Abstract
Inflammation and oxidative stress are associated with atherosclerotic progression. Fibroblast growth factor 21 (FGF21), a regulator of energy metabolism, has been reported to suppress the pathogenesis of atherosclerosis. However, the mechanism of anti-atherosclerotic effects of FGF21 remains unclear and needs to be further investigated. Transcription factor NF-E2-related 2 (Nrf2), a sensitive regulator of oxidative stress, is also associated with atherosclerotic progression. In this study, we investigated whether up-regulation of FGF21 affected inflammation and oxidative stress in atherosclerotic rats and whether the Nrf2-signaling pathway was involved in FGF21-mediated effects. Pathological changes were detected in arterial tissues of rats, and the expression of inflammatory and oxidative stress indicators, vascular endothelial markers, and Nrf2-signaling related protein were measured in the serum or/and arterial tissues of rats. As a result, expression of FGF21 and Nrf2-ARE signaling related proteins were markedly suppressed in arterial tissues of model rats. Thickness of endarteria and infiltrating cells obviously increased in atherosclerotic rats, whereas the increase of FGF21 expression could decrease thickness of endarteria. Moreover, the levels of ET-1, MDA, MCP-1, ICAM-1 and VCAM-1 were significantly higher in model rats than that in normal rats, whereas the levels of NO, GSH and T-AOC were significantly lower. Compared with model rats, up-regulation of FGF21 could increase the expression of Nrf2-ARE signaling related proteins and the level of anti-oxidative indicators, decrease the levels of endothelial dysfunction, and reduce inflammatory indicators. Down-regulation of FGF21 could reverse these actions. Therefore FGF21 reduces inflammation and oxidative stress in atherosclerotic rats via Nrf2-ARE signaling pathway.
Keywords: FGF21, Nrf2, inflammation, oxidative stress, atherosclerosis
Introduction
Atherosclerosis is a common and imperceptible chronic cardiovascular disease, which is an essential cause of morbidity and mortality in coronary heart disease and stroke patients [1]. Atherosclerosis is characterized by lipid deposition and inflammatory cells in large and medium-sized arteries [2,3]. It is generally believed that vascular endothelial dysfunction caused by some factors, such as dyslipidemia, is the major reason for the beginning of atherosclerosis [4] and that subsequent oxidative stress and chronic inflammatory reactions aggravate this progression [5]. Therefore, it is still the focus for researchers to explore how to delay progression of atherosclerosis and seek better effective methods to treat this disease. At present, strategies focusing on anti-oxidative and anti-inflammatory aspects are potential means in the treatment of atherosclerosis.
Oxidative stress is one of the most important pathogenesis of atherosclerosis. Oxidative stress leads to the generation of reactive oxygen species (ROS) and/or downregulation of body’s innate anti-oxidant defense systems [6]. Subsequently, inflammatory reaction, another pathogenesis, may be triggered by ROS and reactive nitrogen species (RNS). Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor which can activate cellular antioxidant defense mechanism through regulating the transcriptional expression of downstream antioxidant genes, such as genes containing antioxidant/electrophile response elements (ARE/EpRE) [7]. Under normal homeostatic conditions, Nrf2 is mainly present in cytoplasm and maintains at a basic level by proteasomal degradation. However, once faced with stress, it can be activated and translocate into the nucleus where it exerts transcriptional activity [8,9]. Active-Nrf2 could inhibit activation of endothelial cells and expression of pro-inflammatory molecules, such as vascular cell adhesion molecule-1 (VCAM-1) both of which contribute to the occurrence of atherosclerosis. A growing body of evidence has proven that the antioxidant effect of Nrf2 signaling pathway is closely associated with development of atherosclerosis [10]. Therefore, induction of Nrf2 signaling could be a new valuable therapeutic strategy for the treatment and prevention of atherosclerosis.
Fibroblast growth factor 21 (FGF21) belongs to the fibroblast growth factor (FGF) superfamily and acts as an essential regulator of energy metabolism. But recent studies showed that the function of FGF21 is not limited to the regulation of metabolism but also involved in multiple physiological processes, such as anti-aging, anti-atherosclerosis, antioxidant, anti-inflammatory progresses [11]. Although it has been well characterized the metabolic functions of FGF21, the pathophysiological actions in atherosclerosis are still unclear. There is evidence to show that exogenous supplementation of FGF21 improves dyslipidemia through reducing the level of low density lipoprotein and enhancing the level of high density lipoprotein cholesterol in mammals with diabetes mellitus [12]. FGF21 also decreases ROS accumulation and endothelial cell damage induced by H2O2 [11]. So whether the FGF21 exhibits anti-oxidant and anti-inflammatory effects through the Nrf2 signaling pathway remains unknown. Here, we investigated the anti-oxidant and anti-inflammatory effects of FGF21 and molecular mechanisms using a rat model of atherosclerosis.
