Abstract
Background: Vasculogenic mimicry (VM) is a new blood supply in malignant tumors and has long been considered as an effective factor in the metastasis and prognosis of many cancers. ALDH1 (a marker of cancer stem cells), Beclin1 (a biomarker of autophagy) and p16 (a suppressor gene of tumor) are all useful predictive factors for many cancer metastases. However, the prognostic and metastatic value of VM, ALDH1, Beclin1, or p16 in oral squamous cell carcinoma (OSCC) is unclear. In this study, we analyzed correlations among VM, ALDH1, Beclin1, and p16 in OSCC, and their respective associations with clinicopathological parameters and survival in OSCC. Methods: Positive rates of VM, ALDH1, Beclin1, and p16 in 186 whole OSCC specimens were detected by immunohistochemical and histochemical staining. Patients’ clinical information was also collected. Results: Positive rates of VM, ALDH1, and Beclin1 were significantly higher, and levels of p16 were significantly lower in OSCC than in normal oral tissues. Positive rates of VM, ALDH1, and Beclin1 were positively associated with tumor grade, primary tumor (pT), lymph node metastasis (LNM), and tumor-node-metastasis (TNM) stage, and inversely with patients overall survival (OS) time. Levels of p16 was negatively associated with grade, pT, LNM, and TNM stage, and positively associated with smoking and alcohol. The p16+ subgroup had significantly longer OS time than did the p16- subgroup. In multivariate analysis, high ALDH1, VM, Beclin1 levels, tumor grade, pT, LNM, TNM stage, and low p16 levels were potential to be independent prognostic factors for OS time in OSCC patients. Conclusions: VM, and the expression of ALDH1, Beclin1, and p16 represent promising markers for metastasis and prognosis, and potential therapeutic targets for OSCC.
Keywords: Oral squamous cell carcinoma, VM, ALDH1, Beclin1, p16, prognosis
Introduction
In 2012, an estimated 300,400 new cases and cause 145,400 deaths from oral cancers occurred worldwide [1]. The most common source of cells in oral cancer is the squamous cell, accounting for more than 90% of oral cancers [2]. Although there are currently integrated sequential therapies, including surgery, chemoradiation, biotherapy, and gene-targeted therapies, the long-term treatment effects of OSCC remain unsatisfactory [3]. Oral squamous cell carcinoma has a 5-year survival rate in the past few decades that did not significantly improve, remaining at less than 50%, which is a low survival rate for malignant tumors [4]. Metastasis and postoperative recurrence are the major reasons [5,6]. Routine anti-angiogenic therapy did not significantly improve patient survival time [7]. Some researchers have found that when endothelium dependent angiogenesis was not sufficient to support the rapid growth of tumor tissue, some cancer cells could mimic endothelial cells and form vascular structure is called vasculogenic mimicry (VM) [8,9]. The VM is a lumen-like structure that provides nutrient and blood supply and promotes metastasis [10]. VM is mainly composed of three structures: stem-like cancer cells, remodeling of the extracellular matrix, and the lumen-like structure which can directly connect with the host microcirculation system [11]. Accumulating studies have shown that cancer-related VM patients are prone to metastasis and have poor prognosis [8-13].
Malignant tumor metastasis and prognosis may be related to cancer stem cells (CSCs) and the cancer stem cell theory proposes that development of tumors is derived from gene mutations creating tumor stem cells, although these cells in tumor cells only a small part, the cell subsets have a permanent potential for self-renewal and multidirectional differentiation that leads to the formation and growth of tumors [14]. Currently, stem cells have been confirmed as the driving force behind the tumor formation, recurrence, and metastasis of many cancers, including breast cancer, colorectal cancer, and laryngeal squamous cell carcinoma [15-17]. Aldehyde dehydrogenase (ALDH), as one of features of functional stem cells, has been used to identify and analyze many tumor stem cells. Human ALDH1 gene expression is present in the cytoplasm, its gene is cloned and located on chromosome 9q21. ALDH1 is a useful marker of cancer stem cells [18,19] and high ALDH1 expression may also be correlated with poor prognosis in breast, ovarian, and lung cancers [18,20,21].
Autophagy is an evolutionarily conserved and lysosomal degradation pathway where the cell self-digests cell components and recycles useful cytoplasmic material [22]. Beclin1 is a vital protein which can initiate autophagy and play an important role in the nucleation stage of cell autophagy. It was the first tumor suppressor gene in mammals which was directly related to autophagy activation [23]. Furthermore, autophagy also plays a cytoprotective effect in tumor therapy [24].
Several molecular epidemiological research studies have shown that human papillomavirus (HPV) infection could be a subtype which might induce head and neck cancers [25]. It has been reported that the expression of P16 protein, a product of p16 tumor suppressor gene in oral tumors, and is closely related to HPV infection and has been suggested by researchers as an alternative detection method for HPV [26,27].
Overall, studies of the correlation between tumor metastasis and prognosis suggest that VM, ALDH1, Beclin1, and p16 affect cancer progression. However, associations among VM, ALDH1, Beclin1, and p16 in OSCC have not been widely reported. In our study, we examined the hypothesis that these factors are mutual correlated, and are related to metastasis and prognosis in OSCC.
