Abstract
RBM5 has been reported to be a candidate tumor suppressor gene which plays an important role in the induction of apoptosis. In this study, we investigated the effect of miR-483-5p on apoptosis of lung cancer cells and the underlying mechanism. We found that the expression of miR-483-5p mRNA was significantly up-regulated in lung cancer compared with adjacent para-cancerous tissues by using real-time PCR. Silencing miR-483-5p promoted A549 cell apoptosis and enhanced caspase-3 activity by flow cytometry with annexin V-FITC/PI staining and caspase-3 activity report kit. Western blotting demonstrated that miR-483-5p mimicked down-regulated RBM5 protein expression and miR-483-5p inhibitor up-regulated RBM5 protein expression. With additional bioinformatics analysis, we confirmed that RBM5 is a target gene of miR-483-5p and is favored for treating NSCLC. The immunohistochemical pattern of RBM5 could be used to predictoutcome for NSCLC. In conclusion, our results support that RBM5 expression can be regulated by miR-483-5p which is a prognostic marker for NSCLC patients. miR-483-5p inhibitor plays a role in lung cancer through targeting RBM5 to induce apoptosis.
Keywords: miR-483-5p, lung cancer, RBM5, A549, apoptosis
Introduction
Non-small cell lung cancer (NSCLC) is the most common malignant tumor of the respiratory system that is threatens human health with high morbidity and mortality [1]. MicroRNAs (miRNAs) are a class of endogenous non-coding single-stranded small RNA molecules that bind to the 3’ untranslated region (3’ UTR) of a target mRNA to inhibit or degrade the translation product and regulate target genes at the post-transcriptional level. miRNAs participate in the regulation of numerous genes in the body and are also involved in cell growth, proliferation and apoptosis [2]. Many studies have demonstrated that miRNAs play important roles in tumorigenesis and the progression of many malignant tumors, including lung cancer [3,4]. Inducing tumor cell apoptosis has become one of the important anti-tumor approaches. Cancer cell apoptosis induced by miRNAs has received attention [5]. MiR-483-5p is confirmed to promote the proliferation and migration of lung cancer cells, but its effect on lung cancer cell apoptosis and the associated mechanism remain unclear [6]. In addition, RBM5 is known to be an effective tumor suppressor gene of lung cancer cells and also can induce lung cancer cell apoptosis [7-9]. According to limited research results, RBM5 is believed to be a potential target of miR-483-5p [10]. However, the regulatory association between miR-483-5p and RBM5 protein expression in lung cancer cells has not been investigated to date. This study aims to elucidate the impact of miR-483-5p on lung cancer cell apoptosis and explore its possible mechanism.
Materials and methods
Cell line and patient samples
Human lung cancer cell line A549 was purchased from Shanghai Institute of Cell Biology. Lung cancer tissues and the corresponding paracancerous tissues (>3 cm from the cancer tumor) were collected from 40 pathologically confirmed NSCLC patients who were treated in the Second Affiliated Hospital of Harbin Medical University from January 2014 to January 2016, including 24 males and 16 females. Prior to surgery, none of the patients received radiotherapy or chemotherapy including molecular targeted therapy. Signed consent was obtained from all patients before operation (with the approval of Harbin Medical University Human Ethnics Committee).
Cell culture
A549 cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum with 100 IU/ml penicillin and 100 μg/ml streptomycin in an incubator at 37°C under 5% CO2. Cells in logarithmic growth phase were collected for subsequent experiments.
Transient transfection
A549 cells were seeded in culture plates and randomly divided into three groups, including transfection group, non-target control (NC) group and non-transfected group, for transfection with a Hsa-miR-483-5p (5’-AAGACGGGAGGAAAGAAGGGAG-3’) mimic, miR-483-5p inhibitor, miR-NC (5’-UUUGUACUACACAAAAGUACUG-3’) and RMB5 siRNA (Guangzhou RiboBio, CHINA) following the user’s manuals of the X-treme GENE siRNA transfection kit (Roche, USA). RNA was mixed with Opti-MEM and incubated at room temperature for five minutes. the cells were cultured for 48 additional hours prior to collection.
Apoptosis assay
After the supernatant removed, the cells were trypsinized and collected to prepare single-cell suspensions. The cells were washed once with PBS and adjusted to a cell concentration of 1 × 106 cells/mL followed by centrifugation at 2000 rpm for ten minutes. After Annexin V-FITC and PI staining (Thermo Fisher Scientific, USA), the cells were loaded by flow cytometer. The experimental data were analyzed by CytExpert.
