Abstract
The routine biochemical parameters for hepatocellular carcinoma (HCC) diagnosis are all protein markers. Serum concentrations of these markers can be affected by some benign diseases. Most of the occurrence of HCC has a background of cirrhosis, posing a great challenge to differential diagnosis of HCC from cirrhosis using traditional biochemical parameters. Values of serum small molecular metabolites for HCC diagnosis are not fully evaluated. In this study, a traditionalmass spectrometry-based screening strategy was employed to profile amino acids and acylcarnitines in blood samples collected from HCC and cirrhosis patients. Each whole blood specimen was sampled on filter paper and dried at room temperature. Metabolites in the dried blood spots were extracted using organic solvent and then concentrated for mass spectrometry analysis. It was found that 11 parameters, including amino acids, acylcarnitines and some of their relevant ratios, could be used to construct a satisfied differential diagnosis model. In this model, most of the relevant amino acids were essential amino acids. It was noticed that short-chain acylcarnitines tended to be risk factors for HCC. Long-chain acylcarnitines seemed to be risk factors for cirrhosis. This study demonstrates the value of mass spectrometry-based analysis for differential diagnosis of HCC and cirrhosis. Improved differential diagnosis ability may be achieved by combined use of traditional protein markers along with metabolite markers.
Keywords: Hepatocellular carcinoma, mass spectrometry, amino acids, carnitine
Introduction
Hepatocellular carcinoma (HCC) is one of the most common primary liver malignancies with poor prognosis [1]. Among the risk factors, cirrhosis is ranked as the leading cause of HCC. Clinically, nearly all patients diagnosed with HCC have a background of cirrhosis [2]. Besides, hepatitis virus infection, alcohol liver diseases and fatty liver diseases contribute to HCC to different extents [3].
To date, advances in HCC diagnosis are encouraging. Percutaneous biopsy is thought to be one of the most reliable measures for HCC diagnosis. But, due to the various unexpected effects, this invasive operation is reluctantly accepted by both patients and clinicians [4]. Owning to the distinguished advantage of non-invasiveness features, imaging modalities such as ultrasound scan, computed tomography, and magnetic resonance imaging are prevailing in clinics and playing key roles in HCC screening and diagnosis [4]. However, the diagnosis accuracy is varied due to the expertise of the clinicians. Compared to imaging techniques, serum biomarkers analysis is thought to be cheaper, more objective, and sensitive [5]. The most widely used biomarkers include, but not limited to, alpha-fetoprotein (AFP), glypican-3, heat shock protein 70, glutamine synthetase, and des-γ-carboxyprothrombin (DCP) [6,7]. The fact that nearly all the clinically utilized biomarkers can be affected by benign diseases limits the diagnosis efficiency of serological analysis. For example, it has been estimated that only half of HCC tumors secrete AFP. Increased serum AFP concentrations can also be found in patients suffering from virus hepatitis, cirrhosis, and neurodegenerative diseases [8,9]. AFP’s diagnosis sensitivity is roughly less than 65% [10]. DCP is reported to be more specific for HCC [11], but its ability to differentiate HCC from cirrhosis is inferior to AFP sometimes [12]. A meta-analysis also indicated the drawback of using glypican-3 alone for differential diagnosis of HCC from cirrhosis [10]. Although combined using various biomarkers could improve the HCC diagnosis accuracy to some extent, the close relationship between HCC and cirrhosis still poses great challenge to accurate HCC diagnosis.
Unlike traditional immunological techniques aimed at large molecular protein detection, mass spectrometry (MS) analysis is a strategy that can precisely quantify small molecule metabolites. Limited by the propensity that one antibody exclusively recognizes one specific antigen, current serum HCC protein biomarkers analysis can only realize one-analysis-for-one-protein. However, MS analysis can simultaneously detect multiple metabolites in a single run. This feature can greatly improve the detection efficiency.