Materials and methods
Specimens
All 186 patients (median age: 60.5 years; range: 38-79 years) who were treated for OSCC were collected from the First Affiliated Hospital of Bengbu Medical College, (China) from January 2009 to December 2011. Samples of the corresponding adjacent normal tissues from all 186 patients were removed. We excluded patients who had received preoperative chemotherapy or radiotherapy. All cases were obtained with written consent. The study was approved by the Ethics Committee of Bengbu Medical College and performed in accordance with the guidelines of the Declaration of Helsinki. We collected patients for whom we had completely clincopathological information and follow-up data (at 6-months intervals by phone, e-mail, or mail). Overall survival (OS) time was calculated from the date of surgery of the patients to his/her death date or December 2016 (mean OS: 59.7 months; range: 8-96 months). Tumor-node-metastasis stage was assessed according to the 7th edition of the American Joint Committee on Cancer (AJCC). Tumors were graded according to World Health Organization (WHO) standards. Specific parameters were provided in Table 1.
Table 1.
Patient characteristics
| Patients characteristics | Frequency (n) | Percentage (%) |
|---|---|---|
| Age (year) | ||
| <60 | 76 | 40.9 |
| ≥60 | 110 | 59.1 |
| Gender | ||
| Male | 104 | 55.9 |
| Female | 82 | 44.1 |
| Smoking | ||
| No | 75 | 40.3 |
| Yes | 111 | 59.7 |
| Alcohol | ||
| No | 66 | 35.5 |
| Yes | 120 | 64.5 |
| Location | ||
| Tongue | 70 | 37.6 |
| Gingiva | 36 | 19.3 |
| Oral floor | 28 | 15.1 |
| Jaw | 25 | 13.4 |
| Buccal mucosa | 15 | 8.1 |
| Others | 12 | 6.5 |
| Grade | ||
| Well | 84 | 45.2 |
| Moderate | 74 | 39.8 |
| Poor | 28 | 15.0 |
| Primary tumor | ||
| T1 | 90 | 48.4 |
| T2 | 56 | 30.1 |
| T3 | 26 | 14.0 |
| T4a | 14 | 7.5 |
| Lymph node metastasis | ||
| N0 | 135 | 72.6 |
| N1 | 37 | 19.9 |
| N2 | 14 | 7.5 |
| TNM stage | ||
| I | 82 | 44.1 |
| II | 48 | 25.8 |
| III | 39 | 21.0 |
| IVA | 17 | 9.1 |
Immunohistochemistry
According to the ElivisionTM Plus detection kit instructions (Lab Vision, USA), we performed immunohistochemistry staining. All OSCC and corresponding normal oral tissues were fixed in 10% buffered formalin, and embedded in paraffin. Continuous 4-µm thick sections were cut. All sections were deparaffinized, dehydrated in xylene and graded alcohol, and then washed with phosphate buffer saline (PBS, pH 7.2) for 10 min. The sections were incubated in methanol containing 3% H2O2 at room temperature for 10 min, for blocking the endogenous peroxidase activity. Tissue sections were placed in citrate buffer (pH 6.0) and then heated to 95°C for antigen repair for 30 minutes. All sections were then quenched with goat serum at RT for 30 min after several washes with PBS, subsequently tissue sections were incubated with mouse monoclonal antibody against human CD34 (Abcam, USA), Beclin1 (Abcam, USA), ALDH1 (Cell Signaling Technology, USA) and p16 (Abcam, USA) at 37°C for 1 h. Periodic Acid-Schiff (PAS)-CD34 dual staining was used to determine endothelial cells in glycosylated basement membranes of vessels, including vessel-like (VM) structure [13]. Yue’s method was used to evaluate VM structure in the OSCC tissues and the control tissues [28]. All sections were counterstained with hematoxylin, dehydrated, air-dried, and mounted. p16 stains were mainly seen in tumor cell nuclear and cytoplasm. Beclin1 and ALDH1 stains were mainly seen in tumor cell cytoplasm.
Evaluation of staining
All staining results were evaluated semi-quantitatively by two experienced pathologists who were blind to patients’ clinical information and follow-up data. In order to avoid potential intra-tumoral heterogeneity of antibody expression, we analyzed ten representative high-power-fields (HPF) from different areas of each OSCC slide. The experimental results were scored according to intensity staining (none staining, 0; weak staining, 1; moderate staining, 2; strong staining, 3) and extent (<11% positive cells mean, 1; 11-50% positive cells mean, 2; 51-75% positive cells mean, 3; >75% positive cells mean, 4). Final scores were obtained by multiplying intensity and extent scores that ranged 0-12. Final scores ≥3 were considered as positive result. For tissue sections that were positive results for all four of VM, ALDH1, Beclin1 and p16, an average value of the final score of each tissue section was taken.
Statistical analysis
Relationships between clinicopathological indices and VM, ALDH1, Beclin1, and p16 were analyzed using Fisher’s exact test or Chi-square test. Association between VM, ALDH1, Beclin1, or p16 was evaluated using Spearman’s correlate test. Effects of VM, ALDH1, Beclin1, or p16 on survival time were analyzed by univariate and multivariate analyses. Independent prognostic factors were analyzed using the multivariate Cox regression model. We used the Kaplan-Meier method with log-rank test to assess correlations between OS time and VM, ALDH1, Beclin1, or p16 results and clinicopathological characteristics, using SPSS 24.0 software for Windows (New York, IBM, USA). A value of P<0.05 was regarded as statistically significant.
Results
Association between VM, ALDH1, Beclin1, and p16 expression and clinicopathological characteristics
To assess the effects of VM, ALDH1, Beclin1, and p16 in OSCC, the experimental results thereof were immunohistochemically detected for both OSCC and corresponding normal oral tissue specimens. Clinicopathological characteristics were compared to these experimental data. The positive result rate of VM findings (small vessel structure, which is a lumen-like in OSCC, the lumen was PAS-positive result but CD34-negative. The VM structure pattern included linear, tubular, and network, etc.) in the OSCC specimens (37.1%, 69/186) was significantly higher than that in the corresponding normal oral tissues (0%, 0/186; P<0.001; Figure 1A and 1B). The positive rate of VM structure in OSCC was positively related to tumor grade, primary tumor, LNM, and TNM stage, but not patients’ age, gender, smoking, alcohol, or location (Table 2).