Materials and methods
Animals
Thirty-two adult male Wistar rats (Rattus norvegicus), eight weeks of age and weighing from 180 g to 200 g, were purchased from Hubei provincial center for disease control and prevention (Wuhan, China). All animals were housed in a pathogen-free environment and under controlled conditions (temperature, 22 ± 2°C and relative humidity 50 ± 2%), with free access to water and food, and regular conversion from dark to light for each 12 h. The animal experimental procedures were performed in accordance the requirements of the Ethics of Animal Experiments.
Experimental design and model establishment of atherosclerosis
The rats were adaptively fed for one week and then randomly divided into four groups (eight for each group): normal control group, model group, model group with Lv-GFP-overexpression FGF21 (Lv-expFGF21 group) and model group with Lv-GFP-shFGF21 (Lv-shFGF21 group). Except for the rats in normal control group, the rest rats in other groups were intraperitoneally injected with 600,000 IU/kg of vitamin D3 (VD3) (Shanghai general pharmaceutical CO.,LTD., China) on the first day and fed daily with high-lipid diet (formulation: 3% cholesterol, 0.3% sodium cholate, 5% egg yolk powder, 0.2% propylthiourcil, 5% sugar, 10% lard, 15% whole milk powder and 61.5% basal diet) for 14 weeks. During this time, the rats in Lv-expFGF21 group or Lv-shFGF21 group were infected with 3×108 transforming unit (TU) of lentivirus particles with FGF21 overexpression plasmid or shRNA interfering plasmid of FGF21 (shFGF21) (Han Heng Biotechnology Co., Ltd., Shanghai, China) by tail vein injection, respectively. The rats in the normal control group were fed with a normal basic diet and injected with an equal volume of normal saline. After 14 weeks, the rats were anesthetized with intraperitoneal injection of chloral hydrate and all animals were euthanized at the same time of day to avoid circadian fluctuations.
Preparation of serum and tissue samples
Serum samples were collected to evaluate the biochemical indicators. The blood samples were placed at 4°C for 1 hour and centrifuged at 4000×g for 10 min. Supernatant was then collected and stored at -20°C before the detection. The aorta tissues of rats in the four groups were stripped after animals were euthanized. A portion of the tissues were frozen at -80°C after elimination of residual blood whereas another part of tissues used for mRNA extraction were placed in 1.5 mL sterile and RNAse-free centrifuge tubes and stored at -80°C.
Measurement of the levels of oxidant indicators and total antioxidant capacity indicator in serum and aorta tissues
The assay kits for the analyses of malondialdehyde (MDA), glutathione (GSH) and total antioxidant capacity (T-AOC) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Both the experimental operation and analyses were conducted according to the manufacturer’s instructions, using a microplate reader (Multiskan MK3, Finnpipette, Finland). The serum samples were directly used for the detection experiments. For tissue samples, tissue homogenate solution was prepared before detection and then supernatant, which could be used for directly detection, was collected after high velocity centrifugation. In addition, serum nitric oxide (NO) was also evaluated according to the manufacturer’s instructions.
Determination of inflammatory indicators in arterial tissues by ELISA assay kits
Rat intercellular adhesion molecule 1 (ICAM-1), monocyte chemotactic protein 1 (MCP-1) and vascular cell adhesion molecule (VCAM-1) ELISA kits were obtained from Bioswamp (Wuhan, China). All tissue samples were pretreated to get tissue lysates before the detection. The concentrations of ICAM-1, MCP-1, and VCAM-1 were evaluated using ELISA kits according to the manufacturers’ instructions. The optical density (OD) value was measured by microplate reader at 450 nm and the concentration of each indicator in the samples was then determined by comparing the OD value of the samples to the standard curve respectively. The contents of endothelin 1 (ET-1) in arterial tissues were measured using the ELISA kit from Bioswamp.
Quantitative real-time PCR (RT-PCR)
Total RNA was extracted from aorta tissue using the Trizol reagent (Invitrogen, California, USA). Quality testing of the total RNA was then performed using agarose gel electrophoresis. First-strand cDNA was synthesis according to the protocol for the super script II first-strand synthesis system (Invitrogen, California, USA) and diluted before the use for quantitative RT-PCR. Subsequently, quantitative RT-PCR was conducted according to the protocol of the QuantiFast SYBR Green PCR Kit (Qiagen, California, USA). The primer sequences were used as follows: Nrf2: F: 5’-ATTCAAGCCGATTAGAGG-3’, R: 5’-ATTGCTCCTTGGACATCA-3’; HO-1: F: 5’-ATGTCCCAGGATTTGTC-3’, R: 5’-CTGCTTGTTTCGCTCT-3’; NQ1: F: 5’-ATGGCGGTGAGAAGAGC-3’, R: 5’-TCCCCTGTGATGTCGTT-3’; γGCS: F: 5’-CAAGTGGGGTGACGAGG-3’, R: 5’-TTGGGTGGTTGGGGTTT-3’; SOD: F: 5’-GGCTTGGTCCTCTTCC-3’, R: 5’-CCCAGCGGGTTGTAGT-3’; GAPDH: F: 5’-CAAGTTCAACGGCACAG-3’, R: 5’-CCAGTAGACTCCACGACAT-3’. Gene expression was normalized through comparison with expression of GAPDH.