The first introduction of MS into the clinical laboratory was for new born screening (NBS) purpose [13]. The current MS-based NBS strategy is to find some inherited disorders by quantifying varied amino acids and acylcarnitines in the dried blood spots (DBSs) samples collected on filter paper. Studies have demonstrated that development and progression of HCC is accompanied by various amino acids and acylcarnitines fluctuating in the circulation [14,15]. Additionally, many inherited disorders are thought to be risk factors to oncogenesis [16]. This implies that metabolic disorders are closely related to malignancies. In this light, the current study tried to employ the MS strategy to profile blood amino acids and acylcarnitines of patients with HCC or cirrhosis. The aims of this study were to test if this MS analysis could be used for differential diagnosis of HCC and to find which metabolites play key roles in separating HCC from cirrhosis.
Material and methods
Samples
This study was approved by Ethnic Committee of The First Affiliated Hospital of Jinzhou Medical University. Informed consent was acquired from each patient. HCC and cirrhosis were diagnosed as described elsewhere [17]. For cirrhosis cases, there were 92 male (age 40-78 years, median 53) and 44 female (age 42-79 years, median 62.5) patients. For HCC patients, 41 males (age 44-78 years, median 61) and 9 females (age 49-72 years, median 63) were enrolled. All patients were randomly divided into a training group (~80% cases) and a prediction group (the left ~20% patients). The training group was used to construct a diagnosis model and the prediction group was utilized to test the applicability of the model. All the tested samples were collected in the form of DBSs as described elsewhere [18]. Briefly, fasting blood samples were collected onto aseptic filter paper by finger puncture. Each specimen paper was dried at room temperature and then stored at 4°C. For each analysis, a DBS disc of 3 mm diameter was punched out.
MS analysis
The DBS sample procession and MS analysis methods were identical to what had been described previously [17]. In brief, the DBS discs were placed into a 96-well plate containing metabolite extraction solution and isotope labeled quantitation standards individually. After the extract was dried by nitrogen flow, 1-butanol, and acetyl chloride were used for metabolite derivatization for each sample. Subsequently, every 100 μl of 80% acetonitrile was employed to redissolve the individually derivatized sample. For metabolite quantitation, tandem MS analysis (MS/MS) was conducted by using AB SCIEX 4000 QTrap system (Framingham, MA). The equipment settings were identical to the previous report [17]. The target metabolites included 23 amino acids, 26 acylcarnitines, and 44 ratios derived from them. The detailed metabolite information could be retrieved from our previous study [17].
Statistical analysis
For HCC and cirrhosis differentiation, a partial least squares-discriminant analysis (PLS-DA) was carried out by employing SIMCA-P v13.0 (Umeå, Sweden) using the MS data. Parameters playing significant roles in group separation were determined by their corresponding variable importance in projection (VIP) [19]. According to the software, parameters of VIP>1 were kept. Differences in metabolite concentrations were evaluated by t-test using MINITAB v17.0 (State College, PA) and P<0.05 was deemed as statistical significance. A binary logistic regression analysis was conducted to construct the differentiation model by using MINITAB v17.0. The utility of the model was evaluated by area under the receiver operating characteristic (ROC) curve.
Results
PLS-DA shows a clear separation trend between HCC and cirrhosis
For HCC and cirrhosis differentiation, a PLS-DA was carried out. The score plot showed that HCC and cirrhosis showed a clear separation trend (Figure 1A). A permutation test based on 100 iterations indicated that no over-fitting occurred in this PLS-DA model, implying the reliability of the model [17] (Figure 1B). This permutation test ensured that some metabolites were of different concentrations in samples of HCC and cirrhosis. Figure 1C showed the metabolites that could be used to differentiate HCC and cirrhosis and their individual VIP values after multivariate analysis.
Figure 1.