Figure 1.
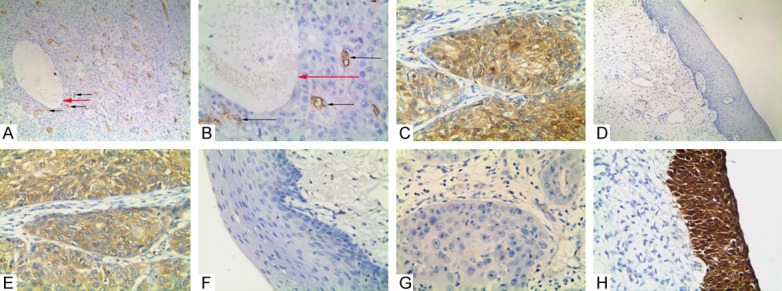
Immunostaining of VM, ALDH1, Beclin1, and p16 in OSCC or the control tissue. A: Positive staining of VM structure in the OSCC tissue (100 magnification, black arrow is microvessel, red arrow is VM structure); B: Positive staining of VM in the OSCC tissue (400 magnification, black arrow is microvessel, red arrow is VM structure); C: Positive staining of ALDH1 in the cytoplasm of cancer cells (400 magnification); D: Negative staining of ALDH1 in the control tissues (100 magnification); E: Positive staining of Beclin1 in the cytoplasm of the cancer cells (400 magnification); F: Negative staining of Beclin1 in the control tissue (400 magnification); G: Negative staining of p16 in the cancer tissue (400 magnification); H: Positive staining of p16 in the cytoplasm and nucleus of the normal oral mucosa epithelial cells (400 magnification).
Table 2.
Association between VM and expression of ALDH1, Beclin1, p16, and clinicopathological characteristics of oral squamous cell carcinoma (OSCC)
| Variables | VM | P | ALDH1 | P | Beclin1 | P | P16 | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| - | + | - | + | - | + | - | + | |||||
| Age (year) | 0.952 | 0.933 | 0.871 | 0.716 | ||||||||
| <60 | 48 | 28 | 32 | 44 | 32 | 44 | 52 | 24 | ||||
| ≥60 | 69 | 41 | 47 | 63 | 45 | 65 | 78 | 32 | ||||
| Gender | 0.177 | 0.213 | 0.130 | 0.920 | ||||||||
| Male | 61 | 43 | 40 | 64 | 38 | 66 | 73 | 31 | ||||
| Female | 56 | 26 | 39 | 43 | 39 | 43 | 57 | 25 | ||||
| Smoking | 0.799 | 0.796 | 0.988 | 0.006 | ||||||||
| No | 48 | 27 | 31 | 44 | 31 | 44 | 44 | 31 | ||||
| Yes | 69 | 42 | 48 | 63 | 46 | 65 | 86 | 25 | ||||
| Alcohol | 0.878 | 0.358 | 0.602 | 0.017 | ||||||||
| No | 42 | 24 | 31 | 35 | 29 | 37 | 39 | 27 | ||||
| Yes | 75 | 45 | 48 | 72 | 48 | 72 | 91 | 29 | ||||
| Location | 0.733 | 0.468 | 0.696 | 0.201 | ||||||||
| Tongue | 48 | 22 | 34 | 36 | 29 | 41 | 48 | 22 | ||||
| Gingiva | 21 | 15 | 10 | 26 | 15 | 21 | 30 | 6 | ||||
| Oral floor | 19 | 9 | 12 | 16 | 15 | 13 | 15 | 13 | ||||
| Jaw | 14 | 11 | 11 | 14 | 8 | 17 | 19 | 6 | ||||
| Buccal mucosa | 8 | 7 | 6 | 9 | 5 | 10 | 10 | 5 | ||||
| Others | 7 | 5 | 6 | 6 | 5 | 7 | 8 | 4 | ||||
| Grade | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||
| Well | 69 | 15 | 54 | 30 | 53 | 31 | 45 | 39 | ||||
| Moderate | 40 | 34 | 24 | 50 | 19 | 55 | 59 | 15 | ||||
| Poor | 8 | 20 | 1 | 27 | 5 | 23 | 26 | 2 | ||||
| Primary tumor | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||
| T1 | 74 | 16 | 66 | 24 | 56 | 34 | 46 | 44 | ||||
| T2 | 34 | 22 | 10 | 46 | 18 | 38 | 49 | 7 | ||||
| T3 | 8 | 18 | 3 | 23 | 1 | 25 | 22 | 4 | ||||
| T4a | 1 | 13 | 0 | 14 | 2 | 12 | 13 | 1 | ||||
| LNM | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||
| N0 | 110 | 25 | 75 | 60 | 73 | 62 | 83 | 52 | ||||
| N1 | 6 | 31 | 4 | 33 | 2 | 35 | 34 | 3 | ||||
| N2 | 1 | 13 | 0 | 14 | 2 | 12 | 13 | 1 | ||||
| TNM stage | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||
| I | 74 | 8 | 65 | 17 | 56 | 26 | 38 | 44 | ||||
| II | 35 | 13 | 10 | 38 | 17 | 31 | 41 | 7 | ||||
| III | 7 | 32 | 4 | 35 | 2 | 37 | 35 | 4 | ||||
| IVA | 1 | 16 | 0 | 17 | 2 | 15 | 16 | 1 | ||||
Similar to VM, ALDH1+ expression was especially higher in OSCC tissues (57.5%, 107/186) than that in the control oral tissues (6.4%, 12/186; P<0.001; Figure 1C and 1D). The positive rate of ALDH1 expression in OSCC was related to tumor grade, primary tumor, LNM and TNM stage, but not patients’ gender, age, alcohol, smoking, or location (Table 2).
