Abstract
Osteoblast-related bone formation is an indispensable part of bone remodeling. Osteoblasts can regulate osteoclast activity through the OPG/RANK/RANKL system. Therefore, studies focus on osteoblast proliferation, differentiation, mineralization, and regulation of osteoclasto genes are important. Leptin is a polypeptide encoded by the obesity gene. In the past, regulatory roles of leptin on sugar and lipid metabolism have been extensively studied. In recent years, leptin has been found to have multiple effects on bone metabolism. However, its role in osteoblasts has not been clarified. In order to investigate the effects of leptin on osteoblasts of adult female SD rats, primary cultured osteoblasts were passaged and divided into control and leptin groups. The effects of leptin on osteoblast proliferation, differentiation, mineralization, and OPG and RANKL mRNA expression were observed. Leptin significantly promoted mineralization of osteoblasts in adult female SD rats, and the mineralized area gradually increased with the increase of leptin concentration. When the concentration of leptin was 100 ng/ml, the mineralized area was the highest (P = 0.001). The expression of RANKL mRNA was also elevated by 10 ng/ml leptin. However, leptin had no significant effect on osteoblast proliferation, secretion of ALP, and expression of OPG mRNA in adult female SD rat osteoblast.
Keywords: Adult female SD rat, osteoblast, leptin, cell proliferation, cell differentiation, cell calcification, osteoprotegerin (OPG), receptor activator of NF-κB Ligand (RANKL)
Introduction
Osteoporosis is caused by an imbalance of bone metabolism. When bone absorption is greater than bone formation, bone loss will occur. The loss of bone mass makes bone fragile and more prone to fracture. The fracture of bone can cause pain, restricted ambulation, bone deformation, disability, and even death. With the aging population, osteoporosis becomes a serious problem to human health, especially post-menopausal women. Bone metabolism mainly depends on the bone formation of osteoblasts and bone absorption of osteoclasts to maintain balance. Post-menopausal osteoporosis occurs as a result of ovarian failure and decreased estrogen levels, leading to reduced formation and increased absorption of bone. The way to maintain bone mass is focused treatment and research in post-menopausal osteoporosis. Because postmenopausal osteoporosis is due to decreased estrogen levels, the initial treatment for this disease has been estrogen replacement therapy. Although hormone replacement therapy can partially alleviate the bone loss, the increased risk of hormone-related cancer and cardiovascular disease restricts the use of the hormone [1]. One of the goals of current research is to find a safe and effective anti-osteoporosis medication.
Leptin is a polypeptide encoded by the OB gene and mainly secreted by the white adipose tissue [2]. Leptin exerts its biological effects through the leptin receptor (ObR). Leptin receptors are widely distributed in the central and peripheral tissues, such as hypothalamus, cerebral cortex, pituitary, uterus, ovary, placenta, liver, kidney, bone, and other organs [3-9]. In the past, the regulatory role of leptin on sugar and lipid metabolism has been extensively studied. Recent studies indicate that leptin has multiple regulatory effects on bone metabolism. Leptin can regulate bone metabolism by the central and peripheral pathway [10]. In vivo studies indicate that leptin exerts a positive effect on bone in obese female leptin receptor deficient Zucker (fa/fa) rats [11]. But leptin inhibits bone formation through a hypothalamic relay in leptin-deficient mice (ob/ob) and leptin receptor-deficient mice (db/db) [8]. Peripheral administration of leptin can prompt bone turnover in male ob/ob mice [10] and reduce ovariectomy-induced bone loss in rats [12]. In vitro, the effect of leptin remains controversial. Some studies have shown that leptin can promote human osteoblast proliferation, mineralization, and reduce osteoblast apoptosis [13-15]. However, some studies have shown that leptin has no effect on osteoblast proliferation and differentiation [16]. Even studies have suggested that leptin may reduce the expression of osteoprotegerin (OPG) and promote bone absorption [17]. The current relationship between leptin and osteoblasts is not clear, therefore this study sets out to observe the effect of leptin on osteoblast proliferation, differentiation, mineralization, and regulation of the bone metabolism related genes OPG/RANKL. This study clarifies the relationship between leptin and osteoblasts in vivo and provides a candidate medication for the treatment of post-menopausal osteoporosis.