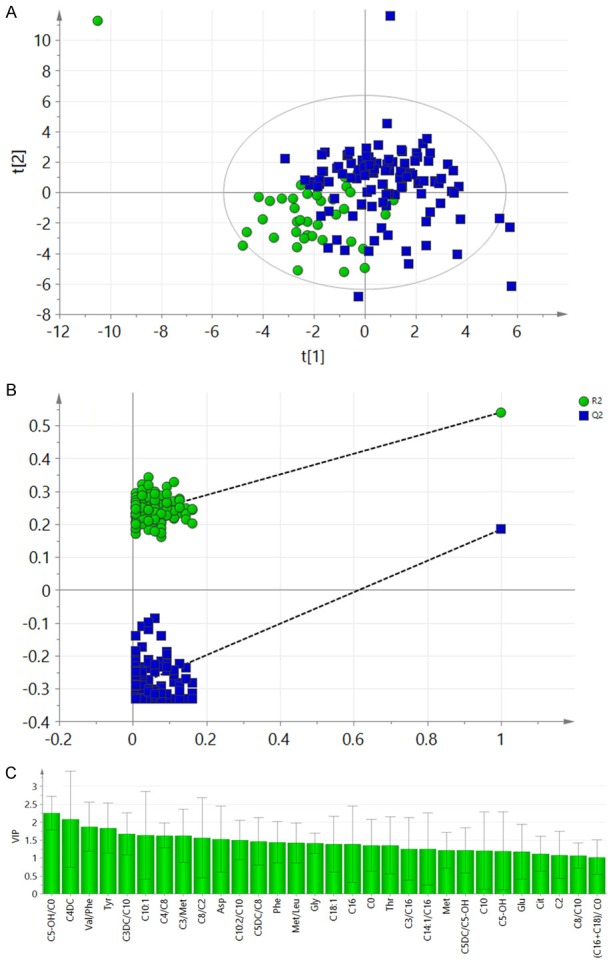
PLS-DA of the training sets data. A: Score plot of the PLS-DA. The circles represented the cirrhosis patients and the rectangles showed the HCC cases. B: The permutation test result of the PLS-DA model. R2 less than 0.4 and Q2 less than zero meant an acceptable model. C: Metabolite parameters with VIP>1.
Metabolite parameters can be used for a satisfied diagnosis model
For differential diagnosis purpose, significantly different metabolites between the HCC and cirrhosis groups (Figure 1C) were subjected to binary regression analysis to construct a diagnosis model. This yielded a regression equation of y = -0.94 - 7.97 C10 + 9.31 C10:1 - 2.71 C18:1 + 21.42 C3/Met - 0.841 C3DC/C10 - 1.048 C4/C8 + 2.53 C4DC + 188.6 C5-OH/C0 + 0.01624 Glu - 0.0944 Thr + 1.003 Val/Phe. When the cutoff value was set to -0.675, the regression model could realize the HCC diagnosis specificity of 88.1% and sensitivity of 87.5% (Figure 2). Figure 3 showed the content difference of the metabolites included in the equation. The individual odds ratios of the parameters were listed in Table 1. By using this model, 78% of the patients in the prediction group could be accurately diagnosed.
Figure 2.
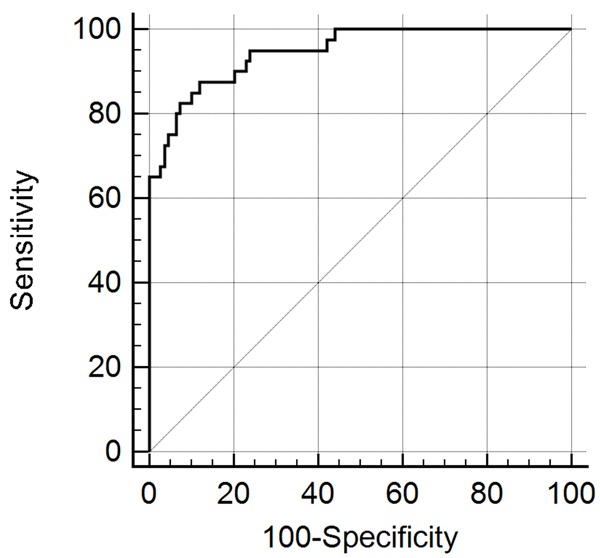
ROC analysis based on the regression model. Area under the curve (AUC) was 0.947 with 95% confidence interval of 0.898 to 0.977.
Figure 3.