The positive rate of Beclin1 expression was higher in OSCC tissues (58.6%, 109/186) than that in the control tissues (5.3%, 10/186; P<0.001; Figure 1E and 1F). The positive rate of Beclin1 expression was significantly associated with primary tumor, tumor grade, LNM, and TNM stage. No correlation was found between Beclin1 expression and patients’ gender, age, smoking, alcohol, or location (Table 2).
The positive expression rate of p16 expression was significantly lower in OSCC tissues (30.1%, 56/186) than that in the control normal tissues (83.9%, 156/186; P<0.001; Figure 1G and 1H). The positive expression rate of p16 was inversely correlated with tumor grade, primary tumor, LNM, TNM stage, smoking, and alcohol. No correlation was found between p16 positive expression and patients’ location, gender, or age (Table 2).
Univariate and multivariate analyses
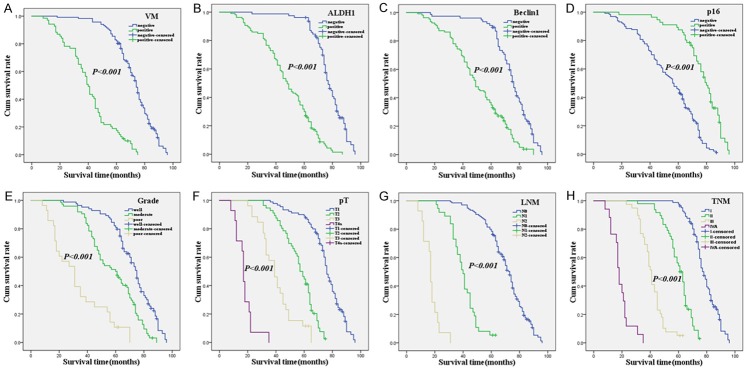
Follow-up data suggested that OST was significantly lower in OSCC patients with VM+ specimens (40.6±17.1 months) compared with VM- patients (71.0±13.5 months; log-rank = 128.741, P<0.001; Figure 2A). Similarly, OST of ALDH1+ patients (47.6±17.9 months) was significantly lower than ALDH1- patients (76.0±11.4 months; log-rank = 106.036, P<0.001; Figure 2B). The OST of Beclin1+ patients (50.0±19.3 months) was significantly shorter than Beclin1- patients (73.4±14.5 months; log-rank = 52.807, P<0.001; Figure 2C). The OST of p16+ (75.6±14.9 months) was significantly higher than those p16- specimens (52.9±19.4 months; log-rank = 53.718, P<0.001; Figure 2D). In univariate analysis, OS time was significantly related to clinicopathological information, including tumor grade (log-rank = 97.222, P<0.001, Figure 2E), primary tumor (log-rank = 369.471, P<0.001, Figure 2F), LNM (log-rank = 383.472, P<0.001, Figure 2G), and TNM stage (log-rank = 434.913, P<0.001, Figure 2H) (Table 3).
Figure 2.
Kaplan-Meier analysis of the survival rate of patients with OSCC. The y-axis represents the percentage of patients; the x-axis, their survival in months. (A) Overall survival of all patients in relation to VM (log-rank = 128.741, P<0.001); (B) Overall survival of all patients in relation to ALDH1 expression (log-rank = 106.036, P<0.001); (C) Overall survival of all patients in relation to Beclin1 expression (log-rank = 52.807, P<0.001); (D) Overall survival of all patients in relation to p16 expression (log-rank = 53.718, P<0.001); In (A-D) analyses, the green line represents patients with positive VM, or ALDH1, or Beclin1, or p16, and the blue line representing the negative VM, or ALDH1, or Beclin1, or p16 group. (E) OS of all patients in relation to tumor grade (log-rank = 97.222, P<0.001, the blue line represents patients with grade 1 group, the green line represents patients with grade 2 group, the brown line represents patients with grade 3 group). (F) OS of all patients in relation to primary tumor (log-rank = 369.471, P<0.001, the blue line represents patients with pT1 group, the green line represents patients with pT2 group, the brown line represents patients with pT3 group, the purple line represents patients with pT4a group). (G) OS of all patients in relation to LNM (log-rank = 383.472, P<0.001, the blue line represents patients with N0 group, the green line represents patients with N1 group, the brown line represents patients with N2 group). (H) OS of all patients in relation to TNM stage (log-rank = 434.913, P<0.001, the blue line represents patient with I stage group, the green line represents patients with II stage group, the brown line represents patients with III stage group, the purple line represents patients with IV A stage group).
Table 3.