Western blot analysis
The total protein in tissue samples were prepared using appropriate amount of RIPA buffer (Solarbio Life Sciences, Beijing, China) containing 1 μM PMSF after grinding and sonication in ice. The content of total protein solution was then quantified using BCA protein assay kit (Beyotime Institute of Biotechnology, Shanghai, China) after high speed centrifugation. 30 μg of total proteins were separated by SDS-PAGE and then the separated proteins were transferred and immobilized on the PVDF membranes (Millipore, Massachusetts, USA). The membranes were incubated with dilution of the primary antibody anti-Nrf2, anti-HO-1, anti-NQO-1, anti-γGCS, anti-SOD (Abcam, Cambridge, England) and anti-GAPDH (Cell Signaling Technology, Danvers, USA) at 4°C overnight after blocked with 5% skim milk. The membranes were then incubated with HRP-conjugated secondary antibody goat anti-rabbit IgG (Bioswamp, Wuhan, China) after which they were washed with TBST buffer three times. Protein bands were detected using chemiluminescent substrate reagents (Millipore, Massachusetts, USA) and imaged using automatic chemiluminescence analyzer (Tanon, Shanghai, China).
Hematoxylin-eosin (H&E) staining
The separated aorta tissues were fixed in 4% paraformaldehyde for 30 min and then embedded into paraffin blocks by conventional dehydration, transparency, dipping and embedding. Sections were sliced for 5 μm from paraffin blocks. These sections were then stained according to routine hematoxylin and eosin staining protocols. The results were observed under optical microscope and photographed.
Statistical analysis
Statistical analysis was performed with SPSS 22.0 statistical software. The measurement data are expressed in the form of the mean ± standard deviation. The data between groups were compared by One-way ANOVA with LSD multiple comparison test. In all cases, values of P<0.05 meant that the differences were statistically significant.
Results
Overexpression of FGF21 improves endothelial dysfunction by affecting the levels of ET-1 and NO in atherosclerotic rats
In order to explore how FGF21 exerts anti-atherosclerotic effects, we first verified pathological changes and FGF21 expression in arterial tissues of normal and model rats and then tried to change the expression of FGF21 in arterial tissue of model rats via using the lentiviral vector with FGF21 overexpression plasmid or shFGF21 plasmid. The results showed that both transcriptional and protein levels of FGF21 were decreased by more than 50% in atherosclerosis rats compared to the normal control rats. Compared to the model group, mRNA and protein levels of FGF21 were significantly increased in the Lv-expFGF21 group and clearly decreased in the Lv-shFGF21 group. (Figure 1A and 1B) Furthermore, H&E staining analysis showed that the thickness of endarteria was noticeably increased and that infiltrating cells obviously existed in arterial tissue of model rats, whereas these pathological phenomena weakened after up-regulation of FGF21 expression (Figure 1C and 1D). In contrast, down-regulation of FGF21 expression significantly aggravated these pathological changes. Vasoconstrictor ET-1 and its synthesis inhibitor NO, also known as a vasodilator, play important roles in endothelial dysfunction in atherosclerosis. As shown in Figure 1E, serum level of ET-1 was 1.82 folds markedly higher in the model group than that in the control group, and overexpression of FGF21 in the Lv-expFGF21 group could inhibit up-regulation of ET-1 in atherosclerotic rats, whereas suppression of FGF21 in the Lv-shFGF21 group aggravated accumulation of serum ET-1 (P<0.001). Moreover, the serum NO levels were opposite with the serum ET-1 levels. The content of NO in the model rats significantly reduced in model rats. But serum NO levels were enhanced when FGF21 expression was up-regulated and reduced again when the expression of FGF21 was suppressed (P<0.001) (Figure 1F). These results indicate that FGF21 has an improvement effect on endothelial dysfunction by regulating serum ET-1 and NO levels.
Figure 1.