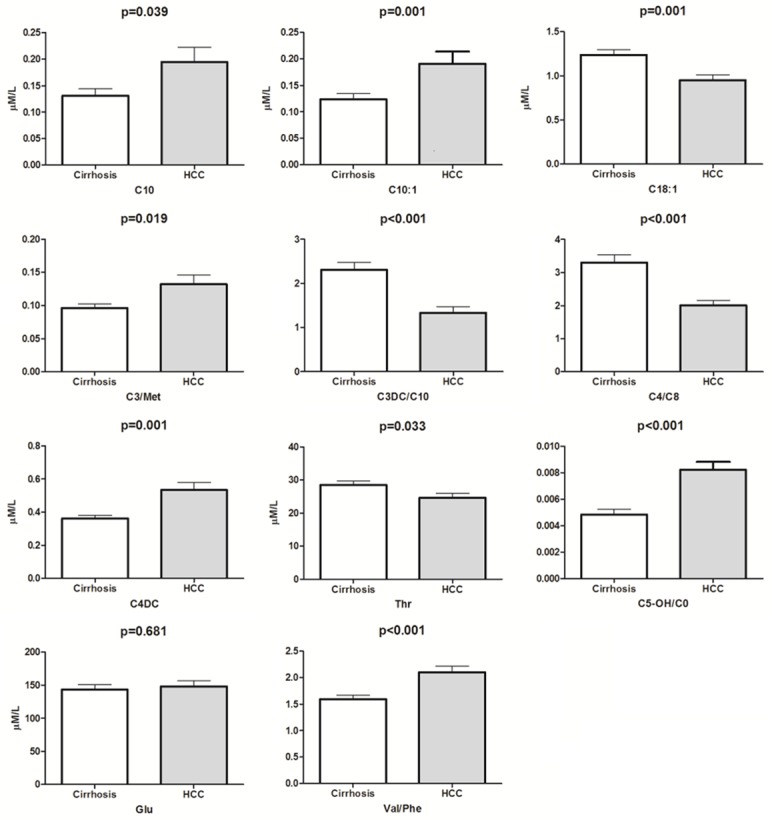
Concentration difference of the metabolite parameters in the regression model. C10: decanoylcarnitine; C3DC/C10: malonylcarnitine/decanoylcarnitine; C10:1: decenoylcarnitine; C18:1: octadecenoylcarnitine; Glu: glutamate; Thr: Threonine; Val/Phe: valine/phenylalanine; C5-OH/C0: 3-Hydroxy-isovalerylcarnitine/carnitine; C4DC: Methylmalonylcarnitine; C4/C8: butyrylcarnitine/octanoylcarnitine and C3/Met: propionylcarnitine/methionine.
Table 1.
The odds ratios of the parameters in the regression model
| Parameter | Odds ratio | 95% confidential interval |
|---|---|---|
| C5-OH/C0 | 7.34×1081 | 7.86×105-7.62×10157 |
| C3/Met | 2.01×109 | 6.55×104-6.16×1013 |
| Val/Phe | 2.73 | 1.02-7.27 |
| Glu | 1.02 | 1.00-1.03 |
| C4DC | 12.49 | 0.60-2.60×102 |
| C10:1 | 1.10×104 | 0.59-2.08×108 |
| C10 | 3.00×10-4 | 0-3.01 |
| Thr | 0.91 | 0.84-0.99 |
| C3DC/C10 | 0.43 | 0.19-0.97 |
| C18:1 | 6.67×10-2 | 8.80×10-3-0.51 |
Discussion
Tumor cells conduct metabolism in an abnormal way compared to their normal counterparts. Patients suffering from various malignancies showed distinct serum metabolites changes. NBS pays attention to the metabolites fluctuation of some amino acids and acylcarnitines. Notably, cancer patients sometimes exhibited serum metabolites change profiles which were comparable to the findings in inherited disorders [20]. Thus, the traditional MS-based NBS strategy was intended to employ in this study to seek potential differential metabolites of HCC and cirrhosis.
This study showed that PLS-DA only exhibited a partial separation of HCC from cirrhosis (Figure 1A). Distinct separation could not be acquired. This might be due to the fact that most of the HCC patients have a background of cirrhosis. Although the partial overlap of the two groups, the PLS-DA model was acceptable. As the permutation test result did not indicate evidence of over-fitting of this PLS-DA (Figure 1B), metabolites played key roles in separating the HCC and cirrhosis groups were defined (Figure 1C) and could be readily used for subsequent differential analysis.