Materials and methods
Materials
Adult female SD rats were 3-4 months old and weighed between 200-250 g. The rats were provided by the Chengdu Dossy Biological Technology Co. Ltd. The main reagents were as follows: Ham’ F12 medium (Hyclone, Thermo Fisher Scientific), leptin (R&D Systems, Inc.), collagenase I (Invitrogen), trypsin (Amresco), fetal bovine serum (Hyclone, Thermo Fisher Scientific), bromodeoxyuridine (BrdU, BD Pharmingen™), purified mouse anti-BrdU (BD Pharmingen™), Cy2-AffiniPure goat anti-mouse IgG (H+L) (Jackson Immuno Research), Dapi (Beyotime Institute of Biotechnology), rat alkaline phosphatase (ALP) kit (R&D Systems, Inc.), Trizol reagent (Invitrogen Corporation), DEPC (Invitrogen Corporation), Prime Script RT reagent (TaKaRa), SsoFastEvaGreenSupermixz (BIO-RAD), and Taq DNA Polymerase (Fermentas, Waltham, MA, USA).
Primary culture of osteoblasts
Before inclusion in the study, vaginal exfoliated cells of all the female adult SD rats were taken for 5 consecutive days. Only the rats with a normal estrous cycle were included. The rats were euthanized with an intraperitoneal injection of pentobarbital sodium (150 mg/kg). Death was confirmed by the absence of the heartbeat. The calvarium was excised and the periosteum was removed. The remaining parietal bone was minced and extensively washed with PBS. Primary osteoblasts were isolated from the calvarium by digestion with collagenase and trypsin. The cells were collected and cultured in F12 culture medium, containing 10% fetal bovine serum, and 100 U/mL penicillin/streptomycin. The culture medium was changed every 3 days. At confluence, the cells were trypsinized, resuspended, and seeded into new flasks. Osteoblasts were identified by alkaline phosphatase expression and the presence of mineral deposition. The third generation osteoblasts were used in the study. The Ethics Committee of the West China Second University Hospital of Sichuan University approved the study.
Group setting
The osteoblasts from adult female SD rats were distributed into 6 groups. Each group was treated with a different concentration of leptin (① control group, ② 0.01 ng/ml leptin group, ③ 0.1 ng/ml leptin group, ④ 1 ng/ml leptin group, ⑤ 10 ng/ml leptin group, ⑥ 100 ng/ml leptin group). According to the group setting, the influence of leptin on the proliferation, differentiation, calcification, and expression of OPG and RANKL mRNA of osteoblast was investigated.
Detection of osteoblast proliferation rate
Osteoblasts from adult female SD rats were seeded into 24-well cell culture plates at 6,000 cells/well and divided into 6 groups. All the cells were cultured with different concentration of leptin according to the group setting. On the 3rd day after cell seeding, BrdU was added and incorporated into the DNA during DNA synthesis. 24 hours later, the cells were fixed. Mouse Anti-BrdU and Cy2-AffiniPure Goat Anti-Mouse IgG were used to label the BrdU. Dapi stained the nucleus. The numbers of total osteoblasts and proliferating osteoblasts were counted by image analysis software (IPP 6.0), and the ratios of proliferating cells in every group were calculated respectively.
ALP detection
The cells were trypsinized and seeded into 6-well cell culture plates at 4*104 cells/well. All the cells were cultured with different concentration of leptin according to the group setting. On the 5th day, the culture medium was collected. The determination was done according to the instruction of the Rat Alkaline Phosphatase (ALP) ELISA Kit.
Osteoblast mineralization
The culture medium was made by α-MEM fortified with 10% fetal calf serum, 8 mM β-glycerolphosphate, and 100 µg/mL ascorbic acid. All the cells were cultured with different concentration of leptin according to the group setting. The cell culture medium was changed every 3 days. Osteoblasts were cultured continuously for 28 days and fixed with 95% ethanol for 30 minutes on the 28th day. Subsequently, the cells were stained with 0.1% alizarin red-Tris-Hcl at pH 4.3 for 30 minutes and washed with distilled water. Images of the mineralized nodules were obtained and the percent surface covered by mineral was estimated by image analysis software (IPP 6.0).
Quantitative real-time PCR experiments (qPCR)
According to the group setting, collection of the osteoblast RNAs in each group was done on the 4th day of culture. Total RNA was extracted using the Trizol reagent according to the manufacturer’s protocol. Isolated RNA was dissolved in RNAase Free dH2O. Concentration and purity of the RNA were determined by spectrophotometer (NonoVue Plus, GE). RNA was reverse transcribed into cDNA according to the Prime Script RT reagent’s instruction (TaKaRa). RT-PCR was performed by C1000™ Thermal Cycler PCR (BIO-RAD). The RT reaction system was incubated at 37°C for 15 min and the template was denatured at 85°C for 5 seconds.