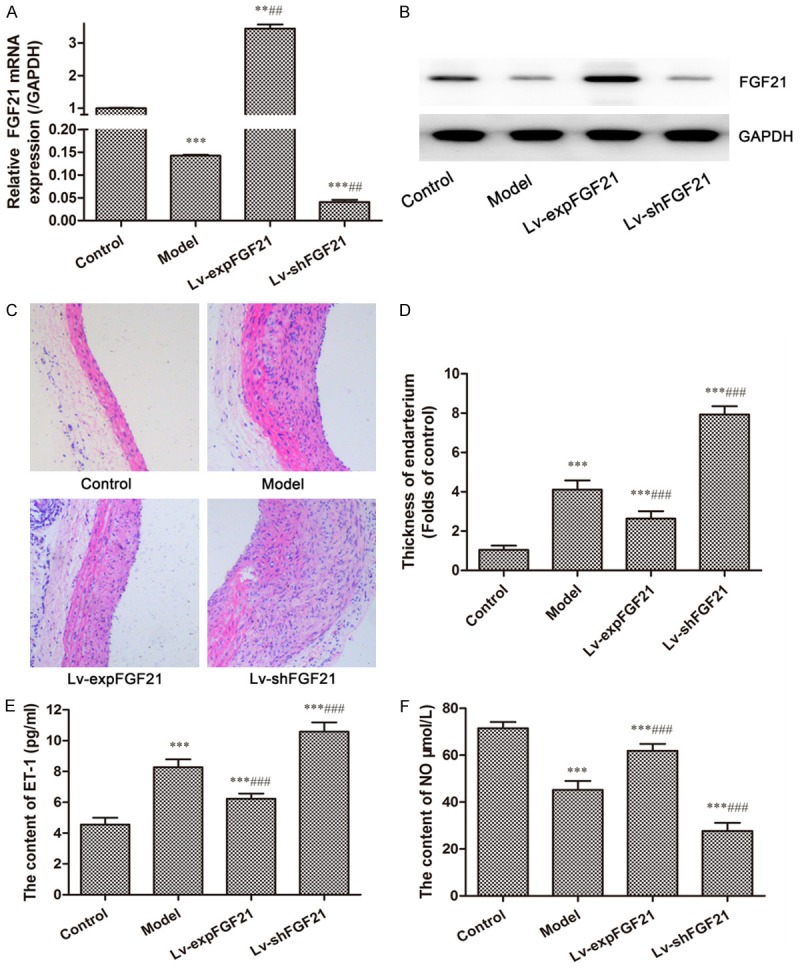
Expression of FGF21, serum levels of ET-1 and NO, and pathological changes in atherosclerotic rats. The expression of FGF21 in arterial tissue of rats in different groups was detected by RT-PCR (A) and Western blot (B). Histopathological observation in arterial tissues were performed by H&E staining analysis (C) (at a 200× magnification) and quantitatively analyzed for the thickness of the endarterium (D). The serum levels of ET-1 (E) and NO (F) were measured using the assay kits according to the manufacturer’s instructions. Data are expressed in the form of mean ± SD for three independent experiments. Compared with the control group, **P<0.01, ***P<0.001; compared with the model group, ##P<0.01, ###P<0.001.
Up-regulation of FGF21 affects inflammatory molecular levels in serum and the contents of oxidative indicators both in serum and tissue
Inflammatory reaction and oxidative stress are two of the key causes during the development of atherosclerosis. In atherosclerotic rats the serum levels of inflammatory molecules, such as MCP-1, ICAM-1, and VCAM-1, are significantly up-regulated compared to that in the control group (P<0.001) (Figure 2). Overexpression of FGF21 remarkably attenuated the increases of serum levels of these inflammatory molecules, whereas inhibition of FGF21 reversed these effects. These findings imply that FGF21 exerts anti-inflammatory effects by decreasing the serum inflammatory molecular levels.
Figure 2.
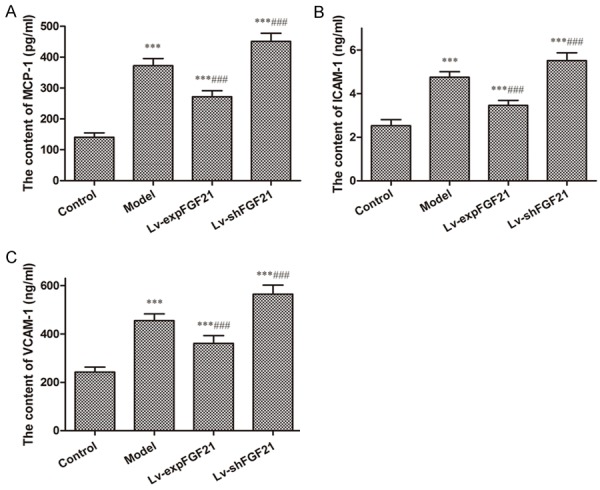
Serum levels of pro-inflammatory molecules MCP-1, ICAM-1, and VCAM-1 in atherosclerotic rats. The levels of MCP-1 (A), ICAM-1 (B) and VCAM-1 (C) were measured in the serum of rats in different groups using assay kit according to the manufacturer’s instructions. Data are expressed in the form of mean ± SD (n=8) for three independent experiments. Compared with the control group, ***P<0.001; compared with the model group, ###P<0.001.