After regression analysis, 6 metabolites including 2 amino acids and 5 ratios were kept in the binary model. Most of them were involved in acylcarnitines. Combined using these parameters really showed comparable diagnosis ability against traditional protein markers as showed in Figure 2. Additionally, except Glu, the others parameters exhibited a statistically significant difference between HCC and cirrhosis after univariate analysis (Figure 3).
In view of their individual odds ratios, C5-OH/C0, C3/Met, and Val/Phe seemed to be the most important risk factors for HCC (Table 1). Increased C5-OH was usually due to the deficiency or inactivity of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) lyase [21]. Currently, there are no reports on how this enzyme affects cancer development. Deficiency of HMG-CoA lyase really caused oxidative stress and contributed to liver damage [22]. Carnitine was reported to favor HCC therapy [23]. Apoptosis is thought to be a protective process against tumor development. In HepG2, one of the typical HCC cell lines, it was found that apoptotic cells showed decreased intercellular C3 [24]. As of Met, a recent report showed that patients suffering from hepatitis C virus infection showed higher serum Met concentrations compared to the HCC patients [25]. This evidence, combined with the findings in this study, coincide with the notion that deregulated lipid metabolism is related to HCC development.
Val and Phe are essential amino acids. The former, a branched-chain amino acid, is mainly metabolized in the muscle and the latter, an aromatic amino acid, is in the liver. Clinically, the ratio of branched-chain/aromatic amino acids is used to evaluate the liver functions state. The lower the level of the ratio, the worse the liver functions. A previous study demonstrated Val/Phe elevated in ischemic diseases [26]. Many solid tumors will suffer from ischemic state due to their rapid proliferation. This might help to explain the increased Val/Phe in malignant liver diseases.
Thr, C3DC/C10 and C18:1 seemed to be the risk factors for cirrhosis (Table 1). Thr is also one of the essential amino acids. A study carried out in Egyptian patients showed no serum Thr difference between cirrhosis and HCC patients [27]. This discrepancy might arise from different diet styles between Western and Eastern people. Additionally, the major reason for Chinese people’s cirrhosis is hepatitis virus infection, especially hepatitis B virus. Whereas, the Western people’s cirrhosis is mainly due to alcohol consumption. Whether the different reasons of cirrhosis might result in serum Thr difference should also be explored.
According to the results of this study, risk factors for HCC are most short- or medium-chain carnitines. Whereas the unfavorable factors for cirrhosis are largely long-chain carnitines (Table 1). C18:1 has been found to be closely linked to fibrosis in a cell model study [28]. A previous report demonstrated that long-chain fatty acid oxidation was impaired in hepatoma cells [29]. This might explain the findings of lower short-chain and long-chain carnitine ratio-C3DC/C10 in the HCC group.
In this study, a traditionally used DBS-based MS analysis was adopted to differentiate HCC and cirrhosis. By combined using 11 parameters, a feasible diagnosis model was formulated and could achieve satisfied HCC and cirrhosis differentiation. The diagnosis ability was comparable to that of the traditional protein biomarkers. If this tactic could be incorporated with traditional serum biomarker analysis, improved differentiation ability might be expected.
Acknowledgements
The study was sponsored by Natural Science Foundation of Liaoning Province (201602206).
Disclosure of conflict of interest
None.