The gene sequence of OPG, RANKL, and GAPDH were obtained from the NCBI gene database of the National Biotechnology Information Center (http://www.ncbi.nlm.nih.gov/). The primers were designed by primer premier 5.0 (PREMIER Biosoft International, Palo Alto, CA). The following PCR primers were used: OPG forward: 5’-CAAAGGCAGGGCATACTTC-3’; reverse: 5’-TTCAATGATGTCCAAGAACACC-3’; RANKL forward: 5’-CATCGGGTTCCCATAAAGTC-3’; reverse: 5’-CTGAAGCAAATGTTGGCGTA-3’; GAPDH forward: 5’-TATCGGACGCCTGGTTAC-3’; reverse: 5’-CTGTGCCGTTGAACTTGC-3’. The PCR was done by Bio-Rad CFX96™ Real-Time PCR System. All experiments were performed in triplicate. The PCR conditions were 50°C for 2 min and 95°C for 2 min followed by 45 cycles with 95°C for 15 s and the optimal annealing temperature for 30 s. After the last cycle a melting curve was scanned for 5 min from 65°C to 95°C.
Statistics
In vitro data were obtained from three separate cultures. All data are expressed as mean ± standard deviation. The differences between groups were analyzed by one-way ANOVA. The software SPSS 21.0 was used and P < 0.05 was considered significant.
Result
Osteoblast proliferation rate
The cells were double fluorescence stained by Dapi and BrdU. The results are shown in Figure 1, in which the green fluorescent labeled B group showed BrdU staining of proliferating cells, blue fluorescent labeled D group showed DAPI staining of survival cells, M group shows is the group B and D combined effect of the picture. Only when the B group and D group staining overlapped, was the cell considered alive and proliferating. The number of total cells, the number of proliferating cells and the proliferating rate of the osteoblasts in different concentrations of leptin group were higher than those in blank group (Table 1), but the statistical analysis showed that there was no statistical significance between the groups, P values were 0.731, 0.939 and 0.784 respectively.
Figure 1.
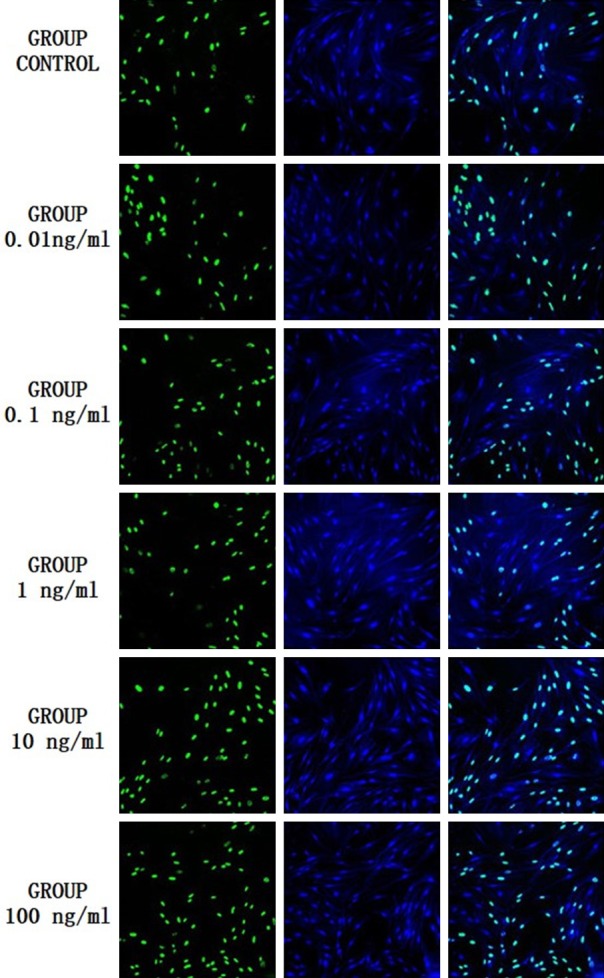
Fluorescent double stain of osteoblast in experimental group and control group. (B group: the green fluorescent showed BrdU staining of proliferating cells; D group: the blue fluorescent showed DAPI staining of survival cells, M group: the group B and D combined effect of the picture).
Table 1.
The value of proliferating rate, ALP and mineralized area in experimental groups and control group
| Proliferating rate (100%) | ALP (U/L) | Mineralized area (µm2) | P value of mineralize area compared with the control group | |
|---|---|---|---|---|
| Control group | 48.19±12.74 | 57.94±0.57 | 104.82±39.02 | / |
| Leptin 0.01 ng/ml | 61.79±15.24 | 54.89±4.25 | 164.47±34.16 | 0.046 |
| Leptin 0.1 ng/ml | 52.79±13.56 | 55.13±3.35 | 167.55±29.40 | 0.038 |
| Leptin 1 ng/ml | 58.47±15.57 | 56.95±0.13 | 169.21±32.67 | 0.034 |
| Leptin 10 ng/ml | 59.12±16.58 | 59.70±1.58 | 179.22±34.36 | 0.017 |
| Leptin 100 ng/ml | 64.32±15.21 | 58.19±4.13 | 218.24±26.25 | 0.001 |
Secretion of alkaline phosphatase (ALP) in osteoblasts
According to the experimental setting, the culture medium was collected on the 5th day, and the concentration of ALP was determined by the Rat Alkaline Phosphatase (ALP) ELISA Kit. The results areshown in Table 1. Statistical analysis show that there is no statistically significant difference in the ability of osteoblasts to secrete ALP in different leptin concentrations and control groups (P = 0.408).