Quantitative real-time PCR
Total RNA was extracted from lung cancer and the corresponding paracancerous tissues and A549 cells using the Trizol method. RNA was reverse-transcribed into cDNA following the instructions of the Prime-Script™ Reverse Transcription Kit. U6 was used as the reference for miRNA, and GAPDH served as the reference for RBM5. The test was repeated three times for each group, and the data were analyzed using the 2-ΔΔCt method. The upstream primer of miR-483-5p was 5’-AAGACGGGAGGAAAGAAGGGAG-3’, and the downstream primer was 5’-GTGCAGGGTCCGAGGTATTC-3’. The upstream primer of U6 was 5’-CGCTTCGGCAGCACATATACTA-3’, and the downstream primer was 5’-CGCTTCACGAATTTGCGTGTCA-3’. The upstream primer of RBM5 was 5’-GCACGACTATAGGCATGACAT-3’, and the downstream primer was 5’-AGTCAAACTTGTCTGCTCCA-3’. The upstream primer of GAPDH was 5’-GAAGGTGAAGGTCGGAGTC-3’, and the downstream primer was 5’-GAAGATGGTGATGGGATTTC-3’.
Caspase-3 protease activity assay
A549 cells were trypsinized and collected. Then, lysis buffer was added and incubated on ice for 30 minutes. The cells were then centrifuged at 15000 rpm for 15 minutes at 4°C, and the supernatant was retained. The subsequent steps were performed in accordance with the instructions of the caspase-3 activity assay kit (KeyGEN Biotech, Nanjing). OD values were measured at 405 nm.
Western blotting
The cells in each group were collected and lysed on ice with RIPA lysis buffer for 30 minutes, then centrifuged at 15000 × g for 15 minutes. The supernatant was collected. Total protein was extracted and denatured at 95°C. After the protein was quantified using the BCA protein concentration assay kit, 50 μg of the protein was taken and subjected to 10% SDS-PAGE gel electrophoresis and membrane transfer. After blocking, the membrane was incubated with the primary antibody (RBM5, 1:500 dilution, Santa Cruz Biotechnology) overnight at 4°C. The membrane was washed three times with TBST and incubated with horseradish peroxidase-labeled secondary antibody (1:2000 dilution) for one hour at room temperature. β-actin served as reference. Odyssey image analysis software was employed to analyze optical density.
Public database mining
An online public database was used for prediction of miRNAs that had RBM5 as a binding target (MicroCosm Targets Version 5, https://www.ebi.ac.uk/) [11]. Survival and protein expression with immunohistochemical staining analysis were obtained from Human Protein Atlas (HPA, https://www.proteinatlas.org) in TCGA NSCLC patients [12].
Statistical analysis
Data were expressed as the mean ± SEM. The non-paired t-test was used to compare two groups, whereas ANOVA was utilized to compare multiple groups. Bonferroni-adjusted t-test was used for multiple paired comparisons. P<0.05 was considered a significant difference. GraphPad Prism 6.0 and SPSS 18.0 were used for data analysis and graphing. For overall survival analyses, the Kaplan-Meier method was used. NSCLC patients in the TCGA database were categorized into “high” or “low” expression group using the RBM5 cutoff value FKPM = 4.8.
Results
The structure and expression of miR-483-5p mRNA in lung cancer tissues
The precursor miRNA stem-loop structure of miRNA-483 with its two mature strands (mir-483-5p and mir-483-3p) is shown in Figure 1A. The expression levels of miR-483-5p mRNA in the lung cancer tissues and the adjacent paracancerous tissues from 40 lung cancer patients were examined using quantitative real-time PCR. In total, 92.5% (37/40) of the lung cancer patients exhibited significantly increased miR-483-5p mRNA expression in lung cancer tissues compared with the corresponding paracancerous tissues (Figure 1B). The patient clinical parameters are summarized in Table 1.
Figure 1.
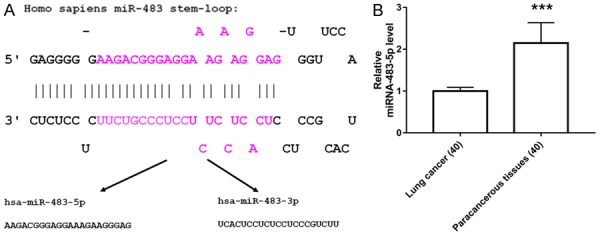
mir-483-5p in NSCLC patients. A: Precursor miRNA stem-loop structure of mir-483 and its two mature strands. B: miR-483-5p expression in 40 cases of NSCLC patients detected by quantitative real-time PCR. **P<0.01 vs. paracancerous tissue.
Table 1.