In addition, the levels of oxidative product, MDA, in serum and arterial tissues was raised in the model rats (P<0.001) (Figure 3A and 3B). In contrast, the contents of antioxidative indicators, such as GSH and T-AOC, were lower in serum and arterial tissues in the model group than that in the control group (P<0.001) (Figure 3C-F). Moreover, the levels of MDA reduced and the levels of GSH and T-AOC increased in the Lv-expFGF21 group both in serum and tissues, whereas the levels of MDA continued to increase and the levels of GSH and T-AOC continued to minimize in the Lv-shFGF21 group. These results reveal that FGF21 enhances anti-oxidative capacity by enhancing expression of anti-oxidative indicators.
Figure 3.
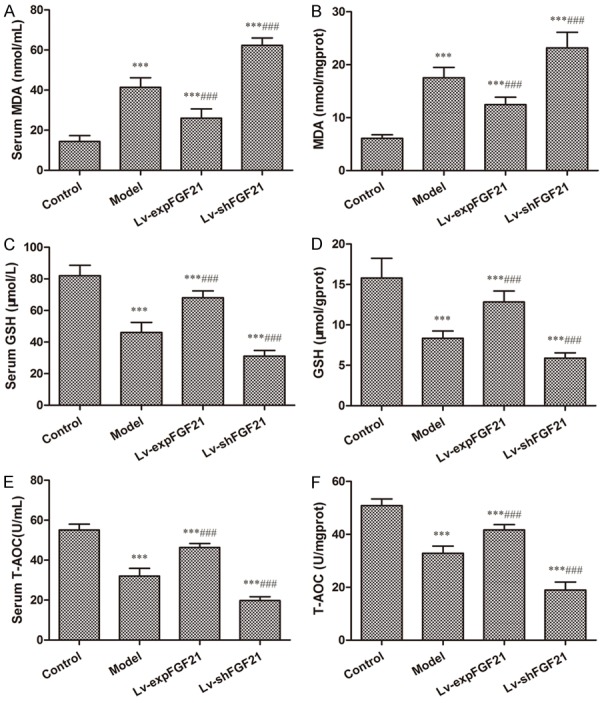
The levels of oxidative and anti-oxidative indicators in serum and arterial tissues of atherosclerotic rats. The levels of MDA (A and B), GSH (C and D) and T-AOC (E and F) were measured in serum and arterial tissues of rats in different groups using ELISA assay kit according to the manufacturer’s instructions. Data are expressed in the form of mean ± SD (n=8) for three independent experiments. Compared with the control group, ***P<0.001; compared with the model group, ###P<0.001.
FGF21 promotes expression of Nrf2-ARE signaling pathway-related proteins in atherosclerotic rats
Nrf2-ARE signaling pathway is one of the critical antioxidant defense pathways. To investigate whether the Nrf2-ARE signaling pathway is involved in the FGF21-mediated anti-oxidative effect, we evaluated expression of proteins involved in this pathway. As shown in Figure 4, compared to the control group, both transcriptional and protein expressions of Nrf2 and its downstream genes, such as HO-1, NQO-1, γGCS, and SOD, were significantly suppressed in arterial tissues of atherosclerotic rats in the model group (P<0.05 for all). Compared to the model group, up-regulation of FGF21 could markedly increase mRNA and protein expression of Nrf2 and downstream genes (P<0.05 for all), whereas opposite effects occurred when the expression of FGF21 was inhibited. Thus, these findings demonstrate that FGF21 can induce activation of the Nrf2-ARE signaling pathway in atherosclerotic rats.
Figure 4.
