transcription and q-PCR (Novoprotein, China). The sequences of primers were as follows: rat CXCL12 upstream, 5’-GTGACGGTAAGCCAGTCAGC-3’; and downstream, 3’-TGCACACTTGTCTGTTGTTGC-5’; rat GAPDH up-stream, 5’-CAGTGCCAGCCTCGTCTCAT-3’; and downstream, 3’-AGGGGCCATCCACAGTCTTC-5’. RT-PCR conditions were as follows: 5 min. at 95°C, followed by 40 cycles of 30 sec. at 95°C, 30 sec. at 57°C, and 30 sec. at 72°C. CT values were normalized to the GAPDH gene and RQ values were viewed as our final results.
Enzyme-linked immunosorbent assay (ELISA)
SCs were cultured in 6-well plates, before treatment of CXCL12, at concentrations of 100 ng/mL for 24 hours. After that, culture supernatants were abandoned and cells were cleaned with PBS three times. Fresh culture medium was then added for 12 hours. Cytokines in the supernatants (CXCL12) were detected by ELISA (MEIMIAN, Jiangsu, China), according to manufacturer instructions. Triplicates were included in each sample and experiments were repeated three times.
Statistical analysis
Data are presented as mean ± standard deviation (SD), obtained from at least three independent experiments. Statistical analysis was conducted by one-way ANOVA and P < 0.05 was regarded to be statistically significant.
Results
Cellular features and identification of Schwann cells
Most cultured Schwann cells displayed a spindle-like or multi-angle shape, with a protuberance growing from both sides, and a spherical nuclei. Almost all of these cultured Schwann cells expressed S-100 and GFAP, two specific biomarkers of Schwann cells (Figure 1A and 1B).
Figure 1.
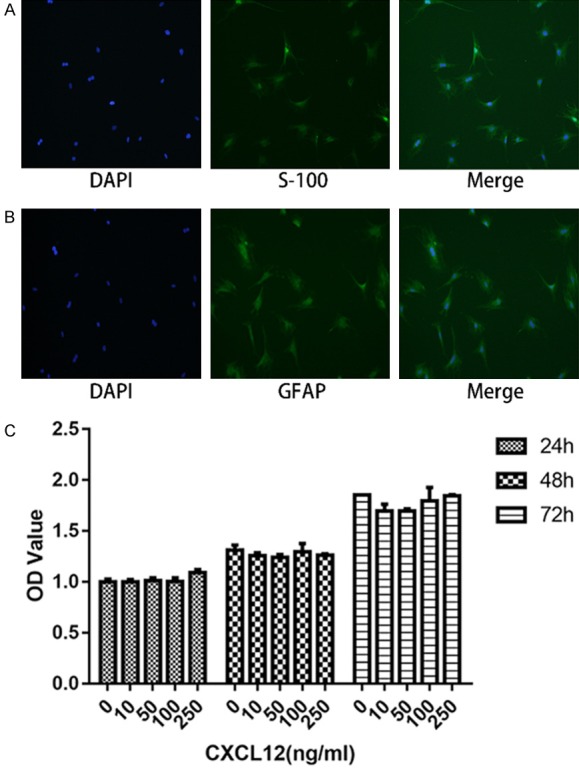
Fluorescent immunocytochemistry of cultured Schwann cells stained by S-100 (A) and GFAP (B), with nuclei counterstained with DAPI. (C) Effects of different CXCL12 concentration on Schwann cell proliferation performed by CCK-8 assay. Data are presented as mean ± SD, n = 3. One-way ANOVA.
CXCL12 has no effect on Schwann cell proliferation
The effects of CXCL12 on SCs proliferation were examined using CCK-8 assay. It can be seen that the number of cells increased as time went by. However, there was no significant difference between CXCL12-treated group and blank control group. These data show that CXCL12 has no significant impact on proliferation of SCs (Figure 1C).
