Abstract
Glioblastoma (GBM) is the most common and aggressive brain tumor in adults. Classical treatment of glioblastoma includes surgical resection followed by radiation and chemotherapy. However, radio-resistance is always a challenge for the treatment. MicroRNA-128 was found at lower expression in glioma tissues compared to normal tissue. Its downstream target gene, Bmi-1, was associated with self-renewal and differentiation of neural stem cells and could promote the growth of glioma. Our previous studies showed that expression of Bmi-1 can increase following exposure to X-ray radiation, implying that Bmi-1 may confer radio-resistance to glioma. However, the mechanism is still unclear. In this study, we found that overexpression microRNA 128 could inhibit growth of glioma cells and expression of Bmi-1 (P<0.05). Following exposure the 8 Gy X-ray, the growth of cells was inhibited in the microRNA-128 overexpression group compared to the control group (P<0.05). Expression of Bmi-1 was also lower (P<0.05) and the ratio of senescent cells was higher (P<0.05) in the microRNA-128 overexpression group than the control group. Thus, our results suggest that overexpression of micro-RNA128 could increase the radio-sensitivity of glioma cells through Bmi-1. This mechanism may inhibit senescent evasion in glioma cells and provides a novel view for how to resolve the radio-resistance of glioma and investigate a new strategy for glioma radiation treatment regimens.
Keywords: Glioma, microRNA-128, Bmi-1, senescence, radiation
Introduction
Glioblastoma (GBM) is the most common and aggressive brain tumor in adults [1]. Therapeutic methods, including surgery, radiation and chemotherapy, have been widely applied in treatment of GBM. However, the overall 5-year survival of GBM is still less than 10%, and the median survival time is less than 2 years [2]. The reason why GBM has a high recurrence incidence and mortality is due to therapy resistance of GBM, especially radio-resistance. Increasing the sensitivity of radiation in GBM can significantly improve the therapeutic effect and benefit the patients’ survival. Therefore, it would be very important to investigate the mechanism of radio-resistance of GBM, and to find the ways to improve the GBM response to radiation.
MicroRNAs are generally 18-24 nucleotides long expressing small non-coding RNAs, which can regulate gene expression through binding target mRNAs [3,4]. Many microRNAs have been demonstrated to play very important roles in cell proliferation [5], differentiation [6], apoptosis [7], senescence [8] and stem cell maintenance [9]. It has been found that microRNAs are involved in many malignant tumors as oncogenes or anti-oncogenes [10,11]. MicroRNA128 distributes in brain specifically, and it was found be down-regulated in glioma [12]. However, the role of microRNA-128 in the radio-resistance of glioma has yet not been elucidated.
B lymphoma mouse Moloney leukemia virus insertion region 1 (Bmi-1) belongs to polycomb protein family. In many cancers, expression of Bmi-1 is up-regulated so that it is regarded as an oncogene, including in glioma [13-15]. The function of Bmi-1 has been demonstrated to be associated with neural stem cell renewal [16]. Our previous studies showed that expression of Bmi-1 can increase following exposure to X-ray radiation, which may imply that Bmi-1 is involved in radio-resistance of glioma [17]. However, the mechanism is still unclear.
Our previous finding showed that microRNA128a and Bmi-1 may be involved in glioma radio-resistance by affecting senescence in human U87 glioma cells [17,18]. In this study, we performed further research to investigate the role of microRNA128a in radio-resistance in glioma cells, and to clarify its mechanism.
Materials and methods
Cell culture
The glioma cell line U87 was obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and cultured in minimum essential medium (GE Healthcare Life Sciences, Logan, UT, USA) containing 10% fetal bovine serum (FBS; GE Healthcare Life Sciences) and incubated at 37°C in a humidified atmosphere of 5% CO2.
Cell radiation
Cell radiation was performed using X-ray radiation by a linear accelerator source (Elekta, Stockholm, Sweden) at a dose rate of 300 cGy/min. Prior to radiation, a radiation plan was designed, and the accuracy of the X-ray radiation doses was verified by a radiation therapy physicist using I’mRT MatriXX 2D-ion chamber array (IBA Dosimetry, Schwarzenbruck, Germany). According to our previous results, the difference of radiation effects was apparent between groups of less than and more than 4 Gy. In the present study, the cells were exposed to various doses of X-ray radiation, including 0 Gy (control group), 2 Gy, and 8 Gy. All groups of cells were continuously incubated following radiation until the experiments were finished.