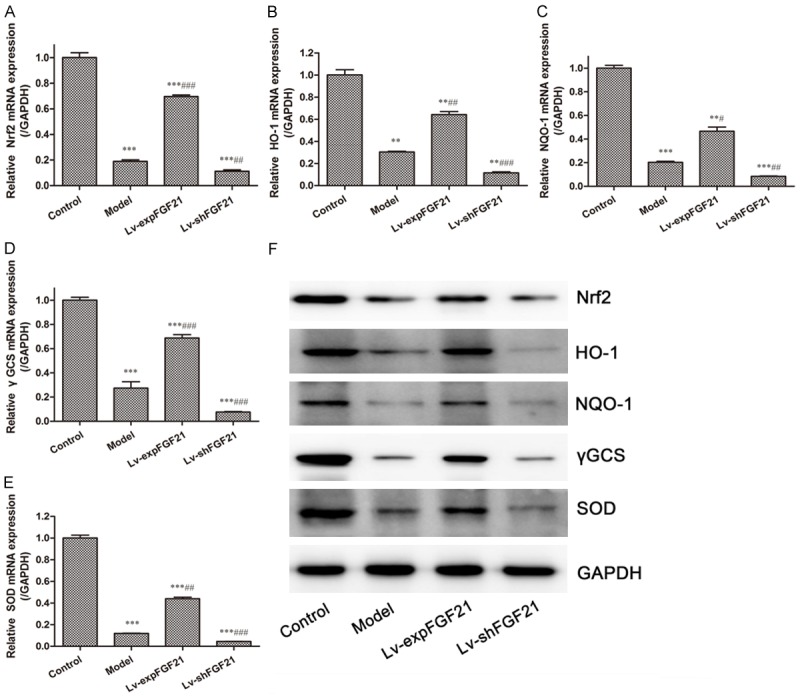
Expression of Nrf-ARE signaling pathway related proteins in arterial tissues of atherosclerotic rats. The transcriptional expression of Nrf2 (A), HO-1 (B), NQO-1 (C), γGCS (D) and SOD (E) in arterial tissues of rats in different groups were detected by RT-PCR. Protein expression of these proteins (F) in arterial tissues was detected by Western blot. Data are expressed in the form of mean ± SD (n=8) for three independent experiments. Compared with the control group, **P<0.01, ***P<0.001; compared with the model group, ##P<0.01, ###P<0.001.
Discussion
Fibroblast growth factor 21 (FGF21), a member of the endocrine FGF subfamily, is predominantly secreted by the liver but also other tissues [13]. During starvation and longtime fasting, FGF21 is induced mainly by the liver to enhance fatty acid oxidation, ketogenesis, and gluconeogenesis. Additionally, serum FGF21 levels are remarkably increased in patients with metabolic disorders [14]. FGF21 participates in metabolic regulation and has been proven to play important roles in mediating glucose and lipid metabolism through binding to the complex receptor between the β-klotho and FGF receptors [15]. The mechanisms that FGF21 acts as a metabolic regulator include interaction between FGF21 and peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1alpha) [16], a key transcriptional regulator of energy homeostasis, and activation of PPAR alpha and AKT/GSK-3 signaling pathways [15,17].
Increasing evidence indicates that FGF21 has multiple protective effects. For instance, endogenous FGF21 is obviously elevated in neurons after treatment with mood stabilizers and exogenous FGF21 protein exerts neuroprotective effects through activation of the AKT/GSK-3 signaling pathway [15]. Some evidence also suggests that FGF21 plays a key role in the prevention of atherosclerosis. The findings from Lin ZF and Pan XB [18] revealed that FGF21 deficiency exacerbated the formation of atherosclerotic plaques and increased mortality in apolipoprotein E-/- mice. Furthermore, they demonstrate that FGF21-mediated protection against atherosclerosis is associated with a decrease of cholesterol synthesis and abduction of adiponectin which improved the hypercholesterolemia in apolipoprotein E-/- mice. In this study we also show that mRNA and protein levels of FGF21 in arterial tissues of atherosclerotic rats are significantly reduced compared to the normal rats. Moreover, there was obvious pathological alteration, such as the incrassation of the thickness of the endarterium and infiltration of inflammatory cells, in arterial tissues of atherosclerotic rats. These demonstrate down-regulation of FGF21 can be associated with the atherosclerosis. Another study also revealed that FGF21 could promote the activation of ABCA1 and ABCG1 in macrophages and enhance cholesterol efflux in macrophages [19], which indicate that FGF21 plays a role in shrinking atherosclerotic plaques.
Endothelial dysfunction is a major initiator of atherosclerotic progression. ET-1 and NO, act as important vasoconstrictors and vasodilators, and are responsible for the regulation of the arterial pressure. The turbulence of synthesis of ET-1 and NO is one of the essential initiators of endothelial dysfunction [20]. In our study the level of serum vasoconstrictor ET-1 was 1.82-fold greater in atherosclerotic rats than that in normal rats, meanwhile the levels of serum NO were 1.58-fold lower. Up-regulation of FGF21 could reverse these changes, including reducing the serum ET-1 level and raising serum NO level, while down-regulation of FGF21 induced opposite results. These findings suggest that FGF21 could improve endothelial dysfunction via affecting expression of vasoconstrictors and vasodilators. Either increasing vasoconstriction expression or reducing vasodilation expression in coronary arteries leads to reduction of blood flow and inadequate oxygen supply, which is harmful for patients with cardiovascular diseases, especially acute coronary syndrome [21].