CXCL12 promotes Schwann cell migration
The function of CXCL12 to Schwann cell migration ability was analyzed by Transwell migration assay. After 12 hours of cell spreading, migratory cells were stained with crystal violet. As shown in Figure 2, the number of migratory cells increased at first and then decreased with an increase of CXCL12 concentration. The largest migration concentration was 100 ng/mL. These results suggest that CXCL12 dose-dependent promotes migration of SCs, in a certain concentration range, and inhibits migration of SCs at higher concentrations.
Figure 2.
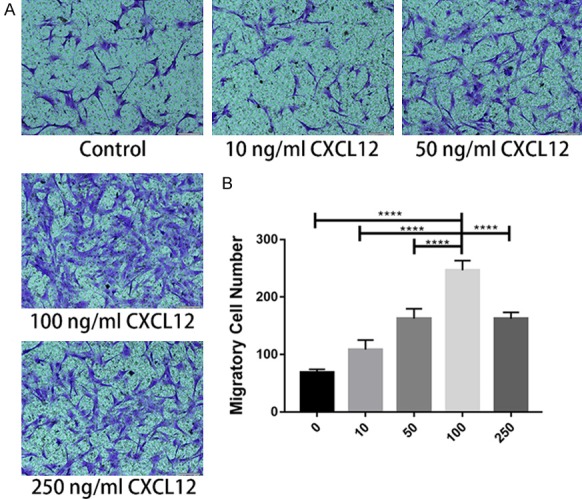
Migratory SCs stained with crystal violet 12 h after induced by different concentration of CXCL12 in Transwell migration assay. Quantitative analysis showing the effect of various concentrations of CXCL12 on SCs migrating. Data are presented as mean ± SD, n = 3, ****P < 0.0001. One-way ANOVA.
Contribution of CXCR4 to CXCL12-induced migration in SCs
To analyze the contribution of CXCR4 to CXCL12-dependent migration in SCs, we employed CXCR4 antagonist AMD3100 (100 ng/mL), which selectively inhibits binding of CXCL12 to CXCR4 without affecting other combinations in SCs. Blocking CXCR4 with AMD3100 completely abolished CXCL12-induced migration (Figure 3A and 3C). Additionally, compared with control group, the number of cell migration was significantly reduced by the addition of AMD3100 group alone. These data show that CXCL12 plays a role in migration relying on its receptor CXCR4.
Figure 3.
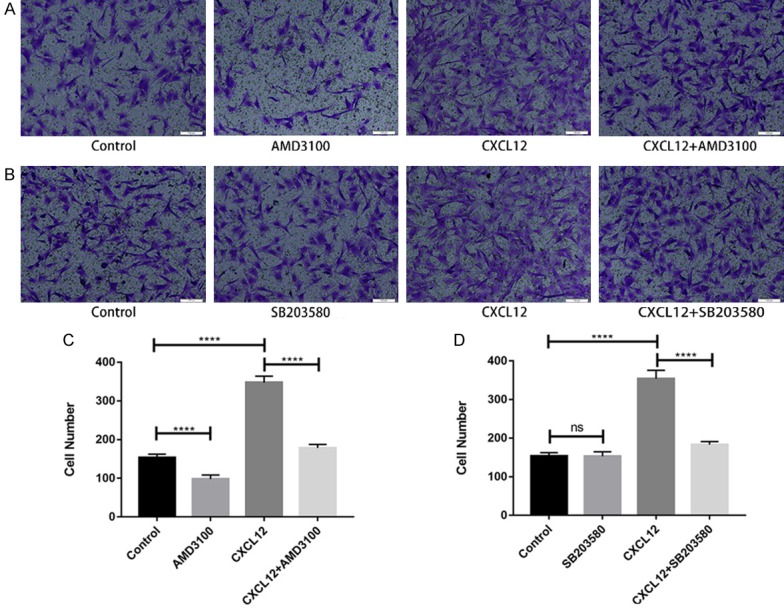
CXCL12 induces migration through receptor CXCR4 (A) and p38MAPK (B) pathway. (C and D) Quantitative analysis showing the effect of AMD3100 and SB203580 on Schwann cells migrating. Data are presented as mean ± SD, n = 3, *P < 0.05, **P < 0.01, ****P < 0.0001. One-way ANOVA.