References
- 1.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Sauzay C, Petit A, Bourgeois AM, Barbare JC, Chauffert B, Galmiche A, Houessinon A. Alpha-foetoprotein (AFP): a multi-purpose marker in hepatocellular carcinoma. Clin Chim Acta. 2016;463:39–44. doi: 10.1016/j.cca.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Ramalho M, Matos AP, AlObaidy M, Velloni F, Altun E, Semelka RC. Magnetic resonance imaging of the cirrhotic liver: diagnosis of hepatocellular carcinoma and evaluation of response to treatment-Part 1. Radiol Bras. 2017;50:38–47. doi: 10.1590/0100-3984.2015.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394–399. doi: 10.3322/caac.21161. [DOI] [PubMed] [Google Scholar]
- 5.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 6.Best J, Bilgi H, Heider D, Schotten C, Manka P, Bedreli S, Gorray M, Ertle J, van Grunsven LA, Dechene A. The GALAD scoring algorithm based on AFP, AFP-L3, and DCP significantly improves detection of BCLC early stage hepatocellular carcinoma. Z Gastroenterol. 2016;54:1296–1305. doi: 10.1055/s-0042-119529. [DOI] [PubMed] [Google Scholar]
- 7.Di Tommaso L, Roncalli M. Tissue biomarkers in hepatocellular tumors: which, when, and how. Front Med (Lausanne) 2017;4:10. doi: 10.3389/fmed.2017.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakao K, Ichikawa T. Recent topics on alpha-fetoprotein. Hepatol Res. 2013;43:820–825. doi: 10.1111/hepr.12052. [DOI] [PubMed] [Google Scholar]
- 9.Schieving JH, de Vries M, van Vugt JM, Weemaes C, van Deuren M, Nicolai J, Wevers RA, Willemsen MA. Alpha-fetoprotein, a fascinating protein and biomarker in neurology. Eur J Paediatr Neurol. 2014;18:243–248. doi: 10.1016/j.ejpn.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Liu JW, Zuo XL, Wang S. Diagnosis accuracy of serum Glypican-3 level in patients with hepatocellular carcinoma and liver cirrhosis: a meta-analysis. Eur Rev Med Pharmacol Sci. 2015;19:3655–3673. [PubMed] [Google Scholar]
- 11.Seo SI, Kim HS, Kim WJ, Shin WG, Kim DJ, Kim KH, Jang MK, Lee JH, Kim JS, Kim HY, Kim DJ, Lee MS, Park CK. Diagnostic value of PIVKA-II and alpha-fetoprotein in hepatitis B virusassociated hepatocellular carcinoma. World J Gastroenterol. 2015;21:3928–3935. doi: 10.3748/wjg.v21.i13.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park SJ, Jang JY, Jeong SW, Cho YK, Lee SH, Kim SG, Cha SW, Kim YS, Cho YD, Kim HS, Kim BS, Park S, Bang HI. Usefulness of AFP, AFP-L3, and PIVKA-II, and their combinations in diagnosing hepatocellular carcinoma. Medicine (Baltimore) 2017;96:e5811. doi: 10.1097/MD.0000000000005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jannetto PJ, Fitzgerald RL. Effective use of mass spectrometry in the clinical laboratory. Clin Chem. 2016;62:92–98. doi: 10.1373/clinchem.2015.248146. [DOI] [PubMed] [Google Scholar]
- 14.Shiozawa S, Usui T, Kuhara K, Tsuchiya A, Miyauchi T, Kono T, Asaka S, Yamaguchi K, Yokomizo H, Shimakawa T, Yoshimatsu K, Katsube T, Naritaka Y. Impact of branched-chain amino acid-enriched nutrient on liver cirrhosis with hepatocellular carcinoma undergoing transcatheter arterial chemoembolization in barcelona clinic liver cancer Stage B: a prospective study. J Nippon Med Sch. 2016;83:248–256. doi: 10.1272/jnms.83.248. [DOI] [PubMed] [Google Scholar]
- 15.Yaligar J, Teoh WW, Othman R, Verma SK, Phang BH, Lee SS, Wang WW, Toh HC, Gopalan V, Sabapathy K, Velan SS. Longitudinal metabolic imaging of hepatocellular carcinoma in transgenic mouse models identifies acylcarnitine as a potential biomarker for early detection. Sci Rep. 2016;6:20299. doi: 10.1038/srep20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ullrich NJ. Inherited disorders as a risk factor and predictor of neurodevelopmental outcome in pediatric cancer. Dev Disabil Res Rev. 2008;14:229–237. doi: 10.1002/ddrr.30. [DOI] [PubMed] [Google Scholar]