Patient characteristics
| Characteristics | N (%) |
|---|---|
| Sex | |
| Male | 22 (55.0%) |
| Female | 18 (45.0%) |
| Median age | 58.6 |
| Tumor location | |
| Left Lung | 25 (62.5%) |
| Right Lung | 15 (37.5%) |
| Tumor size (cm) | |
| ≤2 | 18 (45.0%) |
| >2, ≤5 | 20 (50.0%) |
| >5 | 2 (5.0%) |
| Smoking habits | |
| Nonsmoker | 13 (32.5%) |
| Smoker | 27 (67.5%) |
| Histology | |
| Adenocarcinoma | 26 (65%) |
| Squamous cell carcinoma | 13 (32.5%) |
| Large-cell carcinoma | 1 (2.5%) |
| Histological type | |
| Well differentiated | 31 (77.5%) |
| Poorly differentiated | 9 (22.5%) |
| TNM stage | |
| I-II | 32 (80.0%) |
| III-IV | 8 (20.0%) |
TNM tumor, nodule, metastasis.
Effect of miR-483-5p on the apoptosis of A549 cells
To study the effect of miR-483-5p on A549 cell apoptosis, we examined the transfection efficiency of miR-483-5p using quantitative real-time PCR. Compared with the A549 group, the A549 cells transfected with miR-483-5p mimic exhibited remarkably increased miR-483-5p expression. A549 cells transfected with miR-483-5p inhibitor exhibited significantly reduced miR-483-5p miRNA expression (Figure 2A). Flow cytometry analysis revealed that the apoptosis rate of A549 cells was significantly increased after transfection with miR-483-5p inhibitor (Figure 2B, 2C). In addition, A549 cells showed significantly increased activity of the key protein kinase in the process of apoptosis, caspase-3 [13], after transfection with miR-483-5p inhibitor, which was consistent with the results from flow cytometry (Figure 2D). These results demonstrated that miR-483-5p inhibitor promoted A549 cell apoptosis.
Figure 2.
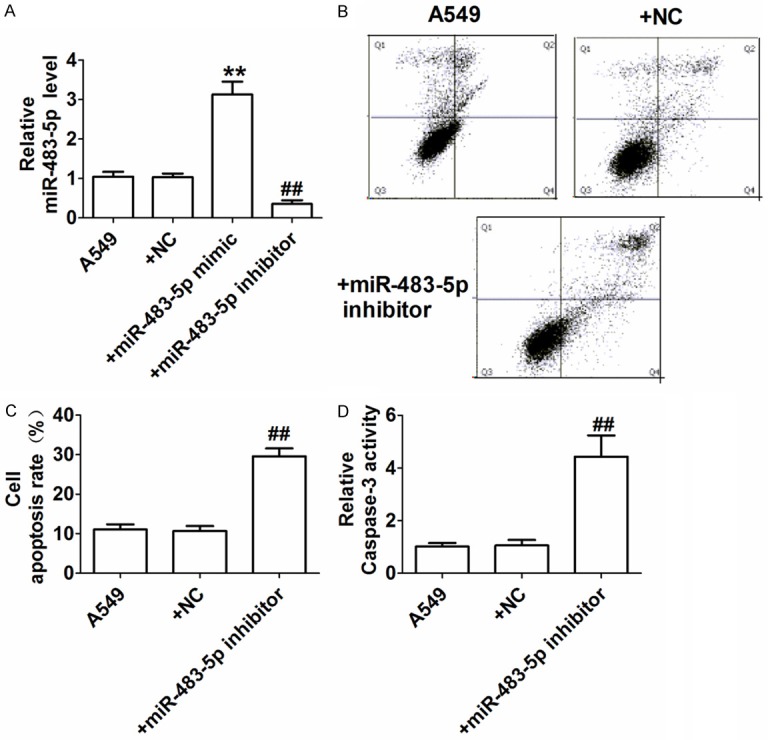
Effect of miR-483-5p on A549 cell apoptosis. A: Expression of miR-483-5p detected by quantitative real-time PCR; B: Representative scatter plot from apoptosis assay in A549 cells via flow cytometry; C: Apoptotic rate (%); D: Caspase-3 activity detected by colorimetric method. **P<0.01 and ##P<0.01 vs. A549 NC group.
Upregulation of RBM5 in A549 cells by miR-483-5p
RBM5 is a target gene of miR-483-5p [10]. However, whether miR-483-5p regulates RBM5 in A549 cells remains unclear. We used western blotting to detect the expression levels of RBM5 protein in A549 cells transfected with miR-483-5p mimic, miR-483-5p inhibitor, and miRNA NC. Compared with the A549 group, RBM5 protein expression was reduced in A549 cells transfected with miR-483-5p mimic and was increased in A549 cells transfected with miR-483-5p inhibitor (Figure 3). Those findings confirmed that RBM5 is a target of miR-483-5p.