Osteoblast mineralization
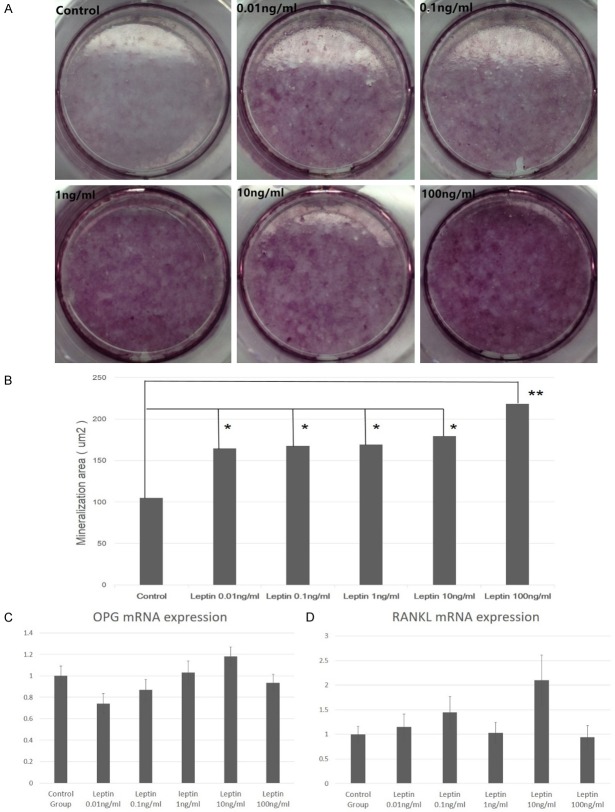
According to the experimental setting, osteoblasts were cultured continuously for 28 days. The mineralization nodules were stained by alizarin red and observed by microscopy. Images of the mineralized nodules were obtained and the percent of the surface covered by mineral was estimated by image analysis software (IPP 6.0). Alizarin red staining results were shown in Figure 2A. Image analysis software (IPP 6.0) on the mineralization area analysis results are shown in Table 1. The results show that the mineralized area of the different concentrations of leptin is significantly higher than that of the control group (P<0.05), and the mineralized area increased gradually with the increase of leptin concentration (Figure 2B and Table 1). When the leptin concentration was 100 ng/ml, the cell mineralization area was the highest (P = 0.001).
Figure 2.
The effect of leptin on mineralization and OPG/RANKL mRNA expression. A. Alizarin red stain of mineralization nodules in experimental group and control group; B. Mineralization area in experimental group and control group (*: P<0.05; **: P<0.005); C. OPG mRNA expression fold change in experimental group and control group; D. RANKL mRNA expression fold change in experimental group and control group.
OPG, RANKL mRNA expression
According to the group setting, acollection of the osteoblast RNAs in each group was done on the 4th day of culture. The expression of OPG gene was increased with the increase of leptin concentration in the low concentration leptin group. When the leptin concentration was 10 ng/ml, OPG expression was the highest (Figure 2C). However, there was no significant difference in OPG gene expression between different leptin concentration groups and control group (P = 0.560).
Expression of RANKL mRNA in adult rat osteoblasts was higher than that in the control group (Figure 2D). When the leptin concentration was 10 ng/ml, the expression of RANKL was significantly higher than that of the other leptin concentration group and the control group (P<0.05). There was no statistically significant difference between the other groups.
Discussion
Leptin concentration setting
In this study, the leptin concentration range was from 0.01 ng/ml, 0.1 ng/ml, 1 ng/ml, 10 ng/ml to 100 ng/ml. Compared with the currently published articles on leptin and osteoblast, the leptin concentration range was consistent with the leptin concentration in the current study [13,18,19]. Further reference was made to the experimental results of leptin on the effective half (ED50) dose of 0.05 to 0.3 ng/mL for leptin receptor transgenic mice pre-B cell proliferation in the leptin product specification. This result means that the leptin concentration setting was reasonable.