Cell transfection
Transfection of microRNA128 was carried out using Lipofectamine 2000 agent (Invitrogen) according to the manufacturer’s instructions. Pre-microRNA-128-1 oligonucleotides (Ambion) and reporter plasmids were transfected into U87 with microRNA-128 at a final concentration of 50 nM. The pri-miR-128-1 lentiviral construct was used for establishing U87 cells constitutively overexpressing miR-128-1, according to the manufacturer’s instructions (System Biosciences).
Cell growth curve
The cells were seeded in 24-well plates at a density of 5×104 cells/0.5 ml on day 0. If the cells need to be irradiated according with the experimental groups, the U87 cells were immediately exposed to X-ray radiation. Every 24 h, the number of cells in three wells was quantified using a cell counter (Inno-AllianceBiotech, Wilmington, DE, USA) and the mean was calculated. The results are presented as the mean ± standard error of three independent experiments.
Cell cycle
To analyze the cell cycle, glioma cells were fixed with 70% ethanol, re-suspended in PBS/1% FBS, and treated with ribonuclease (Beyotime Institute of Biotechnology, Haimen, China). PI was added to the cells and the samples were then analyzed by FCM (Becton-Dickinson). Cell cycle profile analysis of the DNA histograms of integrated red fluorescence was performed with ModFit LT 2.0 (Verity Software, Inc., Topsham, ME, USA).
Cell senescence
Senescence-associated β-galactosidase (SA-β-Gal) staining was used to detect the senescence ratio. SA-β-Gal staining was performed using the SA-β-Gal Kit (Beyotime Institute of Biotechnology) following the manufacturer’s instructions. The cells were considered to be positive when the cytoplasm was stained with SA-β-Gal.
Western blot analysis
Total protein was extracted with lysis buffer (150 mM NaCl, 50 mM Tris with pH 7.4, 1% NP40, 0.1% SDS, 0.5% sodium deoxycholate), supplemented with protease inhibitors (CWBio, Inc., Beijing, China). After lysis on ice for 30 min, the lysate was centrifuged at 10,000×g for 15 min at 4°C. The protein concentration in each sample extract was detected using the bicinchoninic acid assay (CWBio, Inc.). SDS-PAGE was performed on 15% polyacrylamide gels, with 40 μg of protein sample per lane. Following electrophoresis, the protein was transferred to nitrocellulose membranes and incubated in 5% non-fat milk at room temperature for 2 h. Subsequently, the membranes were incubated overnight at 4°C with a primary polyclonal rabbit anti-human antibody for Bmi-1 (sc-10745; Santa Cruz Biotechnology, Dallas, TX, USA). Subsequent to being washed three times using PBS, the membrane was incubated with an appropriate concentration of horseradish peroxidase-conjugated anti-rabbit secondary antibody (CWBio, Inc.) for 2 h. Following additional three washes with PBS, the specific protein band was visualized using an enhanced chemiluminescence kit (CWBio, Inc.).
RT-PCR analysis
Total RNA was extracted from U87 glioma cells in accordance with the instructions Ultrapure RNA kit (CwBio, Inc., Beijing, China) for Bmi-1 or the miRNApure Mini kit (CwBio, Inc.) for microRNA-128. The RNA was reverse transcribed using the HiFi-MMLV cDNA kit (CwBio, Inc.) for Bmi-1 or the miRNA cDNA kit (CwBio, Inc.) for microRNA-128. The cDNA was subsequently amplified using UltraSYBR Mixture (with ROX; CwBio, Inc.) for Bmi-1 or the Real-Time PCR Assay kit (CwBio, Inc.) for microRNA-128. β-actin (ACTB) mRNA (for Bmi-1) or small nuclear RNA U6 (for microRNA-128) expression levels were used as an internal control to normalize the data. The primers for Bmi-1 were obtained from CwBio, Inc. (cat. no. CW0900). The primers for microRNA-128 were also synthesized by CwBio, Inc. The primers used in the present study were as follows: Bmi-1, forward 5’-TCC ACC TCT TCT TGT TTG CCT-3’, and reverse 5’-GAA GAA GTT GCT GAT GAC CCA-3’. U6, forward 5’-GCT TCG GCA GCA CAT ATA CTA AAAT-3’; and miRNA-128a, forward 5’-CAC AGT GAA CCG GTC TCTTT-3’. All methods were conducted according to the manufacturer’s instructions.