Results of univariate analyses of overall survival (OS) time
| Variables | n | Mean OS (months) | Log-rank | P value |
|---|---|---|---|---|
| VM | 128.741 | <0.001 | ||
| Negative | 117 | 71.0±13.5 | ||
| Positive | 69 | 40.6±17.1 | ||
| ALDH1 | 106.036 | <0.001 | ||
| Negative | 79 | 76.0±11.4 | ||
| Positive | 107 | 47.6±17.9 | ||
| Beclin1 | 52.807 | <0.001 | ||
| Negative | 77 | 73.4±14.5 | ||
| Positive | 109 | 50.0±19.3 | ||
| p16 | 53.718 | <0.001 | ||
| Negative | 130 | 52.9±19.4 | ||
| Positive | 56 | 75.6±14.9 | ||
| Age (year) | 0.192 | 0.661 | ||
| <60 | 76 | 59.9±21.3 | ||
| ≥60 | 110 | 59.6±20.7 | ||
| Gender | 0.095 | 0.758 | ||
| Male | 104 | 58.1±21.6 | ||
| Female | 82 | 61.8±20.1 | ||
| Smoking | 0.063 | 0.802 | ||
| No | 75 | 59.4±21.7 | ||
| Yes | 111 | 59.9±20.5 | ||
| Alcohol | 0.753 | 0.386 | ||
| No | 66 | 59.7±22.3 | ||
| Yes | 120 | 59.7±20.2 | ||
| Location | 8.966 | 0.110 | ||
| Tongue | 70 | 65.1±19.5 | ||
| Gingiva | 36 | 53.0±20.5 | ||
| Oral floor | 28 | 61.2±22.4 | ||
| Jaw | 25 | 56.8±18.9 | ||
| Buccal mucosa | 15 | 57.2±21.9 | ||
| Others | 12 | 53.8±24.5 | ||
| Grade | 97.222 | <0.001 | ||
| Well | 84 | 70.7±15.1 | ||
| Moderate | 74 | 57.5±17.2 | ||
| Poor | 28 | 32.5±18.5 | ||
| Primary tumor | 369.471 | <0.001 | ||
| T1 | 90 | 74.1±13.0 | ||
| T2 | 56 | 56.1±11.5 | ||
| T3 | 26 | 40.2±12.3 | ||
| T4a | 14 | 17.6±6.5 | ||
| LNM | 383.472 | <0.001 | ||
| N0 | 135 | 69.5±13.6 | ||
| N1 | 37 | 40.2±10.0 | ||
| N2 | 14 | 17.3±5.8 | ||
| TNM stage | 434.913 | <0.001 | ||
| I | 82 | 77.1±9.1 | ||
| II | 48 | 59.4±9.1 | ||
| III | 39 | 41.2±8.7 | ||
| IVA | 17 | 18.8±6.8 |
Multivariate analysis demonstrated that VM+, ALDH1+, Beclin1+, and/or p16+ specimens, and tumor grade, primary tumor, TNM stage and LNM, were independent prognostic factors for OSCC (Table 4).
Table 4.
Results of multivariate analyses of overall survival (OS) time
| Variables | B | SE | P | RR | 95% CI |
|---|---|---|---|---|---|
| Grade | 0.394 | 0.158 | 0.013 | 1.483 | 1.087-2.022 |
| pT | 0.474 | 0.195 | 0.015 | 1.606 | 1.095-2.355 |
| LNM | 0.829 | 0.350 | 0.018 | 2.291 | 1.155-4.545 |
| TNM stage | 0.865 | 0.329 | 0.008 | 2.376 | 1.247-4.525 |
| VM | 1.220 | 0.241 | <0.001 | 3.388 | 2.111-5.438 |
| ALDH1 | 0.799 | 0.253 | 0.002 | 2.222 | 1.353-3.651 |
| Beclin1 | 0.460 | 0.213 | 0.031 | 1.583 | 1.043-2.405 |
| P16 | -0.615 | 0.236 | 0.009 | 0.541 | 0.341-0.859 |
Correlation among VM, and expression of ALDH1, Beclin1, and p16 in OSCC
Spearman correlation coefficient analysis indicated that negative correlations between p16+ expression and that of VM (r = -0.310, P<0.001), ALDH1 (r = -0.432, P<0.001), or Beclin1 (r = -0.353, P<0.001). Expression of ALDH1 was positively associated with a positive rate of VM (r = 0.480, P<0.001) and Beclin1 (r = 0.492, P<0.001). The expression of VM and Beclin1 showed a positive correlation (r = 0.465, P<0.001) (Table 5).
Table 5.
Correlation among VM, expression of ALDH1, Beclin1, and p16 in OSCC
| Variables | VM | r | P | ALDH1 | r | P | P16 | r | P | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| - | + | - | + | - | + | |||||||
| Beclin1 | 0.465 | <0.001@ | 0.492 | <0.001@ | -0.353 | <0.001* | ||||||
| - | 69 | 8 | 55 | 22 | 39 | 38 | ||||||
| + | 48 | 61 | 24 | 85 | 91 | 18 | ||||||
| VM | 0.480 | <0.001@ | -0.310 | <0.001* | ||||||||
| - | 71 | 46 | 69 | 48 | ||||||||
| + | 8 | 61 | 61 | 8 | ||||||||
| ALDH1 | -0.432 | <0.001* | ||||||||||
| - | 37 | 42 | ||||||||||
| + | 93 | 14 | ||||||||||
negative association;
positive association.
Discussion
As a highly heterogeneous malignant tumor, OSCC can affect the reproducibility of biomarker assessment. In this research, we found that VM was positively correlated with grade, primary tumor, LNM, and TNM stage. Moreover, Kaplan-Meier survival analysis indicates that VM+ OSCC patients had significantly lower OS time than did VM- patients. Our studies suggest that VM should play a vital role in OSCC progression and metastasis, and also should be considered as a very useful biomarker for clinical treatment. Some studies indicate that VM is one of the reasons for the failure of anti-angiogenesis therapy, and should be considered as a potential therapeutic target for OSCC [29,30]. Some other researchers showed similar results [9-13].