CXCL12 supports and protects activation of P38 MAPK signaling cascades
As demonstrated in Figure 3B and 3D, treatment with SB203580, a p38 inhibitor, decreased the migrated cells in groups co-treated with CXCL12. Furthermore, levels of p-p38 in groups treated with CXCL12 was higher than that of incubation with blank groups alone. Expression of total p38 remained unchangeable across different groups (Figure 4A). These data suggest that CXCL12 functions to protect or restore the activation level of p38 MAPK signaling cascades blocked by SB203580 in SCs.
Figure 4.
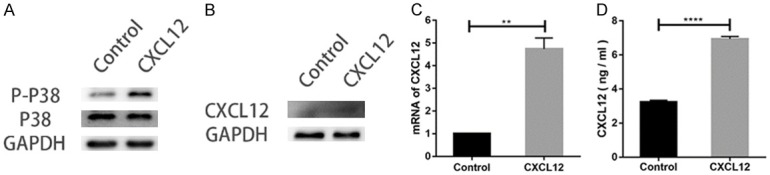
CXCL12 promotes the p38 phosphorylation on SCs (A). Expression of CXCL12 in SCs analyzed by Western blot (B), q RT-PCR (C), and ELISA (D). Data are presented as mean ± SD, n = 3, **P < 0.01, ****P < 0.0001. One-way ANOVA.
CXCL12 promotes autocrine of CXCL12 in SCs
To study why the use of AMD3100 alone could reduce mobility of SCs, we performed Western blot, RT-PCR, and ELISA to detect the level of CXCL12. All three methods showed that expression of CXCL12 was significantly increased after chemokine treatment (Figure 4B-D).
Discussion
Schwann cells play a crucial role in the regeneration of PNS [1]. A full understanding of the molecular regulation mechanism of Schwann cells would contribute to treatment of PNI. Our experiments, for the first time, demonstrated that CXCL12 promotes migration of Schwann cells by positively affecting secretion of CXCL12 via acting on the CXCR4 receptor and p38MAPK.
RSC96 SCs are cell lines that evolved from rat Schwann cells and have been used in several experiments to study Schwann cell migration [10-14]. However, there are many differences between cell lines and primary cells, especially in migration direction [15], so we chose the primary extraction of Schwann cells for experimental study. Herein, we found that CXCL12 attracts SCs in a dose-dependent manner and has the greatest effect at a concentration of 100 ng/mL, consistent with a previous study at 100 ng/mL for optimal physiological concentrations [16,17]. When the concentration reached 250 ng/mL, however, the number of cell migration decreased. We speculated that this may be related to the saturation of CXCR4 receptor on Schwann cell membranes. In addition, CXCR7, another receptor of CXCL12, may also play an important regulatory role. CXCL12 has an increased affinity for CXCR7 when CXCL12 concentrations are too high whereas CXCR7 operates as a sensor for CXCL12 and adjusts CXCR4 protein levels [18,19]. The detailed relationship between these two receptors in SCs requires further elucidation.
As a chemokine, CXCL12 is generally considered to promote cell proliferation, particularly significant in stem cell and tumor research [20,21]. Our study found that the proliferation activity of Schwann cells is almost invariably affected by CXCL12 for up to three days. Tumor cells and stem cells grow rapidly and cell proliferation is their main phenotype, but this is easily changed by external intervention. The main characteristic of SCs is the formation of myelin. Proliferation is not the main phenotype and, thus, is not easily affected.
Ju DT and Lv J reported that the p38MAPK pathway is closely related to SCs migration [11,13]. Our experiments also confirm that p38 MAPK pathway is involved in migration of SCs and migrated cells are significantly reduced after SB203580 treatment. Nevertheless, whether CXCL12 participates in cell migration through other pathways of MAPK, such as ERK1/2, JNK, and ERK5, remains unknown and further experimentation is needed.
During our experiment, we found that, compared with the control group, AMD3100 group significantly reduced the number of SCs migration. Further experiments found that CXCL12 can promote secretion of CXCL12 after CXCL12 acts on SCs, thus, forming a positive feedback regulation. In this process, the regulatory signal can be amplified in a short time, promoting migration of more SCs to CXCL12. This is of major importance in the study of repair of peripheral nerve injury. If CXCL12 is slowly released at the lesion, only a small amount of CXCL12 can play a pivotal role.