The mRNA and miRNA expression levels were measured by qRT-PCR using a LC-480II thermal cycler (Roche Diagnostics, Basel, Switzerland). The cycling conditions were as follows: 95°C for 10 min, followed by 40 cycles of amplification at 95°C for 15 sec and 60°C for 60 sec. The relative miRNA and mRNA expression levels were calculated using the ΔΔCT method.
Statistics
Statistical analysis was done using SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL, USA). The results are expressed as mean ± standard deviation. Student’s t-test (unpaired) and one-way ANOVA was used to evaluate the statistically significant differences. P<0.05 was considered statistically significant.
Results
Overexpression of microRNA-128 can inhibit cell growth of U87 glioma cells
Following transduction with a lentiviral vector containing the primary transcript of microRNA-128 (pre-miR-128-1), the U87 glioma cells could produce high levels of mature microRNA-128 (Figure 1A). The U87 cells of transduced group (pre-miR128) and negative control group (NC) were then cultured and observed. The proliferation of pre-miR128 group was inhibited in comparison with NC group (Figure 1B).
Figure 1.
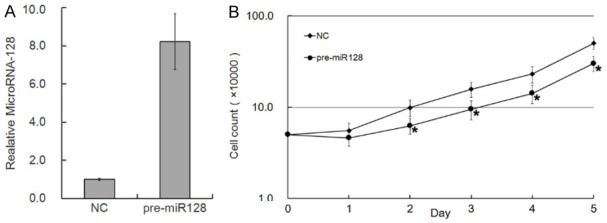
Overexpression of microRNA-128 inhibits cell growth of U87 glioma cells. A: Real-time PCR analysis of microRNA-128 in U87 cells after transfection with pre-mi128 or negative control for 48 h. Pre-miR-128 and negative control (NC) were transfected into U87 using Lipofectamine 2000 (Invitrogen). B: Cell growth curve of U87 cells after treatment with negative control or miR-128 as above. Values represent means ± standard deviation (SD) for three wells. * means p value ≤0.05, all compared with control.
Overexpression of microRNA-128 can increase the radio-sensitivity by increasing cellular senescence in U87 glioma cells
In our previous study, U87 glioma cells showed no cell apoptosis but cellular senescence following exposure to X-ray radiation [17]. To investigate whether overexpression of microRNA-128 could affect cellular radio-sensitivity, the U87 cells of NC and transduced groups were exposed to 2 and 8 Gy X-ray radiation. Cell growth was inhibited more significantly in the transduced group than the NC group (Figure 2A). Meanwhile, cellular senescence was increased in the transduced group (Figure 2B, 2C).
Figure 2.
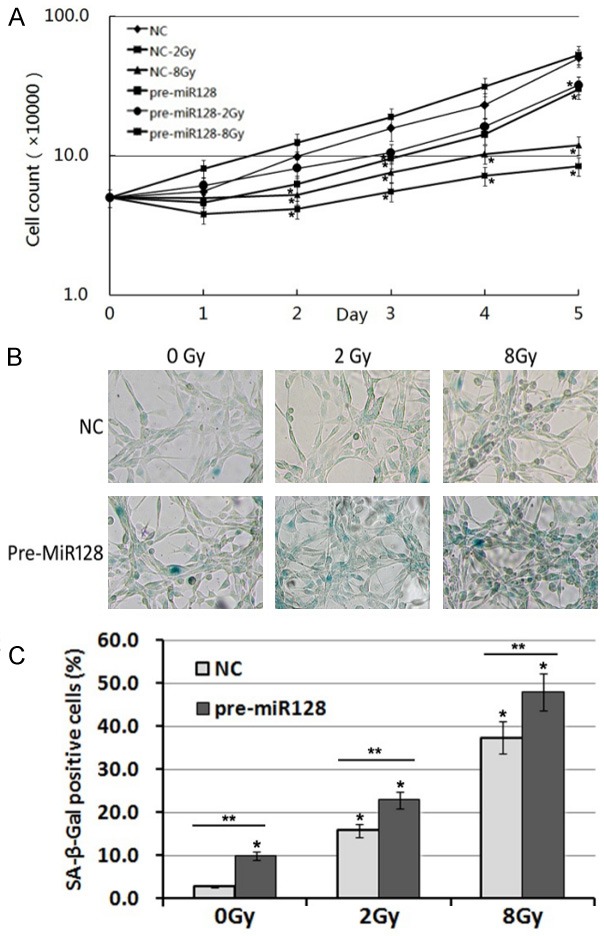
Overexpression of microRNA-128 increases radio-sensitivity by increasing cellular senescence in U87 glioma cells. A: Cell growth curve of U87 cells exposed to 2 and 8 Gy X-ray radiation after treatment with negative control or miR-128 as above. Values represent mean ± standard deviation (SD) for three wells. *mean p value ≤0.05, all compared with negative control. B: Following 72 h of exposure to X-ray radiation at 2 and 8 Gy doses, the U87 glioma cells were treated by SA-β-Gal staining and images were captured under phase-contrast microscopy. C: Under the microscope the SA-β-Gal positive cells were counted and the quantitative results are presented as the mean ± standard deviation of three independent experiments. *P<0.05 vs. control. **P<0.05 comparison among groups.