ALDH1, a marker for CSCs, and also an intracellular enzyme that helps detoxify and metabolize many endogenous and exogenous in various cancers [18-21]. In this study, ALDH1 expression was positively associated with tumor grade, primary tumor, LNM, and TNM stage. In addition, Kaplan-Meier survival analysis showed that ALDH1+ OSCC patients had significantly lower OST than did ALDH1- patients. These results demonstrated that overexpression of ALDH1 should promote OSCC invasion, metastasis, and mean poor prognosis. Our findings are consistent with other researches, and including those of oral cancers and other cancers [18-21,31,32].
Beclin1 is a representative tumor gene of autophagy that can be either monoallelically deleted or display reduced expression in various cancers [23]. Furthermore, autophagy may also promote the survival of starved tumor cells in regions of the tumor with a poor blood supply [33], Autophagy may also play a tumor cytoprotective role during anticancer therapy [34]. In this study, Beclin1 expression was positively related to tumor grade, primary tumor, LNM, and TNM stage. Moreover, Kaplan-Meier survival curve demonstrated that Beclin1+ OSCC patients had significantly lower OST than did Beclin1- patients. The above results suggested that overexpression of Beclin1 should play an important role in the process of invasion, metastasis and prognosis of OSCC. Our results are similar to other studies [24,35-37].
P16 is thought to be a tumor suppressor gene which can inhibit cell proliferation by blocking the cell cycle [38-40]. Moreover, p16 and HPV infection are closely related to the extent and it can be used as an alternative HPV test [26,27]. Findings in this study also showed that p16/HPV expression was significantly lower in OSCC tissues than that in control tissues, and its expression was negatively correlated with smoking, alcohol, tumor grade, primary tumor, LNM and TNM stage. Furthermore, Kaplan-Meier survival demonstrated that OSCC patients with p16/HPV+ samples had significantly longer survival time than did p16/HPV- patients. These findings suggest that down-regulation of p16 should promote OSCC progression and metastasis, and previous research is similar to our experimental results [38-42].
TNM stages of OSCC provide therapeutic strategies for patients but not provide exhaustive biological behavior information about OSCC. Therefore, it is vital to find effective and novel biomarkers to predict OSCC biological behavior, metastasis, and patients’ prognosis. In our study, multivariate analysis showed that VM, expression of ALDH1, Beclin1, and p16, as well as TNM stages, tumor grade, primary tumor, and LNM, are independent prognostic factors for OSCC patients (Table 4). Our findings thus demonstrate that VM, ALDH1, Beclin1, and p16 are reliable biomarkers for OSCC, and especially in predicting metastasis and prognosis.
Oral squamous cell carcinoma (OSCC) is the most common pathological type of oral cancer. When the tumor grows to a certain size, tumor cells can induce angiogenesis. But when the blood supply by angiogenesis cannot meet the need of tumor rapid growth, some stem-like cancer cells can mimic endothelial cells and form vasculogenic mimicry [9,13]. CSCs can induce angiogenesis to provide adequate nutrition and oxygen for rapid tumor growth [43], and can apparently differentiate tumor cells and endothelial cells [44], thus, CSCs can mimic endothelial cells to form VM to overcome environment stress in tumor tissue. At the same time, CSCs protect the genome from damage and thus maintain their own self-renewal capacity. In the process of tumorigenesis, autophagy protects the stability of the genome by delaying the injury/repair cycle and protecting CSCs homeostasis [45,46]. Thus, autophagy contributes to the stability of CSCs, and promotes VM formation. p16 can inhibit the cell cycle and promote cell senescence [38-40,47]. Cell senescence can inhibit the proliferation of cells damaged by DNA, so p16 can also inhibit the proliferation of tumor stem cells by inducing cellular senescence [48]. Damage to tumor stem cells further leads to decrease in VM formation in the tumor tissue. Overall, our results indicate that a complex relationship between the VM, ALDH1, Beclin1, and p16 in tumor progression. Combined with the results of our present study, we have reason to believe that the interrelationships between these factors are related to metastasis and prognosis in OSCC.
Conclusions
In summary, low expression of p16 combined with high expression of VM, ALDH1, and Beclin1 was found to be associated with metastasis and poor prognosis in OSCC. Furthermore, combined detection of VM, ALDH1, Beclin1, and p16 are valuable factors of metastasis and prognosis in OSCC.