Figure 3.
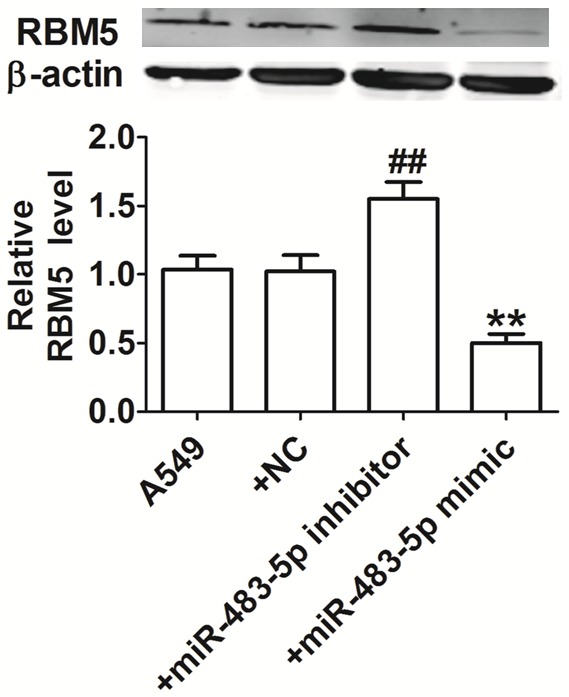
Effect of miR-483-5p on RBM5. β-actin serves as an internal control. The intensity of the band was assessed by ImageJ. **P<0.01 and ##P<0.01 vs. A549 NC group.
Effect of miR-483-5p on apoptosis of A549 cells through targeting RBM5
To investigate the role of miR-483-5p in RBM5 regulation, we examined RBM5 mRNA expression in A549 cells transfected with RBM5 siRNA or miR-483-5p inhibitor plus RBM5 siRNA via qRT-PCR. Compared with the A549 group, RBM5 mRNA was significantly decreased in A549 cells transfected with RBM5 siRNA or miR-483-5p inhibitor and RBM5 siRNA (Figure 4A). RBM5 induces apoptosis in lung cancer cells [7]. To investigate the effect of miR-483-5p on A549 cell apoptosis by targeting RBM5, we examined cell apoptosis in each group using flow cytometry and caspase-3 activity assays. A549 cells transfected with miR-483-5p inhibitor exhibited significantly reduced apoptosis rates, whereas the apoptosis rate of A549 cells transfected with miR-483-5p inhibitor and RBM5 siRNA was significantly reduced compared with cells transfected with miR-483-5p inhibitor alone (Figure 4B, 4C). The caspase-3 activity of A549 cells transfected with the miR-483-5p inhibitor was significantly increased, whereas the caspase-3 activity of A549 cells transfected with miR-483-5p inhibitor and RBM5 siRNA was significantly reduced compared with cells transfected with the miR-483-5p inhibitor alone (Figure 4D). The above resultsindicate that the miR-483-5p inhibitor promotes A549 cell apoptosis by targeting RBM5.
Figure 4.
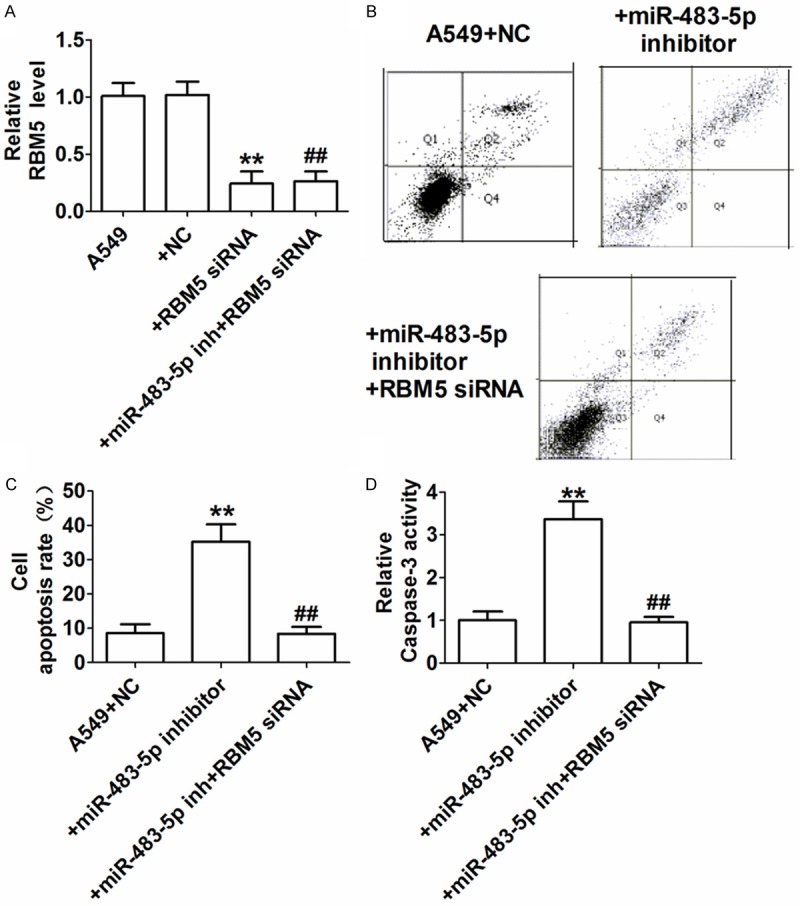
Effect of miR-483-5p on A549 cell apoptosis through targeting RBM5. A: RBM5 levels determined by qRT-PCR. **P<0.01 and ##P<0.01 compared with the A549 group. B: Representative scatter plot of the apoptosis of A549 cells from flow cytometry. C: Apoptosis rate (%). **P<0.01 vs. A549+NC group, ##P<0.01 vs. A549+miR-483-5p inhibitor group. D: Caspase-3 activity determined by colorimetric assay. **P<0.01 vs. A549+NC group, ##P<0.01 vs. A549+miR-483-5p inhibitor group.