Overexpression of microRNA-128 can decrease Bmi-1 expression following exposure to X-ray radiation in U87 glioma cells
In our previous study, Bmi-1 expression could be increased following exposure to X-ray radiation in a dose-dependent manner. Meanwhile, microRNA-128 expression was decreased [18]. To investigate the relationship between microRNA-128 and Bmi-1, the expression of Bmi-1 was detected by Western blot (Figure 3A) and RT-PCR (Figure 3B). The results show that overexpression of microRNA-128 can significantly decrease Bmi-1 expression of both protein and mRNA. Following 72 h of exposure to X-ray radiation, the expression of Bmi-1 increased significantly at 8 Gy dose in NC group, which was consistent with our previous result [17,18]. However, in the pre-MicroRNA128 group, expression of Bmi-1 was inhibited (Figure 3C, 3D).
Figure 3.
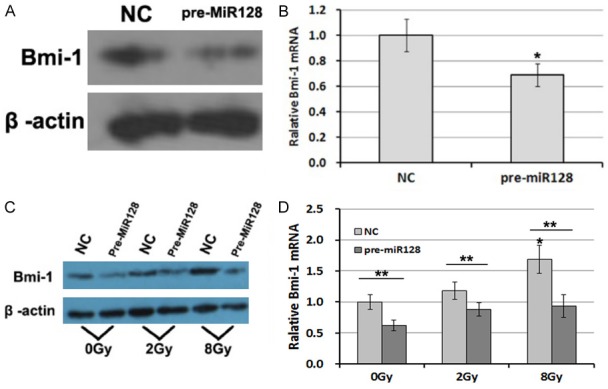
Overexpression of microRNA-128 can decrease Bmi-1 expressing following exposure to X-ray radiation in U87 glioma cells. Bmi-1 expression of U87 cells after treatment with negative control or miR-128 as above, through (A) Western blot & (B) RT-PCR. Following 72 h of exposure to X-ray radiation at 2 and 8 Gy doses, the expression of Bmi-1 of U87 glioma cells were detected by (C) Western blot & (D) RT-PCR. The quantitative results are presented as the mean ± standard deviation of three independent experiments. *P<0.05 vs. control. **P<0.05 comparison among groups.