Cell proliferation
The effect of leptin on the proliferation of osteoblasts is not consistent with the current data. Some studies have suggested that leptin has an effect on the proliferation of osteoblasts. J Cornish et al. [19] used neonatal osteoblasts to observe the effect of leptin for 24 hours and found that leptin could promote osteoblast proliferation. Burguera [20] observed that leptin used for 4 hours can promote the proliferation of human osteosarcoma cell lines. Both studies observed only an effect of leptin on osteoblast proliferation for a short period of time (within 24-48 hours), but the long-term effects of leptin were not known. Further studies have focused on the long-term effects of leptin, and some studies have shown that leptin may prompt the osteoblast proliferation at the early stage, but long-term use of leptin has no effect on osteoblast proliferation. In the Gordeladze’s [13] study, leptin 100 ng/ml was used in osteoblasts of male osteoarthritis in vitro. When leptin was used for 48 hours, cell proliferation was promoted significantly. But when the effect of leptin was last for 14 days and 35 days, the promotion effect on proliferation was not statistically significant. In Iwamoto’s study [21], it was found that 100 ng/ml leptin could significantly promote human osteoblasts for 24 hours, but this proliferative effect did not have statistical significance after 72 hours. In our study, it was found that leptin had no effect on osteoblast proliferation on the 4th day. This result was consistent with the above findings. However, this study did not observe the short-term effects of leptin, so the short-term effects of leptin on osteoblasts remain to be confirmed.
ALP secretion
In vitro, osteoblast growth can be divided into three stages, from proliferation (relative undifferentiation), extracellular matrix maturation (cell differentiation), to matrix mineralization. In the early stage of osteoblast differentiation, alkaline phosphatase is expressed the most. In the later stage of differentiation, the osteoblasts gradually gointo the mineralization period, and ALP activity is decreased. It is believed that the peak of ALP secretion is between 4th to the 7th day. In this study, the 5th day, which was the early stage of osteoblast differentiation, was chosen for the alkaline phosphatase (ALP) measurement. Yang et al. [22] observed that the ALP levels reached the peak on the 5th day and gradually decreased in newborn SD rat osteoblasts. Declercq Ha’s study [23] found that 3-month-old adult Wistar rats on the 4th day of ALP levels were higher than the 14 days and 21 days. Han P [24] also observed that the secretion of ALP began to rise from the first day, reach the peak on the 7th day and then gradually decreased. However, this study showed that leptin had no effect on the ability of adult female SD rat osteoblasts to secrete ALP. In the study of Gordeladze et al. [13], it was found that the use of 100 ng/mL leptin in primary osteoblasts from osteoarthritis for 14 days resulted in a significant increase in ALP mRNA expression. In view of the current literature showing thatleptin’s effect on the osteoblast secretion of ALP was less, more research is need to give insight into this issue.
Mineralization
In this study, we found that the mineralized area of osteoblasts in different concentrations of leptin was significantly higher than that in the control group (P<0.05), and the mineralized area increased gradually with the increase of leptin concentration. When the leptin concentration was 100 ng/ml, the mineralization area was the highest (P = 0.001). Therefore, we speculate that leptin can promote osteoblast mineralization. This conclusion is consistent with the published in vitro study. Gordeladze et al. [13] used leptin (100 ng/ml) to treat the osteoblasts from osteoarthritis and found that leptin group mineral nodules were significantly more than the control group (P<0.05) after continuous intervention for 28 and 35 days. Reseland et al. [15] found that leptin 100 ng/ml intervention for continuous 35-44 days can improve human osteoblast mineralization capacity about 6-fold (8%-42%), osteogenic sarcoma cell lines 788T 2.5-fold, KPDXM 4-fold and OHS 2-fold respectively. In the study of Handschin et al. [25], it was demonstrated that osteoblasts treated with leptin 100 ng/ml, 500 ng/ml and 1000 ng/ml for 14 consecutive days could significantly promote mineralized nodulesand this was positively correlated with the dose of leptin. Quantitative studies have shown that the content of Ca45 in the cell matrix of the leptin group is also significantly higher than that of the control group and is positively correlated with the dose of leptin. Although all of these results are from human osteoblast studies, all of them demonstrate that leptin can prompt mineralization of osteoblast in vitro.
In vivo studies demonstrate that the decrease of bone formation in ob/ob mice can be rectified by subcutaneous administration of leptin [25]. Steppan, et al. [26] foundthat 50 µg leptin administration couldlead to a significant increase in femoral length, total body bone area, bone mineral content, and bone density in ob/ob mice. Hamrick, et al. [27] use leptin 5 µg/day and 10 µg/day for consecutive 14 days and also found that leptin 10 µg/day increased whole body BMC by >30% in the ob/ob mice. However, this phenomenon was not observed in therat. In Stunes’ study [28], leptin 100 µg/d or 200 µg/d or saline was given to female Fischer rats by continuous infusion for 9 weeks. It is found that body weight was significantly lower in rats receiving leptin compared with controls. But no significant differences in femoral BMD were observed.