Acknowledgements
This work was supported by the Nature Science Foundation of Anhui Province (No. 1708085MH230) and the Nature Science Key Program of College and University of Anhui Province (No. KJ2017A224) and the Graduate Research Innovation Program of Bengbu Medical College (No. Byycxz1728 and No. Byycx1705).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Mayne ST, Morse DE, Winn DM. Cancers of the oral cavity and pharynx. In: Schottenfeld D, Fraumeni JF, editors. Cancer epidemiology and prevention. 3rd edition. New York: Oxford University Press; 2006. pp. 674–696. [Google Scholar]
- 3.Rodriguez CP, Adelstein DJ. Survival trends in head and neck cancer: opportunities for improving outcomes. Oncologist. 2010;15:921–923. doi: 10.1634/theoncologist.2010-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nohata N, Hanazawa T, Kikkawa N, Mutallip M, Sakurai D, Fujimura L, Kawakami K, Chiyomaru T, Yoshino H, Enokida H, Nakagawa M, Okamoto Y, Seki N. Tumor suppressive microRNA-375 regulates oncogene AEG-1/MTDH in head and neck squamous cells carcinoma (HNSCC) J Hum Genet. 2011;56:595–601. doi: 10.1038/jhg.2011.66. [DOI] [PubMed] [Google Scholar]
- 5.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends-an update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 6.Boscolo-Rizzo P, Da Mosto MC, Rampazzo E, Giunco S, Del Mistro A, Menegaldo A, Baboci L, Mantovani M, Tirelli G, De Rossi A. Telomeres and telomerase in head and neck squamous cell carcinoma: from pathogenesis to clinical implications. Cancer Metastasis Rev. 2016;35:457–474. doi: 10.1007/s10555-016-9633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vredenburgh JJ, Desjardins A, Herndon JE, Dowell JM, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Wagner M, Bigner DD, Friedman AH, Friedman HS. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 8.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–52. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.EI Hallani S, Boisselier B, Peglion F, Rousseau A, Colin C, Idbaih A, Marie Y, Mokhtari K, Thomas JL, Eichmann A, Delattre JY, Maniotis AJ, Sanson M. A new alternative mechanism in glioblastoma vascularization: tubular vasculogenic mimicry. Brain. 2010;133:973–82. doi: 10.1093/brain/awq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang S, Guo H, Zhang D, Zhang W, Zhao X, Ren Z, Sun B. Microcirculation patterns in different stages of melanoma growth. Oncol Rep. 2006;15:15–20. [PubMed] [Google Scholar]
- 11.Sun W, Fan YZ, Zhang WZ, Ge CY. A pilot histomorphology and hemodynamic of vasculogenic mimicry in gallbladder carcinomas in vivo and in vitro. J Exp Clin Cancer Res. 2011;30:46. doi: 10.1186/1756-9966-30-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao N, Sun BC, Zhao XL, Wang Y, Meng J, Che N, Dong XY, Gu Q. Role of Bcl-2 and its associated miRNAs in vasculogenic mimicry of hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8:15759–68. [PMC free article] [PubMed] [Google Scholar]
- 13.Wu S, Yu L, Wang D, Zhou L, Cheng Z, Chai D, Ma L, Tao Y. Aberrant expression of CD133 in non-small cell lung cancer and its relationship to vasculogenic mimicry. BMC Cancer. 2012;12:535. doi: 10.1186/1471-2407-12-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adorno-Cruz V, Kibria G, Liu X, Doherty M, Junk DJ, Guan D, Hubert C, Venere M, Mulkearns-Hubert E, Sinyuk M, Alvarado A, Caplan AI, Rich J, Gerson SL, Lathia J, Liu H. Cancer stem cells: targeting the roots of cancer, seeds of metastasis, and sources of therapy resistance. Cancer Res. 2015;75:924–929. doi: 10.1158/0008-5472.CAN-14-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han Z, Gong X, Zhu B, Wu S, Yu L, Song W, Wang D. Expression of ALDH1, MACC1, and KAI1 in the triple-negative breast cancer and their clinical significance. Int J Clin Exp Pathol. 2017;10:5655–5664. [Google Scholar]
- 16.Huang Y, Huang Y, Liu D, Wang T, Bai G. Flt-1-positive cells are cancer-stem like cells in colorectal carcinoma. Oncotarget. 2017;8:76375–76384. doi: 10.18632/oncotarget.19403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song W, Zhou L, Gong X, Wu S, Yu L, Zhu B, Wang D. Expression of ORAOV1, ABCG2, and KiSS-1 associate with prognosis in laryngeal squamous cell carcinoma. Int J Clin Exp Med. 2017;10:14623–14631. [Google Scholar]
- 18.Croker AK, Rodriguez-Torres M, Xia Y, Pardhan S, Leong HS, Lewis JD, Allan AL. Differential functional roles of ALDH1A1 and ALDH1A3 in mediating metastatic behavior and therapy resistance of human breast cancer cells. Int J Mol Sci. 2017;18:2039. doi: 10.3390/ijms18102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gasparetto M, Smith CA. ALDHs in normal and malignant hematopoietic cells: potential new avenues for treatment of AML and other blood cancers. Chem Biol Interact. 2017;276:46–51. doi: 10.1016/j.cbi.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Choi YJ, Park JH, Han JW, Kim E, Jae-Wook O, Lee SY, Kim JH, Gurunathan S. Differential cytotoxic potential of silver nanoparticles in human ovarian cancer cells and ovarian cancer stem cells. Int J Mol Sci. 2016;17:2077. doi: 10.3390/ijms17122077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou L, Yu L, Zhu B, Wu S, Song W, Gong X, Wang D. Metastasis-associated in colon cancer-1 and aldehyde dehydrogenase 1 are metastatic and prognostic biomarker for non-small cell lung cancer. BMC Cancer. 2016;16:876. doi: 10.1186/s12885-016-2903-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 23.Tsuchihara K, Fujii S, Esumi H. Autophagy and cancer: dynamism of the metabolism of tumor cells and tissues. Cancer Lett. 2009;278:130–138. doi: 10.1016/j.canlet.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 24.Moreau K, Luo S, Rubinsztein DC. Cytoprotective roles for autophagy. Curr Opin Cell Biol. 2010;22:206–211. doi: 10.1016/j.ceb.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. 2012;100:1–441. [PMC free article] [PubMed] [Google Scholar]