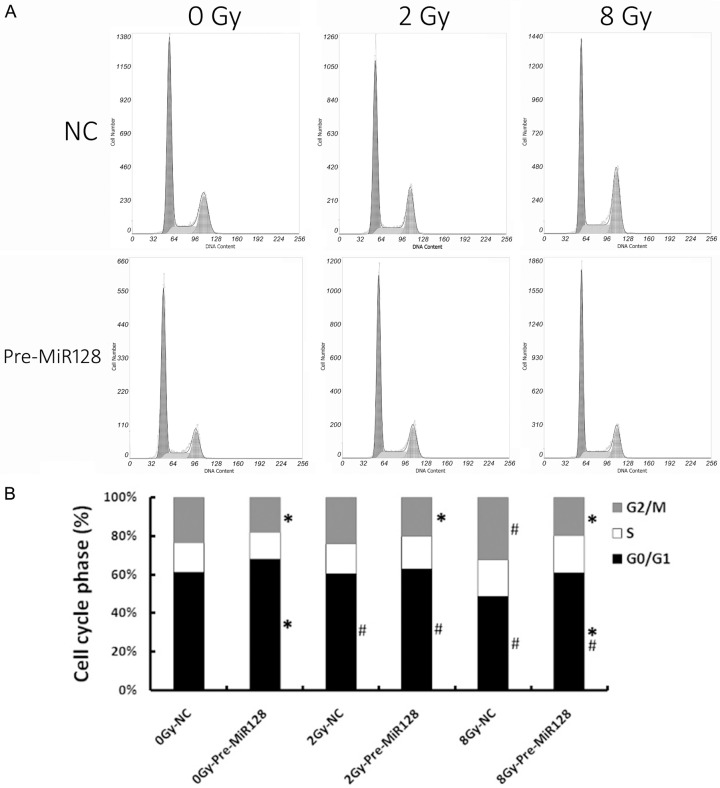
In our previous study, we showed that Bmi-1 may be involved in regulation of cell cycle phase. After treatment with negative control or miR-128 as above, U87 cells were exposed to 2 Gy and 8 Gy X-ray radiations respectively. Following 72 h of exposure to X-ray radiation, cell cycle phase was detected and analyzed by flow cytometry (Figure 4A). In NC group, the results of cell cycle phase was accordance with the previous study [17]. In the pre-MicroRNA128 group, the manner of cell cycle phase was different from the NC group because there was not significant changes regardless of radiation dose (Figure 4B). In comparison to the matched NC group, the percent of G2/M phase was decreased in pre-MicroRNA128 group, whereas the percent of G0/G1 phase was increased.
Figure 4.
Overexpression of microRNA-128 can affect cell cycle following exposure to X-ray radiation in U87 glioma cells. Following 72 h exposure to X-ray radiation, cell cycle status of U87 cells was analyzed through flow cytometry (A). The statistical results are shown here (B). Quantitative results are presented as the mean ± standard deviation of three independent experiments. *P<0.05 vs. The matched NC. #P<0.05 vs. 0Gy in groups.
Discussion
Radio-resistance is always a challenge for the treatment of glioma. Classical treatment of glioblastoma includes surgical resection followed by radiation and chemotherapy. Many patients tumors recurred in one year even if they had a good response in the previous treatment [19]. Apoptosis plays an important role in tumor suppression after radiation [20], but recently some researchers revealed that radiation can induced senescence instead of apoptosis in some tumors, such as breast cancer, lung cancer and colon cancer [21,22].
Cellular senescence plays an important role in tumor suppression [23]. Collado et al. found that normal cells lost the proliferative capacity and stayed irreversibly in a stable form of cell cycle at the ending stage in some situation. In contrast, tumor cells were believed to lose the ability of senescence [24]. In some situations, such as radiation or chemotherapy, tumor cells can be induced to undergo senescence again, which can help suppress the proliferation of tumor [25]. In our previous study, we determined that U87MG, an glioblastoma cell line, only underwent senescence following exposure to radiation instead of apoptosis [17]. In the present study, our results showed that increasing microRNA-128 in glioma cells can prevent them from senescent evading before and after exposure to radiation. Cellular senescence could be increased resulting in the growth of glioblastoma, which can be induced by radiation or genetic manipulation.
MicroRNA-128 was found to be lower in glioma tissues compared to normal tissue [26,27]. It is reported that microRNA128 is expressed abundantly in mature differentiated neurons but is absent in neural stem cells, which are associated with differentiation of neural cells [28]. It is known microRNA-128 could be as a tumor suppressor in glioblastoma and medulloblastoma [12,29]. One of the mechanisms is through the suppression towards to Bmi-1. Bmi-1 is the downstream target gene of mircoRNA128 and is associated with self-renewal and differentiation of neural stem cells [16,30] and can promote glioma growth [15].
The aim of the present study was to elucidate whether microRNA128 could alter cellular response to X-ray radiation and regulate senescence through its downstream gene Bmi-1. Senescence was characterized by irreversible cell cycle arrest, especially G0/G1 phase. After exposure to the radiation, it was found overexpression microRNA-128 in U87MG could suppress expression of Bmi-1 at the level of both mRNA and protein. Meanwhile, the ratio of senescent cells increased significantly. Through the analysis of cell cycle, the cells in G0/G1 phase was more in pre-microRNA128 than in control group following 8 Gy X-ray radiations. This evidence indicates that microRNA128 can change radio-resistance of glioma through the Bmi-1 associated cellular senescent pathway. Bmi-1 can control the tumor development through the Ink4a/Arf pathway in glioma [15]. p16Ink4a is one of the hallmarks of cellular senescence [31]. CDK4, a key enzyme in G1-S transformation, can be inhibited by p16Ink4a. In theory, if the Bmi-1 expression increases, it will suppress p16Ink4a resulting in unregulated CDK4, leading to S phase and evasion and senescence. Therefore, the mechanism of overexpression of microRNA-128 intervenes through inhibition of Bmi-1, so that glioma cells are forced to undergo senescence again.