In summary, our findings demonstrate that CXCL12 recognizes CXCR4 receptors in SCs and promotes cell migration through the p38MAPK pathway. This indicates that the CXCL12/CXCR4 system is likely to become a new therapeutic target for clinical application, promoting regeneration and repair of peripheral nerve injuries.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (No. 8167050708) and Medical Cross Research Fund Project of Shanghai Jiao Tong University (No. YG2016ZD11).
Disclosure of conflict of interest
None.
References
- 1.Heinen A, Lehmann H, Küry P. Negative regulators of schwann cell differentiation-novel targets for peripheral nerve therapies? J Clin Immunol. 2013;33(Suppl 1):S18–26. doi: 10.1007/s10875-012-9786-9. [DOI] [PubMed] [Google Scholar]
- 2.Gomez-Sanchez J, Pilch K, van der Lans M, Fazal S, Benito C, Wagstaff L, Mirsky R, Jessen K. After nerve injury, lineage tracing shows that myelin and remak schwann cells elongate extensively and branch to form repair schwann cells, which shorten radically on remyelination. J Neurosci. 2017;37:9086–9099. doi: 10.1523/JNEUROSCI.1453-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou X, Liang Q, Wu Y. Transplantation of Schwann cells co-cultured with brain-derived neurotrophic factor for the treatment of experimental autoimmune neuritis. J Neuroimmunol. 2013;263:83–90. doi: 10.1016/j.jneuroim.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Ciutat D, Calderó J, Oppenheim R, Esquerda J. Schwann cell apoptosis during normal development and after axonal degeneration induced by neurotoxins in the chick embryo. J Neurosci. 1996;16:3979–3990. doi: 10.1523/JNEUROSCI.16-12-03979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li M, Hale J, Rich J, Ransohoff R, Lathia J. Chemokine CXCL12 in neurodegenerative diseases: an SOS signal for stem cell-based repair. Trends Neurosci. 2012;35:619–628. doi: 10.1016/j.tins.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Q, Zhang M, Li Y, Xu D, Wang Y, Song A, Zhu B, Huang Y, Zheng J. CXCR7 mediates neural progenitor cells migration to CXCL12 independent of CXCR4. Stem Cells. 2015;33:2574–2585. doi: 10.1002/stem.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moridi I, Mamillapalli R, Cosar E, Ersoy G, Taylor H. Bone marrow stem cell chemotactic activity is induced by elevated CXCl12 in endometriosis. Reprod Sci. 2017;24:526–533. doi: 10.1177/1933719116672587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Negro S, Lessi F, Duregotti E, Aretini P, La Ferla M, Franceschi S, Menicagli M, Bergamin E, Radice E, Thelen M, Megighian A, Pirazzini M, Mazzanti C, Rigoni M, Montecucco C. CXCL12α/SDF-1 from perisynaptic Schwann cells promotes regeneration of injured motor axon terminals. EMBO Mol Med. 2017;9:1000–1010. doi: 10.15252/emmm.201607257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu T, Shields LB, Zhang YP, Feng SQ, Shields CB, Cai J. CXCL12/CXCR4/CXCR7 chemokine axis in the central nervous system: therapeutic targets for remyelination in demyelinating diseases. Neuroscientist. 2017;23:627–648. doi: 10.1177/1073858416685690. [DOI] [PubMed] [Google Scholar]
- 10.Huang CY, Kuo WW, Shibu MA, Hsueh MF, Chen YS, Tsai FJ, Yao CH, Lin CC, Pan LF, Ju DT. Citrus medica var. sarcodactylis (Foshou) activates fibroblast growth factor-2 signaling to induce migration of RSC96 Schwann cells. Am J Chin Med. 2014;42:443–452. doi: 10.1142/S0192415X14500293. [DOI] [PubMed] [Google Scholar]