- 26.Lydiatt WM, Patel SG, O’Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, Loomis AM, Shah JP. Head and neck cancers-major changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:122–137. doi: 10.3322/caac.21389. [DOI] [PubMed] [Google Scholar]
- 27.Gröbe A, Hanken H, Kluwe L, Schöllchen M, Tribius S, Pohlenz P, Clauditz T, Grob T, Simon R, Sauter G, Heiland M, Blessmann M. Immunohistochemical analysis of expression, HPV infection and its prognostic utility in oral squamous cell carcinoma. J Oral Pathol Med. 2013;42:676–81. doi: 10.1111/jop.12086. [DOI] [PubMed] [Google Scholar]
- 28.Yue WY, Chen ZP. Does vasculogenic mimicry exist in astrocytoma? J Histochem Cytochem. 2005;53:997–1002. doi: 10.1369/jhc.4A6521.2005. [DOI] [PubMed] [Google Scholar]
- 29.Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–9. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan JJ, Cai J, Guo YF, Bian XW, Yu SC. ALDH1A3, a metabolic target for cancer diagnosis and therapy. Int J Cancer. 2016;139:965–975. doi: 10.1002/ijc.30091. [DOI] [PubMed] [Google Scholar]
- 32.Khorrami S, Zavaran Hosseini A, Mowla SJ, Malekzadeh R. Verification of ALDH activity as a biomarker in colon cancer stem cells-derived HT-29 cell line. Iran J Cancer Prev. 2015;8:e3446. doi: 10.17795/ijcp-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathew R, White E. Autophagy in tumorigenesis and energy metabolism: friend by day, foe by night. Curr Opin Genet Dev. 2011;21:113–119. doi: 10.1016/j.gde.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreau K, Luo S, Rubinsztein DC. Cytoprotective roles for autophagy. Curr Opin Cell Biol. 2010;22:206–211. doi: 10.1016/j.ceb.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei H, Wei S, Gan B, Peng X, Zou W, Guan JL. Suppression of autophagy by FIP200 deletion inhibits mammary tumorigenesis. Genes Dev. 2011;25:1510–1527. doi: 10.1101/gad.2051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Won KY, Kim GY, Kim YW, Song JY, Lim SJ. Clinicopathologic correlation of beclin-1 and bcl-2 expression in human breast cancer. Hum Pathol. 2010;41:107–12. doi: 10.1016/j.humpath.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Z, Han F, Yang S, Wu J, Zhan W. Oxamatemediated inhibition of lactate dehydrogenase induces protective autophagy in gastric cancer cells: involvement of the Akt-mTOR signaling pathway. Cancer Lett. 2015;358:17–26. doi: 10.1016/j.canlet.2014.11.046. [DOI] [PubMed] [Google Scholar]
- 38.Dey B, Raphael V, Khonglah Y, GiriLynrah K. Expression of cyclin D1 and p16 in esophageal squamous cell carcinoma. Middle East J Dig Dis. 2015;7:220–225. [PMC free article] [PubMed] [Google Scholar]
- 39.Su L, Wang H, Miao J, Liang Y. Clinicopathological significance and potential drug target of CDKN2A/p16 in endometrial carcinoma. Sci Rep. 2015;5:13238. doi: 10.1038/srep13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu Y, Zhang X, Zhang J. Inhibition of breast tumor cell growth by ectopic expression of p16/INK4A via combined effects of cell cycle arrest, senescence and apoptotic induction, and angiogenesis inhibition. J Cancer. 2012;3:333–344. doi: 10.7150/jca.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reiter PL, Brewer NT, Smith JS. Human papillomavirus knowledge and vaccine acceptability among a national sample of heterosexual men. Sex Transm Infect. 2010;86:241–246. doi: 10.1136/sti.2009.039065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klussmann JP, Mooren JJ, Lehnen M, Claessen SM, Stenner M, Huebbers CU, Weissenborn SJ, Wedemeyer I, Preuss SF, Straetmans JM, Manni JJ, Hopman AH, Speel EJ. Genetic signatures of HPV-related and unrelatedo carcinoma and their prognostic implications. Clin Cancer Res. 2009;15:1779–1786. doi: 10.1158/1078-0432.CCR-08-1463. [DOI] [PubMed] [Google Scholar]
- 43.Hilbe W, Dirnhofer S, Oberwasserlechner F, Schmid T, Gunsilius E, Hilbe G, Wöll E, Kähler CM. CD133 positive endothelial progenitor cells contribute to the tumour vasculature in non-small cell lung cancer. J Clin Pathol. 2004;57:965–969. doi: 10.1136/jcp.2004.016444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang R, Chadalavad K, Wilshire J, Kowalik U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C, Tabar V. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 45.Song YJ, Zhang SS, Guo XL, Sun K, Han ZP, Li R, Zhao QD, Deng WJ, Xie XQ, Zhang JW, Wu MC, Wei LX. Autophagy contributes to the survival of CD133+ liver cancer stem cells in the hypoxic and nutrient-deprived tumor microenvironment. Cancer Lett. 2013;339:70–81. doi: 10.1016/j.canlet.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 46.Naka K, Hirao A. Maintenance of genomic integrity in hematopoietic stem cells. Int J Hematol. 2011;93:434–439. doi: 10.1007/s12185-011-0793-z. [DOI] [PubMed] [Google Scholar]
- 47.Mowla SN, Lam EW, Jat PS. Cellular senescence and aging: the role of B-MYB. Aging Cell. 2014;13:773–779. doi: 10.1111/acel.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qian X, Wagner S, Ma C, Klussmann JP, Hummel M, Kaufmann AM, Albers AE. ALDH1-positive cancer stem-like cells are enriched in nodal metastases of oropharyngeal squamous cell carcinoma independent of HPV status. Oncol Rep. 2013;29:1777–84. doi: 10.3892/or.2013.2340. [DOI] [PubMed] [Google Scholar]