Identification of potential miRNAs targeting on RBM5 and RBM5-NSCLC patients’ survival analysis (from TCGA Database)
We use MicroCosm Targets Version 5 (https://www.ebi.ac.uk/) to identify potential miRNAs which may have the target on RMB5. 61 miRNAs were found to have the match result including miR-483-5p (Figure 5A). The details of those miRNAs are shown in Supplementary Table 1. In addition, we found that RBM5 is a favored gene for NSCLC patients (TCGA database, total patient number = 994) with the cutoff FKPM value = 4.8 (Figure 5B, 5C).
Figure 5.
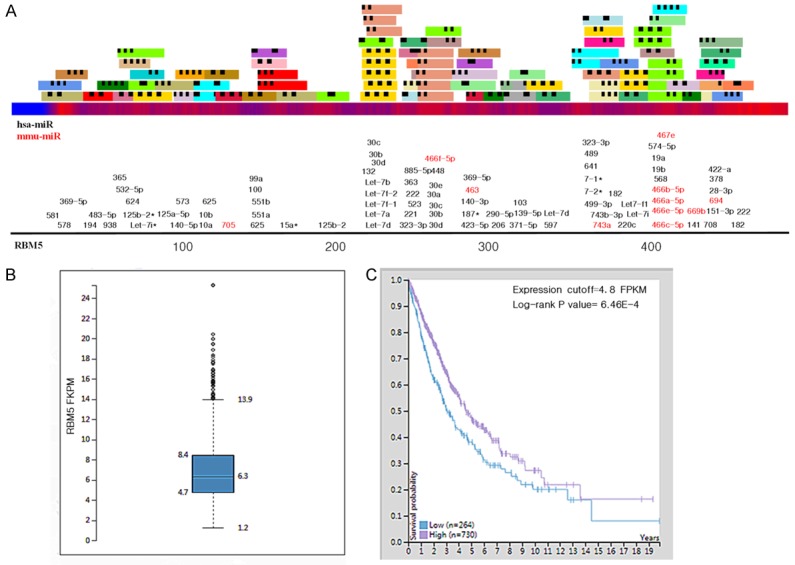
Prediction of miRNAs targeting RBM5: RBM5 expression is associated with poor survivals in NSCLC patients. A: Identification of potential interactions between known miRNAs and RBM5, black number = has-mir, red number = mmu-mir. B: RBM5 RNA-seq expression level based on TCGA database. C: Survival analyses of RBM5 in the TCGA NSCLC patient data set with the cutoff value = 4.8 FPKM.
Correlation between RBM5 and its IHC expression pattern in NSCLC patient from (HPA)
We obtained the IHC expression data from HPA for normal lung tissue and representative NSCLC patients. The IHC result shows us that in both normal lung tissue (Figure 6A) and NSCLC tumor tissue (lung adenocarcinoma and lung squamous cell carcinoma) the nuclear expression was the same. Also, the high and low expression level pattern in NSCLC patients could be used as a prognostic prediction biomarker (Figure 6B-E).
Figure 6.