In vivo and in vitro studies onthe effect of leptin on bone were not completely consistent. It is speculated that interactions between osteoblasts and osteoclasts must be taken into account in in vivo studies. Since the activity of osteoblasts and osteoclasts are coupled to each other, it is not known whether leptin acts directly on osteoclasts or indirectly via osteoblasts. Therefore, we also need to further explore the role of leptin on osteoblast, especially the osteoblast-mediated factors such as OPG/RANKL.
OPG/RANKL mRNA expression
Bone metabolism is balanced by the effect of osteoblast and osteoclast and there are many interactions between osteoblast and osteoclast. OPG/RANKL/RANK (Osteoprotegerin/Nuclear factor-kappa B ligand/Nuclear factor-kappa B) system is believed to participate in this course. Osteoblast can produce both RANKL and OPG which compete with each other to bind with RANK on the osteoclast to regulate the function of osteoclasts. RANKL can prompt differentiation of osteoclasts and their precursor cells and inhibit apoptosis of osteoclasts. While OPG has opposite functions such as inhibition of osteoclast differentiation, maturation, and induces apoptosis [29,30].
A study about Chinese post-menopausal women shows that the genetic polymorphisms of OPG and RANK were associated with bone mineral density [31]. The level of OPG in serum may be an independent risk factor in predicting hip fracture in postmenopausal women [32]. The elevated RANKL/OPG ratio may be related to the etiology of postmenopausal osteoporosis [33].
In in vivo studies, Stunes et al. [28] find that leptin has no impact on the serum OPG, RANKL, and the OPG/RANKL ratio in female Fischer rats. In Martin’s study [34], it has been found that leptin can prevent disuse-induced bone loss by elevating the expression of OPG and OPG/RANKL ratio. In vitro study by Lamghari et al. [35] demonstrated that low dose of leptin (12 ng/ml) can increase the expression of RANKL and the high dose of leptin (24 ng/ml) can inhibit the expression of RANKL in MC3T3-E1 cell line. Meanwhile leptin had no effect on OPG expression. The finding in OPG expression is consistent with our findings. Liu et al. [36] also studied the effect of leptin on MC3T3-E1 cell line. It has been found that leptin treatment at 10, 20, 40, 80 and 160 ng/mL can prompt the expression of OPG mRNA and reduce RANKL mRNA expression in a concentration-dependent manner. In Li’s study [17], the same concentrations of leptin were used as Liu’s study [36]. But this study didnot mention what kind of cells were used and got an opposite result compared with the Liu’s study [36]. In this study, it is found that with the increase of leptin concentration, osteoblast OPG mRNA expression decreased. Although in our study, it showed the downward trend of OPG expression after the treatment with leptin, there was no significance.
According to the current literature, the effects of leptin on the expression of OPG and RANKL mRNA in osteoblasts remain a controversy. However, most studies still indicate that leptin can regulate bone metabolism by affecting osteoblast OPG and RANKL mRNA expression.
Conclusion
In this study, we took a systematic approachtoward the effect of leptin on osteoblasts, from cell proliferation, differentiation, and mineralization to mRNA expression. It was confirmed that leptin can promote osteoblast mineralization and down-regulate the RANKL mRNA expression. However, leptin did not affect proliferation and differentiation of osteoblasts in adult female SD rats. The modulation function of leptin in bone metabolism thus need further study, especially focused on the osteoclast.
Acknowledgements
The research was supported by the National Natural Science Foundation of China (NO. 30772324, NO. 81070464, NO. 81671421 http://www.nsfc.gov.cn/), Specialized Research Fund for Doctoral Subject of College and University (NO. 20100181110007 http://www.cutech.edu.cn/cn/index.htm), Health and Family Planning commission of Sichuan Province Research Project (NO. 16PJ234, http://www.scwst.gov.cn/). The SCU-CUHK Joint Laboratory for Reproductive Medicine, West China Second Hospital gave the technological support.
Disclosure of conflict of interest
None.