In conclusion, the results of our study suggest that overexpression of microRNA-128 can increase the radio-sensitivity of glioma cells through its target, Bmi-1. The possible mechanism to inhibit senescent evasion of glioma cells. These data provide a novel view on solving radio-resistance of glioma and suggest a new strategy for glioma radiation treatment regimens.
Acknowledgements
The present study was supported by the Seed Foundation of the Second Hospital of Shandong University, China (grant no. S2015010005), the Natural Science Foundation of Shandong Province, China (grant no. ZR2011HL019), the Key Research and Development Plan of Shandong Province, China (grant no. 2015GSF118054).
Disclosure of conflict of interest
None.
References
- 1.Wang Y, Jiang T. Understanding high grade glioma: molecular mechanism, therapy and comprehensive management. Cancer Lett. 2013;331:139–46. doi: 10.1016/j.canlet.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 2.Tran B, Rosenthal MA. Survival comparison between glioblastoma multiforme and other incurable cancers. J Clin Neurosci. 2010;17:417–21. doi: 10.1016/j.jocn.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Makeyev EV, Maniatis T. Multilevel regulation of gene expression by microRNAs. Science. 2008;319:1789–90. doi: 10.1126/science.1152326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Sirotkin AV, Lauková M, Ovcharenko D, Brenaut P, Mlyncek M. Identification of microRNAs controlling human ovarian cell proliferation and apoptosis. J Cell Physiol. 2010;223:49–56. doi: 10.1002/jcp.21999. [DOI] [PubMed] [Google Scholar]
- 6.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–6. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 7.Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36. doi: 10.1016/s0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- 8.Martinez I, Almstead LL, DiMaio D. MicroRNAs and senescence. Aging (Albany NY) 2011;3:77–8. doi: 10.18632/aging.100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatfield SD, Shcherbata HR, Fischer KA, Nakahara K, Carthew RW, Ruohola-Baker H. Stem cell division is regulated by the microRNA pathway. Nature. 2005;435:974–8. doi: 10.1038/nature03816. [DOI] [PubMed] [Google Scholar]
- 10.Stahlhut Espinosa CE, Slack FJ. The role of microRNAs in cancer. Yale J Biol Med. 2006;79:131–40. [PMC free article] [PubMed] [Google Scholar]
- 11.Ruan K, Fang X, Ouyang G. MicroRNAs: novel regulators in the hallmarks of human cancer. Cancer Lett. 2009;285:116–26. doi: 10.1016/j.canlet.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 12.Ciafrè SA, Galardi S, Mangiola A, Ferracin M, Liu CG, Sabatino G, Negrini M, Maira G, Croce CM, Farace MG. Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem Biophys Res Commun. 2005;334:1351–8. doi: 10.1016/j.bbrc.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 13.Chowdhury M, Mihara K, Yasunaga S, Ohtaki M, Takihara Y, Kimura A. Expression of Polycomb-group (PcG) protein BMI-1 predicts prognosis in patients with acute myeloid leukemia. Leukemia. 2007;21:1116–22. doi: 10.1038/sj.leu.2404623. [DOI] [PubMed] [Google Scholar]
- 14.Zhang FB, Sui LH, Xin T. Correlation of Bmi-1 expression and telomerase activity in human ovarian cancer. Br J Biomed Sci. 2008;65:172–7. doi: 10.1080/09674845.2008.11732824. [DOI] [PubMed] [Google Scholar]