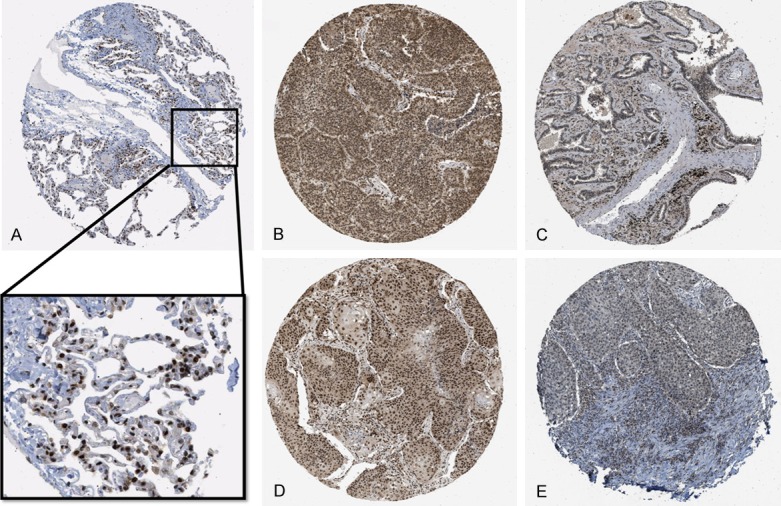
IHC expression pattern of RBM5 in normal lung tissue and NSCLC from HPA database. A: Normal lung tissue expression of RBM5: the magnified area shows a strong expression pattern in the nuclei of pneumocytes. B: Representative strong expression of RBM5 IHC in lung adenocarcinoma. C: Representative weak expression in lung adenocarcinoma. D: Representative strong expression in lung squamous cell carcinoma. E: Representative weak expression in lung squamous cell carcinoma.
Discussion
Non-small cell lung cancer (NSCLC) is the most common lung cancer. Although the diagnosis and treatment of lung cancer continue to improve, the mortality rate remains high [1]. Therefore, it is of utmost importance to study the mechanism underlying the tumorigenesis and progression of lung cancer and identify gene targets for its diagnosis and treatment. In recent years, many studies have demonstrated that microRNAs are involved in the development and progression of lung cancer. A comprehensive analysis indicated that miR-21-5p and miR-30d-5p could be used as tumor markers of lung cancer. Upregulation of miR-483-5p is associated with the development and progression of lung cancer and promotes the metastasis and invasion of lung cancer cells through targeting RhoGDI1 and ALCAM [6]. In this study, we compared miR-483-5p mRNA expression in lung cancer tissues and corresponding paracancerous tissues from NSCLC patients and found significantly increased miR-483-5p mRNA in lung cancer. The above findings demonstrated that miR-483-5p is probably involved in lung cancer tumorigenesis, and its specific mechanism awaits further studies.
Tumorigenesis results from imbalanced cell proliferation and apoptosis [14]. Inducing tumor cell apoptosis has become one of the important anti-tumor approaches [15]. Although NSCLC is sensitive to certain chemotherapy and target therapy, the use of drugs has been limited due to their serious adverse reactions and high risk of refractory cancer [16]. miRNAs have received increasing attention given their anti-tumor activity by inducing tumor cell apoptosis [5]. MiR-107-5p promotes NSCLC cell apoptosis through acting on epidermal growth factor receptor (EGFR) [17]. In addition, miR-139-5p promotes NSCLC cell apoptosis by downregulating the oncogene c-Met [18]. Thus far, the role of miR-483-5p regulation in NSCLC is still unknown. As there are some studies indicated that miR-483-5p may work as a good serum biomarker for malignant disease, more research is needed to explore the mechanisms and contributions of miR-483-5p [19-24]. Therefore, we aimed to test significant different expressed mir-483-5p on its role in lung cancer apoptosis. Our study found that A549 cells transfected with the miR-483-5p inhibitor exhibited significantly increased apoptotic rate and caspase-3 activity, indicating that inhibition of miR-483-5p promotes A549 cell apoptosis and exerts an anti-lung cancer effect.
RMB5, also known as H37/LUCA-15, is a RNA-binding protein with the function of inducing apoptosis. Several studies show that RMB5 overexpression inhibited tumor growth and metastasis by inducing tumor cell apoptosis, including lung cancer [25,26]. Previous studies have demonstrated that miR-483-5p may directly bind to the region of RBM5 [10]. We also confirmed this binding by our bioinformatic analysis result. In our study, A549 cells transfected with the miR-483-5p mimic exhibited significantly increased RBM5 protein levels, whereas A549 cells transfected with the miR-483-5p inhibitor exhibited significantly decreased RBM5 protein levels, indicating that miR-483-5p regulates RBM5 protein in A549 cells. Our results suggest that miR-483-5p inhibitor promoted A549 cell apoptosis by targeting RBM5 to exert its anti-lung cancer function. Moreover, as RMB5 is a favored gene for NSCLC, it is necessary to know the regulation of this gene and IHC pattern, which may help the clinicians to predict the outcome.