- 17.Feng X, Song P, Bie P, Jiang P, Ma K, Li X, Wang S, Wang Z, Tang W, Zheng S. Des-gamma-Carboxyprothrombin plasma level in diagnosis of hepatocellular carcinoma in a Chinese population undergoing surgery. Med Sci Monit. 2016;22:1663–1672. doi: 10.12659/MSM.895483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q, Sun T, Cao Y, Gao P, Dong J, Fang Y, Fang Z, Sun X, Zhu Z. A dried blood spot mass spectrometry metabolomic approach for rapid breast cancer detection. Onco Targets Ther. 2016;9:1389–1398. doi: 10.2147/OTT.S95862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rombouts C, Hemeryck LY, Van Hecke T, De Smet S, De Vos WH, Vanhaecke L. Untargeted metabolomics of colonic digests reveals kynurenine pathway metabolites, dityrosine and 3-dehydroxycarnitine as red versus white meat discriminating metabolites. Sci Rep. 2017;7:42514. doi: 10.1038/srep42514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qu Q, Zeng F, Liu X, Wang QJ, Deng F. Fatty acid oxidation and carnitine palmitoyltransferase I: emerging therapeutic targets in cancer. Cell Death Dis. 2016;7:e2226. doi: 10.1038/cddis.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santarelli F, Cassanello M, Enea A, Poma F, D’Onofrio V, Guala G, Garrone G, Puccinelli P, Caruso U, Porta F, Spada M. A neonatal case of 3-hydroxy-3-methylglutaric-coenzyme A lyase deficiency. Ital J Pediatr. 2013;39:33. doi: 10.1186/1824-7288-39-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leipnitz G, Vargas CR, Wajner M. Disturbance of redox homeostasis as a contributing underlying pathomechanism of brain and liver alterations in 3-hydroxy-3-methylglutaryl-CoA lyase deficiency. J Inherit Metab Dis. 2015;38:1021–1028. doi: 10.1007/s10545-015-9863-3. [DOI] [PubMed] [Google Scholar]
- 23.Iwasa M, Sugimoto R, Ishihara T, Sekoguchi-Fujikawa N, Yoshikawa K, Mifuji-Moroka R, Tanaka H, Kobayashi Y, Hasegawa H, Takei Y. Usefulness of levocarnitine and/or branched-chain amino acids during invasive treatment for hepatocellular carcinoma. J Nutr Sci Vitaminol (Tokyo) 2015;61:433–440. doi: 10.3177/jnsv.61.433. [DOI] [PubMed] [Google Scholar]
- 24.Halama A, Riesen N, Moller G, Hrabe de Angelis M, Adamski J. Identification of biomarkers for apoptosis in cancer cell lines using metabolomics: tools for individualized medicine. J Intern Med. 2013;274:425–439. doi: 10.1111/joim.12117. [DOI] [PubMed] [Google Scholar]
- 25.Baniasadi H, Gowda GA, Gu H, Zeng A, Zhuang S, Skill N, Maluccio M, Raftery D. Targeted metabolic profiling of hepatocellular carcinoma and hepatitis C using LC-MS/MS. Electrophoresis. 2013;34:2910–2917. doi: 10.1002/elps.201300029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Z, Zhu Z, Cao Y, Wang L, Sun X, Dong J, Fang Z, Fang Y, Xu X, Gao P, Hongzhi S. Rapid and sensitive differentiating ischemic and hemorrhagic strokes by dried blood spot based direct injection mass spectrometry metabolomics analysis. J Clin Lab Anal. 2016;30:823–830. doi: 10.1002/jcla.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osman D, Ali O, Obada M, El-Mezayen H, El-Said H. Chromatographic determination of some biomarkers of liver cirrhosis and hepatocellular carcinoma in Egyptian patients. Biomed Chromatogr. 2017:31. doi: 10.1002/bmc.3893. [DOI] [PubMed] [Google Scholar]
- 28.McCoin CS, Knotts TA, Ono-Moore KD, Oort PJ, Adams SH. Long-chain acylcarnitines activate cell stress and myokine release in C2C12 myotubes: calcium-dependent and -independent effects. Am J Physiol Endocrinol Metab. 2015;308:E990–E1000. doi: 10.1152/ajpendo.00602.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prip-Buus C, Bouthillier-Voisin AC, Kohl C, Demaugre F, Girard J, Pegorier JP. Evidence for an impaired long-chain fatty acid oxidation and ketogenesis in Fao hepatoma cells. Eur J Biochem. 1992;209:291–298. doi: 10.1111/j.1432-1033.1992.tb17288.x. [DOI] [PubMed] [Google Scholar]