- 15.Bruggeman SW, Hulsman D, Tanger E, Buckle T, Blom M, Zevenhoven J, van Tellingen O, van Lohuizen M. Bmi1 controls tumor development in an Ink4a/Arf-independent manner in a mouse model for glioma. Cancer Cell. 2007;12:328–41. doi: 10.1016/j.ccr.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 16.Zencak D, Lingbeek M, Kostic C, Tekaya M, Tanger E, Hornfeld D, Jaquet M, Munier FL, Schorderet DF, van Lohuizen M, Arsenijevic Y. Bmi1 loss produces an increase in astroglial cells and a decrease in neural stem cell population and proliferation. J Neurosci. 2005;25:5774–83. doi: 10.1523/JNEUROSCI.3452-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye L, Wang C, Yu G, Jiang Y, Sun D, Zhang Z, Yu X, Li X, Wei W, Liu P, Cheng J, DU B, Hu L. Bmi-1 induces radioresistance by suppressing senescence in human U87 glioma cells. Oncol Lett. 2014;8:2601–2606. doi: 10.3892/ol.2014.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye L, Yu G, Wang C, Du B, Sun D, Liu J, Qi T, Yu X, Wei W, Cheng J, Jiang Y. MicroRNA128a, BMI1 polycomb ring finger oncogene, and reactive oxygen species inhibit the growth of U87 MG glioblastoma cells following exposure to Xray radiation. Mol Med Rep. 2015;12:6247–54. doi: 10.3892/mmr.2015.4175. [DOI] [PubMed] [Google Scholar]
- 19.Hirose Y, Berger MS, Pieper RO. Abrogation of the Chk1-mediated G(2) checkpoint pathway potentiates temozolomide-induced toxicity in a p53-independent manner in human glioblastoma cells. Cancer Res. 2001;61:5843–9. [PubMed] [Google Scholar]
- 20.Ross GM. Induction of cell death by radiotherapy. Endocr Relat Cancer. 1999;6:41–4. doi: 10.1677/erc.0.0060041. [DOI] [PubMed] [Google Scholar]
- 21.Byun HO, Han NK, Lee HJ, Kim KB, Ko YG, Yoon G, Lee YS, Hong SI, Lee JS. Cathepsin D and eukaryotic translation elongation factor 1 as promising markers of cellular senescence. Cancer Res. 2009;69:4638–47. doi: 10.1158/0008-5472.CAN-08-4042. [DOI] [PubMed] [Google Scholar]
- 22.Lee JJ, Lee JH, Ko YG, Hong SI, Lee JS. Prevention of premature senescence requires JNK regulation of Bcl-2 and reactive oxygen species. Oncogene. 2010;29:561–75. doi: 10.1038/onc.2009.355. [DOI] [PubMed] [Google Scholar]
- 23.Wu CH, van Riggelen J, Yetil A, Fan AC, Bachireddy P, Felsher DW. Cellular senescence is an important mechanism of tumor regression upon c-Myc inactivation. Proc Natl Acad Sci U S A. 2007;104:13028–33. doi: 10.1073/pnas.0701953104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat Rev Cancer. 2010;10:51–7. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roninson IB. Tumor cell senescence in cancer treatment. Cancer Res. 2003;63:2705–15. [PubMed] [Google Scholar]
- 26.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–8. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Chao T, Li R, Liu W, Chen Y, Yan X, Gong Y, Yin B, Liu W, Qiang B, Zhao J, Yuan J, Peng X. MicroRNA-128 inhibits glioma cells proliferation by targeting transcription factor E2F3a. J Mol Med (Berl) 2009;87:43–51. doi: 10.1007/s00109-008-0403-6. [DOI] [PubMed] [Google Scholar]
- 28.Smirnova L, Gräfe A, Seiler A, Schumacher S, Nitsch R, Wulczyn FG. Regulation of miRNA expression during neural cell specification. Eur J Neurosci. 2005;21:1469–77. doi: 10.1111/j.1460-9568.2005.03978.x. [DOI] [PubMed] [Google Scholar]
- 29.Venkataraman S, Alimova I, Fan R, Harris P, Foreman N, Vibhakar R. MicroRNA 128a increases intracellular ROS level by targeting Bmi-1 and inhibits medulloblastoma cancer cell growth by promoting senescence. PLoS One. 2010;5:e10748. doi: 10.1371/journal.pone.0010748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fasano CA, Dimos JT, Ivanova NB, Lowry N, Lemischka IR, Temple S. shRNA knockdown of Bmi-1 reveals a critical role for p21-Rb pathway in NSC self-renewal during development. Cell Stem Cell. 2007;1:87–99. doi: 10.1016/j.stem.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Hara E, Smith R, Parry D, Tahara H, Stone S, Peters G. Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol Cell Biol. 1996;16:859–67. doi: 10.1128/mcb.16.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]