Furthermore, oxidative stress plays a key role in atherogenesis and lipid oxidation products accelerate disease progression via triggering vascular inflammation [22,23]. Oxidation products can activate endothelial cells to specifically bind to monocytes and other pro-inflammatory mediators such as TNF-α and IL-1 also promote the activation of endothelial cells and adhesion of monocytes and neutrophils through activating the NF-κB signaling pathway which can mediate the increasing expression of adhesion molecules such as ICAM-1 and VCAM-1. These lead to the occurrence of an acute or chronic inflammatory response [23]. Thus, oxidative stress plays a key role in the initiation of acute or chronic inflammation. The results in our study show that expression of the oxidation product MDA also increased both in serum and arterial tissues of model rats. In addition, expression of the antioxidative indicator GSH and total antioxidative capacity (T-AOC) sharp reduced, suggesting the existence of high levels of oxidative stress in atherosclerotic rats. Likewise, serum levels of inflammatory response mediators markedly increased in atherosclerosis, characterized by the elevation of the expression of MCP-1, VCAM-1, and ICAM-1.
Transcription factor NF-E2-related 2 (Nrf2) exerted its anti-oxidative effects by promoting the expression of key anti-oxidative enzymes. Nrf2-ARE signaling pathway could be activated to protect vascular cells from injury of oxidative stress and shear stress. Some studies has proven that Nrf2-ARE signaling pathway plays a protective effect against atherosclerosis by activating cellular antioxidant defense mechanism [10]. Interestingly, a recent study has reported that the overall effect of Nrf2-signaling also contributed to the development of atherosclerosis in mice [24], while the mechanism remained unknown. Even so, the results of our study showed the mRNA and protein expression of Nrf2 and downstream genes are inhibited in arterial tissue in atherosclerotic rats, indicating downregulation of Nrf2-signaling is positively related to atherogenesis. In addition, FGF21 was considered as a key regulator of inflammation and oxidative stress cell response via enhancing the capacity of Nrf2-mediated anti-oxidative response [25,26]. So FGF21-mediated anti-atherosclerotic effect might be associated with Nrf2 signaling pathway. Our study demonstrates that up-regulation of FGF21 could promote expression of the Nrf2-ARE signaling pathway-related proteins in model rats, simultaneously accompanied by reduction of the levels of inflammatory molecules and an increase of anti-oxidative capacity. Downregulation of FGF21 reversed these actions. These data indicate the FGF21 could promote the expression of Nrf2-signaling pathway-related protein in atherosclerotic rats.
In conclusion, our study demonstrates that up-regulation of FGF21 improves endothelial dysfunction, attenuates expression of pro-inflammatory molecules and enhances anti-oxidative capacity during atherogenesis in rats. Furthermore, expression of Nrf2-signal pathway related protein was up-regulated after expression of FGF21 was increased in atherosclerotic rats and these effects disappeared in FGF21-knockdown atherosclerotic rats. These results indicate that FGF21 is an essential factor in the protection against atherogenesis via enhancing Nrf2-ARE signaling pathway and is expected to lay the foundation for providing a new target in the treatment of atherosclerosis.
Acknowledgements
This work was supported by the grants from Science and Technology Planning Project of Hubei Province and Youth Foundation of Wuhan University of Science and Technology.
Disclosure of conflict of interest
None.
References
- 1.Rafieian-Kopaei M, Setorki M, Doudi M, Baradaran A, Nasri H. Atherosclerosis: process, indicators, risk factors and new hopes. Int J Prev Med. 2014;5:927–946. [PMC free article] [PubMed] [Google Scholar]
- 2.Baldrighi M, Mallat Z, Li X. NLRP3 inflammasome pathways in atherosclerosis. Atherosclerosis. 2017;267:127–138. doi: 10.1016/j.atherosclerosis.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 3.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 4.Profumo E, Buttari B, D’Arcangelo D, Tinaburri L, Dettori MA, Fabbri D, Delogu G, Riganò R. The nutraceutical dehydrozingerone and its dimer counteract inflammation- and oxidative stress-induced dysfunction of in vitro cultured human endothelial cells: a novel perspective for the prevention and therapy of atherosclerosis. Oxid Med Cell Longev. 2016;2016:1–12. doi: 10.1155/2016/1246485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anwaier G, Chen C, Cao Y, Qi R. A review of molecular imaging of atherosclerosis and the potential application of dendrimer in imaging of plaque. Int J Nanomedicine. 2017;12:7681–7693. doi: 10.2147/IJN.S142385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL. Oxidative stress in atherosclerosis. Curr Atheroscler Rep. 2017;19:42–52. doi: 10.1007/s11883-017-0678-6. [DOI] [PubMed] [Google Scholar]