- 11.Ju DT, Kuo WW, Ho TJ, Paul CR, Kuo CH, Viswanadha VP, Lin CC, Chen YS, Chang YM, Huang CY. Protocatechuic acid from alpinia oxyphylla induces schwann cell migration via ERK1/2, JNK and p38 activation. Am J Chin Med. 2015;43:653–665. doi: 10.1142/S0192415X15500408. [DOI] [PubMed] [Google Scholar]
- 12.Ju DT, Liao HE, Shibu MA, Ho TJ, Padma VV, Tsai FJ, Chung LC, Day CH, Lin CC, Huang CY. Nerve regeneration potential of protocatechuic acid in RSC96 schwann cells by induction of cellular proliferation and migration through IGF-IR-PI3K-Akt signaling. Chin J Physiol. 2015;58:412–419. doi: 10.4077/CJP.2015.BAD340. [DOI] [PubMed] [Google Scholar]
- 13.Lv J, Sun X, Ma J, Ma X, Zhang Y, Li F, Li Y, Zhao Z. Netrin-1 induces the migration of Schwann cells via p38 MAPK and PI3K-Akt signaling pathway mediated by the UNC5B receptor. Biochem Biophys Res Commun. 2015;464:263–268. doi: 10.1016/j.bbrc.2015.06.140. [DOI] [PubMed] [Google Scholar]
- 14.Ma Y, Ge S, Zhou D, Zhang H, Sun L, Wang X, Su J. [Rat bone marrow mesenchymal stem cells promote apoptosis and inhibit proliferation and migration of RSC96 cells] . Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017;33:190–195. [PubMed] [Google Scholar]
- 15.Ji Y, Shen M, Wang X, Zhang S, Yu S, Chen G, Gu X, Ding F. Comparative proteomic analysis of primary schwann cells and a spontaneously immortalized schwann cell line RSC 96: a comprehensive overview with a focus on cell adhesion and migration related proteins. J Proteome Res. 2012;11:3186–3198. doi: 10.1021/pr201221u. [DOI] [PubMed] [Google Scholar]
- 16.Liu YJ, Wang J, Chi ZB. [Comparison of chemotaxis of different concentrations of SDF1, bFGF, IL-8 on bone marrow mesenchymal stem cell] . Shanghai Kou Qiang Yi Xue. 2015;24:275–279. [PubMed] [Google Scholar]
- 17.Pasquier J, Abu-Kaoud N, Abdesselem H, Madani A, Hoarau-Vechot J, Thawadi HA, Vidal F, Couderc B, Favre G, Rafii A. SDF-1alpha concentration dependent modulation of RhoA and Rac1 modifies breast cancer and stromal cells interaction. BMC Cancer. 2015;15:569. doi: 10.1186/s12885-015-1556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Li G, Stanco A, Long JE, Crawford D, Potter GB, Pleasure SJ, Behrens T, Rubenstein JL. CXCR4 and CXCR7 have distinct functions in regulating interneuron migration. Neuron. 2011;69:61–76. doi: 10.1016/j.neuron.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sánchez-Alcañiz JA, Haege S, Mueller W, Pla R, Mackay F, Schulz S, López-Bendito G, Stumm R, Marín O. Cxcr7 controls neuronal migration by regulating chemokine responsiveness. Neuron. 2011;69:77–90. doi: 10.1016/j.neuron.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Gravina GL, Mancini A, Colapietro A, Vitale F, Vetuschi A, Pompili S, Rossi G, Marampon F, Richardson PJ, Patient L, Patient L, Burbidge S, Festuccia C. The novel CXCR4 antagonist, PRX177561, reduces tumor cell proliferation and accelerates cancer stem cell differentiation in glioblastoma preclinical models. Tumour Biol. 2017;39:1010428317695528. doi: 10.1177/1010428317695528. [DOI] [PubMed] [Google Scholar]
- 21.Du L, Feng R, Ge S. PTH/SDF-1alpha cotherapy promotes proliferation, migration and osteogenic differentiation of human periodontal ligament stem cells. Cell Prolif. 2016;49:599–608. doi: 10.1111/cpr.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]