In summary, we demonstrated that knockdown of miR-483-5p promoted apoptosis of lung cancer cell line A549 through targeting RBM5. We will increase our patient numbers and obtain more survival-related data to analyze the correlations between prognosis and clinical parameters in the future. However, we hope our findings can provide a potentialnew target for diagnosis and treatment of NSCLC using miR-483-5p to influence RMB5 in the future.
Acknowledgements
The authors thank Yifei Cui of Department of Pathology for assistance in technique supporting. This work was supported by grants from National Natural Science Foundation of China (Grant NO. 81670459) to Maomao Zhang and Nation Natural Science Foundation of China (Grant No. 81572472 and No. 81773161) to Mian Guo. Project of Innovative Scientific Research at Harbin Medical University (Grant No. 2016lczx17 to Fei Wang and Shan Yu).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Arima C, Kajino T, Tamada Y, Imoto S, Shimada Y, Nakatochi M, Suzuki M, Isomura H, Yatabe Y, Yamaguchi T, Yanagisawa K, Miyano S, Takahashi T. Lung adenocarcinoma subtypes definable by lung development-related miRNA expression profiles in association with clinicopathologic features. Carcinogenesis. 2014;35:2224–2231. doi: 10.1093/carcin/bgu127. [DOI] [PubMed] [Google Scholar]
- 3.Hu L, Ai J, Long H, Liu W, Wang X, Zuo Y, Li Y, Wu Q, Deng Y. Integrative microRNA and gene profiling data analysis reveals novel biomarkers and mechanisms for lung cancer. Oncotarget. 2016;7:8441–8454. doi: 10.18632/oncotarget.7264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gasparini P, Cascione L, Landi L, Carasi S, Lovat F, Tibaldi C, Ali G, D’Incecco A, Minuti G, Chella A, Fontanini G, Fassan M, Cappuzzo F, Croce CM. microRNA classifiers are powerful diagnostic/prognostic tools in ALK-, EGFR-, and KRAS-driven lung cancers. Proc Natl Acad Sci U S A. 2015;112:14924–14929. doi: 10.1073/pnas.1520329112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iorio MV, Croce CM. microRNA involvement in human cancer. Carcinogenesis. 2012;33:1126–1133. doi: 10.1093/carcin/bgs140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song Q, Xu Y, Yang C, Chen Z, Jia C, Chen J, Zhang Y, Lai P, Fan X, Zhou X, Lin J, Li M, Ma W, Luo S, Bai X. miR-483-5p promotes invasion and metastasis of lung adenocarcinoma by targeting RhoGDI1 and ALCAM. Cancer Res. 2014;74:3031–3042. doi: 10.1158/0008-5472.CAN-13-2193. [DOI] [PubMed] [Google Scholar]
- 7.Shao C, Zhao L, Wang K, Xu W, Zhang J, Yang B. The tumor suppressor gene RBM5 inhibits lung adenocarcinoma cell growth and induces apoptosis. World J Surg Oncol. 2012;10:160. doi: 10.1186/1477-7819-10-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li P, Wang K, Zhang J, Zhao L, Liang H, Shao C, Sutherland LC. The 3p21.3 tumor suppressor RBM5 resensitizes cisplatin-resistant human non-small cell lung cancer cells to cisplatin. Cancer Epidemiol. 2012;36:481–489. doi: 10.1016/j.canep.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Oh JJ, Razfar A, Delgado I, Reed RA, Malkina A, Boctor B, Slamon DJ. 3p21.3 tumor suppressor gene H37/Luca15/RBM5 inhibits growth of human lung cancer cells through cell cycle arrest and apoptosis. Cancer Res. 2006;66:3419–3427. doi: 10.1158/0008-5472.CAN-05-1667. [DOI] [PubMed] [Google Scholar]
- 10.Yang ZG, Ma XD, He ZH, Guo YX. miR-483-5p promotes prostate cancer cell proliferation and invasion by targeting RBM5. Int Braz J Urol. 2017;43:1060–1067. doi: 10.1590/S1677-5538.IBJU.2016.0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36:D154–158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Ponten F. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 13.Cui R, Kim T, Fassan M, Meng W, Sun HL, Jeon YJ, Vicentini C, Tili E, Peng Y, Scarpa A, Liang G, Zhang YK, Chakravarti A, Croce CM. MicroRNA-224 is implicated in lung cancer pathogenesis through targeting caspase-3 and caspase-7. Oncotarget. 