References
- 1.Alexander IM. Pharmacotherapeutic management of osteoporosis and osteopenia. Nurse Pract. 2009;34:30–40. doi: 10.1097/01.NPR.0000352286.81981.0e. quiz 41. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 3.Kaminski T, Smolinska N, Gajewska A, Siawrys G, Okrasa S, Kochman K, Przala J. Leptin and long form of leptin receptor genes expression in the hypothalamus and pituitary during the luteal phase and early pregnancy in pigs. J Physiol Pharmacol. 2006;57:95–108. [PubMed] [Google Scholar]
- 4.Lin J, Barb CR, Matteri RL, Kraeling RR, Chen X, Meinersmann RJ, Rampacek GB. Long form leptin receptor mRNA expression in the brain, pituitary, and other tissues in the pig. Domest Anim Endocrinol. 2000;19:53–61. doi: 10.1016/s0739-7240(00)00064-3. [DOI] [PubMed] [Google Scholar]
- 5.Morioka T, Asilmaz E, Hu J, Dishinger JF, Kurpad AJ, Elias CF, Li H, Elmquist JK, Kennedy RT, Kulkarni RN. Disruption of leptin receptor expression in the pancreas directly affects beta cell growth and function in mice. J Clin Invest. 2007;117:2860–2868. doi: 10.1172/JCI30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang W, Dedousis N, Bandi A, Lopaschuk GD, O’Doherty RM. Liver triglyceride secretion and lipid oxidative metabolism are rapidly altered by leptin in vivo. Endocrinology. 2006;147:1480–1487. doi: 10.1210/en.2005-0731. [DOI] [PubMed] [Google Scholar]
- 7.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 8.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 9.Cioffi JA, Shafer AW, Zupancic TJ, Smith-Gbur J, Mikhail A, Platika D, Snodgrass HR. Novel B219/OB receptor isoforms: possible role of leptin in hematopoiesis and reproduction. Nat Med. 1996;2:585–589. doi: 10.1038/nm0596-585. [DOI] [PubMed] [Google Scholar]
- 10.Goldstone AP, Howard JK, Lord GM, Ghatei MA, Gardiner JV, Wang ZL, Wang RM, Girgis SI, Bailey CJ, Bloom SR. Leptin prevents the fall in plasma osteocalcin during starvation in male mice. Biochem Biophys Res Commun. 2002;295:475–481. doi: 10.1016/s0006-291x(02)00697-6. [DOI] [PubMed] [Google Scholar]
- 11.Tamasi JA, Arey BJ, Bertolini DR, Feyen JH. Characterization of bone structure in leptin receptor-deficient Zucker (fa/fa) rats. J Bone Miner Res. 2003;18:1605–1611. doi: 10.1359/jbmr.2003.18.9.1605. [DOI] [PubMed] [Google Scholar]
- 12.Burguera B, Hofbauer LC, Thomas T, Gori F, Evans GL, Khosla S, Riggs BL, Turner RT. Leptin reduces ovariectomy-induced bone loss in rats. Endocrinology. 2001;142:3546–3553. doi: 10.1210/endo.142.8.8346. [DOI] [PubMed] [Google Scholar]
- 13.Gordeladze JO, Drevon CA, Syversen U, Reseland JE. Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: impact on differentiation markers, apoptosis, and osteoclastic signaling. J Cell Biochem. 2002;85:825–836. doi: 10.1002/jcb.10156. [DOI] [PubMed] [Google Scholar]
- 14.Mutabaruka MS, AouladAissa M, Delalandre A, Lavigne M, Lajeunesse D. Local leptin production in osteoarthritis subchondral osteoblasts may be responsible for their abnormal phenotypic expression. Arthritis Res Ther. 2010;12:R20. doi: 10.1186/ar2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reseland JE, Syversen U, Bakke I, Qvigstad G, Eide LG, Hjertner O, Gordeladze JO, Drevon CA. Leptin is expressed in and secreted from primary cultures of human osteoblasts and promotes bone mineralization. J Bone Miner Res. 2001;16:1426–1433. doi: 10.1359/jbmr.2001.16.8.1426. [DOI] [PubMed] [Google Scholar]
- 16.Li SQ, Zhao HL, Wei SQ, et al. Effect of Leptin on proliferation and function of human osteoblast. Journal of West China University of Medical Science. 2001;02:240–2. [PubMed] [Google Scholar]
- 17.Li XJ, Wei SQ, Li SQ, et al. Effects of leptin on osteoblast osteoprotegerin mRNA expression. Chinese Journal of Osteoporosis. 2004;04 47-9+37. [Google Scholar]
- 18.Li SQ, Zhao HL, Wei SQ, et al. Effect of Leptin on proliferation and function of human osteoblast. Journal of West China University of Medical Science. 2001;32:240–2. [PubMed] [Google Scholar]
- 19.Cornish J, Callon KE, Bava U, Lin C, Naot D, Hill BL, Grey AB, Broom N, Myers DE, Nicholson GC, Reid IR. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002;175:405–415. doi: 10.1677/joe.0.1750405. [DOI] [PubMed] [Google Scholar]