- 7.Ooi BK, Goh BH, Yap WH. Oxidative stress in cardiovascular diseases: involvement of Nrf2 antioxidant redox signaling in macrophage foam cells formation. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18112336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Bryan HK, Olayanju A, Goldring CE, Park BK. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem Pharmacol. 2013;85:705–717. doi: 10.1016/j.bcp.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Ruotsalainen AK, Inkala M, Partanen ME, Lappalainen JP, Kansanen E, Makinen PI, Heinonen SE, Laitinen HM, Heikkila J, Vatanen T, Horkko S, Yamamoto M, Yla-Herttuala S, Jauhiainen M, Levonen AL. The absence of macrophage Nrf2 promotes early atherogenesis. Cardiovasc Res. 2013;98:107–115. doi: 10.1093/cvr/cvt008. [DOI] [PubMed] [Google Scholar]
- 11.Yan J, Wang J, Huang H, Huang Y, Mi T, Zhang C, Zhang L. Fibroblast growth factor 21 delayed endothelial replicative senescence and protected cells from H2O2-induced premature senescence through SIRT1. Am J Transl Res. 2017;9:4492–4501. [PMC free article] [PubMed] [Google Scholar]
- 12.Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–781. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 13.Seo JA, Kim NH. Fibroblast growth factor 21: a novel metabolic regulator. Diabetes Metab J. 2012;36:26–28. doi: 10.4093/dmj.2012.36.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Fang Q, Gao F, Fan J, Zhou J, Wang X, Zhang H, Pan X, Bao Y, Xiang K, Xu A, Jia W. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J Hepatol. 2010;53:934–940. doi: 10.1016/j.jhep.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Leng Y, Wang Z, Tsai LK, Leeds P, Fessler EB, Wang J, Chuang DM. FGF-21, a novel metabolic regulator, has a robust neuroprotective role and is markedly elevated in neurons by mood stabilizers. Mol Psychiatry. 2015;20:215–223. doi: 10.1038/mp.2013.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, Burgess SC. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Lin Z, Pan X, Wu F, Ye D, Zhang Y, Wang Y, Jin L, Lian Q, Huang Y, Ding H, Triggle C, Wang K, Li X, Xu A. Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory element-binding protein-2 and induction of adiponectin in mice. Circulation. 2015;131:1861–1871. doi: 10.1161/CIRCULATIONAHA.115.015308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin XL, He XL, Zeng JF, Zhang H, Zhao Y, Tan JK, Wang Z. FGF21 increases cholesterol efflux by upregulating ABCA1 through the ERK1/2-PPARgamma-LXRalpha pathway in THP1 macrophage-derived foam cells. DNA Cell Biol. 2014;33:514–521. doi: 10.1089/dna.2013.2290. [DOI] [PubMed] [Google Scholar]
- 20.Smiljic S. The clinical significance of endocardial endothelial dysfunction. Medicina (Kaunas) 2017;53:295–302. doi: 10.1016/j.medici.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Kirkby NS, Hadoke PW, Bagnall AJ, Webb DJ. The endothelin system as a therapeutic target in cardiovascular disease: great expectations or bleak house? Br J Pharmacol. 2008;153:1105–1119. doi: 10.1038/sj.bjp.0707516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berliner JA, Leitinger N, Tsimikas S. The role of oxidized phospholipids in atherosclerosis. J Lipid Res. 2009;50(Suppl):S207–212. doi: 10.1194/jlr.R800074-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leitinger N. Oxidized phospholipids as triggers of inflammation in atherosclerosis. Mol Nutr Food Res. 2005;49:1063–1071. doi: 10.1002/mnfr.200500086. [DOI] [PubMed] [Google Scholar]
- 24.Freigang S, Ampenberger F, Spohn G, Heer S, Shamshiev AT, Kisielow J, Hersberger M, Yamamoto M, Bachmann MF, Kopf M. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur J Immunol. 2011;41:2040–2051. doi: 10.1002/eji.201041316. [DOI] [PubMed] [Google Scholar]
- 25.Yu Y, He J, Li S, Song L, Guo X, Yao W, Zou D, Gao X, Liu Y, Bai F, Ren G, Li D. Fibroblast growth factor 21 (FGF21) inhibits macrophage-mediated inflammation by activating Nrf2 and suppressing the NF-kappaB signaling pathway. Int Immunopharmacol. 2016;38:144–152. doi: 10.1016/j.intimp.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 26.Gomez-Samano MA, Grajales-Gomez M, Zuarth-Vazquez JM, Navarro-Flores MF, Martinez-Saavedra M, Juarez-Leon OA, Morales-Garcia MG, Enriquez-Estrada VM, Gomez-Perez FJ, Cuevas-Ramos D. Fibroblast growth factor 21 and its novel association with oxidative stress. Redox Biol. 2017;11:335–341. doi: 10.1016/j.redox.2016.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]