2015;6:21802–21815. doi: 10.18632/oncotarget.5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Shi M, Hou S, Ding B, Liu L, Ji X, Zhang J, Deng Y. MiR-483-5p suppresses the proliferation of glioma cells via directly targeting ERK1. FEBS Lett. 2012;586:1312–1317. doi: 10.1016/j.febslet.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 15.Mohammad RM, Muqbil I, Lowe L, Yedjou C, Hsu HY, Lin LT, Siegelin MD, Fimognari C, Kumar NB, Dou QP, Yang H, Samadi AK, Russo GL, Spagnuolo C, Ray SK, Chakrabarti M, Morre JD, Coley HM, Honoki K, Fujii H, Georgakilas AG, Amedei A, Niccolai E, Amin A, Ashraf SS, Helferich WG, Yang X, Boosani CS, Guha G, Bhakta D, Ciriolo MR, Aquilano K, Chen S, Mohammed SI, Keith WN, Bilsland A, Halicka D, Nowsheen S, Azmi AS. Broad targeting of resistance to apoptosis in cancer. Semin Cancer Biol. 2015;35(Suppl):S78–S103. doi: 10.1016/j.semcancer.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xue Y, Hou S, Ji H, Han X. Evolution from genetics to phenotype: reinterpretation of NSCLC plasticity, heterogeneity, and drug resistance. Protein Cell. 2017;8:178–190. doi: 10.1007/s13238-016-0330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang P, Liu X, Shao Y, Wang H, Liang C, Han B, Ma Z. MicroRNA-107-5p suppresses non-small cell lung cancer by directly targeting oncogene epidermal growth factor receptor. Oncotarget. 2017;8:57012–57023. doi: 10.18632/oncotarget.18505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun C, Sang M, Li S, Sun X, Yang C, Xi Y, Wang L, Zhang F, Bi Y, Fu Y, Li D. Hsa-miR-139-5p inhibits proliferation and causes apoptosis associated with down-regulation of c-Met. Oncotarget. 2015;6:39756–39792. doi: 10.18632/oncotarget.5476. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Zhang Z, Ge S, Wang X, Yuan Q, Yan Q, Ye H, Che Y, Lin Y, Zhang J, Liu P. Serum miR-483-5p as a potential biomarker to detect hepatocellular carcinoma. Hepatol Int. 2013;7:199–207. doi: 10.1007/s12072-012-9341-z. [DOI] [PubMed] [Google Scholar]
- 20.Qiao Y, Ma N, Wang X, Hui Y, Li F, Xiang Y, Zhou J, Zou C, Jin J, Lv G, Jin H, Gao X. MiR-483-5p controls angiogenesis in vitro and targets serum response factor. FEBS Lett. 2011;585:3095–3100. doi: 10.1016/j.febslet.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 21.Zheng XH, Cui C, Ruan HL, Xue WQ, Zhang SD, Hu YZ, Zhou XX, Jia WH. Plasma microRNA profiling in nasopharyngeal carcinoma patients reveals miR-548q and miR-483-5p as potential biomarkers. Chin J Cancer. 2014;33:330–338. doi: 10.5732/cjc.013.10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chabre O, Libe R, Assie G, Barreau O, Bertherat J, Bertagna X, Feige JJ, Cherradi N. Serum miR-483-5p and miR-195 are predictive of recurrence risk in adrenocortical cancer patients. Endocr Relat Cancer. 2013;20:579–594. doi: 10.1530/ERC-13-0051. [DOI] [PubMed] [Google Scholar]
- 23.Shen J, Wang A, Wang Q, Gurvich I, Siegel AB, Remotti H, Santella RM. Exploration of genome-wide circulating microRNA in hepatocellular carcinoma: miR-483-5p as a potential biomarker. Cancer Epidemiol Biomarkers Prev. 2013;22:2364–2373. doi: 10.1158/1055-9965.EPI-13-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qu X, Zhao M, Wu S, Yu W, Xu J, Xu J, Li J, Chen L. Circulating microRNA 483-5p as a novel biomarker for diagnosis survival prediction in multiple myeloma. Med Oncol. 2014;31:219. doi: 10.1007/s12032-014-0219-x. [DOI] [PubMed] [Google Scholar]
- 25.Rintala-Maki ND, Sutherland LC. LUCA-15/RBM5, a putative tumour suppressor, enhances multiple receptor-initiated death signals. Apoptosis. 2004;9:475–484. doi: 10.1023/B:APPT.0000031455.79352.57. [DOI] [PubMed] [Google Scholar]
- 26.Akhtar MJ, Ahamed M, Khan MA, Alrokayan SA, Ahmad I, Kumar S. Cytotoxicity and apoptosis induction by nanoscale talc particles from two different geographical regions in human lung epithelial cells. Environ Toxicol. 2014;29:394–406. doi: 10.1002/tox.21766. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