- 20.Burguera B, Brunetto A, Garcia-Ocana A, Teijeiro R, Esplen J, Thomas T, Couce ME, Zhao A. Leptin increases proliferation of human osteosarcoma cells through activation of PI(3)-K and MAPK pathways. Med Sci Monit. 2006;12:BR341–349. [PubMed] [Google Scholar]
- 21.Iwamoto I, Fujino T, Douchi T. The leptin receptor in human osteoblasts and the direct effect of leptin on bone metabolism. Gynecol Endocrinol. 2004;19:97–104. doi: 10.1080/09513590412331284389. [DOI] [PubMed] [Google Scholar]
- 22.Yang XY, Liu CH, Liangxing , et al. Effect of mineralizing fluid on the proliferation and differentiation of rat osteoblasts. West China Journal Stomatology. 2008;06:656–9. [PubMed] [Google Scholar]
- 23.Declercq HA, Verbeeck RM, De Ridder LI, Schacht EH, Cornelissen MJ. Calcification as an indicator of osteoinductive capacity of biomaterials in osteoblastic cell cultures. Biomaterials. 2005;26:4964–4974. doi: 10.1016/j.biomaterials.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 24.Han P, Ji WP, Zhao CL, Zhang XN, Jiang Y. Improved osteoblast proliferation, differentiation and mineralization on nanophase Ti6Al4V. Chin Med J (Engl) 2011;124:273–279. [PubMed] [Google Scholar]
- 25.Turner RT, Kalra SP, Wong CP, Philbrick KA, Lindenmaier LB, Boghossian S, Iwaniec UT. Peripheral leptin regulates bone formation. J Bone Miner Res. 2013;28:22–34. doi: 10.1002/jbmr.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steppan CM, Crawford DT, Chidsey-Frink KL, Ke H, Swick AG. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept. 2000;92:73–78. doi: 10.1016/s0167-0115(00)00152-x. [DOI] [PubMed] [Google Scholar]
- 27.Hamrick MW, Della-Fera MA, Choi YH, Pennington C, Hartzell D, Baile CA. Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptindeficient ob/ob mice. J Bone Miner Res. 2005;20:994–1001. doi: 10.1359/JBMR.050103. [DOI] [PubMed] [Google Scholar]
- 28.Stunes AK, Westbroek I, Gordeladze JO, Gustafsson BI, Reseland JE, Syversen U. Systemic leptin administration in supraphysiological doses maintains bone mineral density and mechanical strength despite significant weight loss. Endocrinology. 2012;153:2245–2253. doi: 10.1210/en.2011-1848. [DOI] [PubMed] [Google Scholar]
- 29.Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004;292:490–495. doi: 10.1001/jama.292.4.490. [DOI] [PubMed] [Google Scholar]
- 30.Vega D, Maalouf NM, Sakhaee K. CLINICAL Review #: the role of receptor activator of nuclear factor-kappaB (RANK)/RANK ligand/osteoprotegerin: clinical implications. J Clin Endocrinol Metab. 2007;92:4514–4521. doi: 10.1210/jc.2007-0646. [DOI] [PubMed] [Google Scholar]
- 31.Shang M, Lin L, Cui H. Association of genetic polymorphisms of RANK, RANKL and OPG with bone mineral density in Chinese periand postmenopausal women. Clin Biochem. 2013;46:1493–1501. doi: 10.1016/j.clinbiochem.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 32.LaCroix AZ, Jackson RD, Aragaki A, Kooperberg C, Cauley JA, Chen Z, Leboff MS, Duggan D, Wactawski-Wende J. OPG and sRANKL serum levels and incident hip fracture in postmenopausal Caucasian women in the Women’s Health Initiative Observational Study. Bone. 2013;56:474–481. doi: 10.1016/j.bone.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu XJ, Shen L, Yang YP, Zhu R, Shuai B, Li CG, Wu MX. Serum beta-catenin levels associated with the ratio of RANKL/OPG in patients with postmenopausal osteoporosis. Int J Endocrinol. 2013;2013:534352. doi: 10.1155/2013/534352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin A, de Vittoris R, David V, Moraes R, Begeot M, Lafage-Proust MH, Alexandre C, Vico L, Thomas T. Leptin modulates both resorption and formation while preventing disuse-induced bone loss in tail-suspended female rats. Endocrinology. 2005;146:3652–3659. doi: 10.1210/en.2004-1509. [DOI] [PubMed] [Google Scholar]
- 35.Lamghari M, Tavares L, Camboa N, Barbosa MA. Leptin effect on RANKL and OPG expression in MC3T3-E1 osteoblasts. J Cell Biochem. 2006;98:1123–1129. doi: 10.1002/jcb.20853. [DOI] [PubMed] [Google Scholar]
- 36.Liu AR, Dong J. Effect of leptin on proliferation and OPG/RANKL mRNA expression of osteoblast MC3T3-E1. Chinese Journal of Osteoporosis. 2008;8:552–5. [Google Scholar]