Abstract
Background: Cholesterol gallstone is commonly observed in patients with gallbladder disorders. Interstitial cells of Cajal (ICCs) in the gallbladder are important for regulating gallbladder motility and have a close relationship with cholelithiasis. Aim: The aim of this study was to explore changes in the distribution of gallbladder ICCs during cholesterol gallstone formation. Materials and methods: Thirty guinea pigs were randomly divided into three groups: the control group and study groups. Animals in study groups were fed on high cholesterol diet for 4 weeks or 8 weeks. Animals in the control groups were fed on a standard diet for 8 weeks. Immunohistochemistry was performed to observe the shape, size, morphology, and numbers of ICCs from the neck of the gallbladder to the fundus of the gallbladder, and terminal deoxynucleotidyl transferase dUTP nick-end labeling was performed to detect apoptosis in ICCs from the upper part of the gallbladder to the lower part of the gallbladder. Results: There were no differences in the shape, size, and morphology of the gallbladder ICCs in all groups. Cholesterol gallstones formed in guinea pigs fed on high cholesterol diet. The numbers of gallbladder ICCs were significantly decreased from the neck of the gallbladder to the fundus of the gallbladder, and gallbladder ICC apoptosis was significantly increased from the upper part of the gallbladder to the lower part of the gallbladder in both guinea pigs fed on high cholesterol diet (all P<0.05). Conclusion: Cholesterol gallstone formation reduced the density of gallbladder ICCs and increased the frequency of apoptotic gallbladder ICCs from the neck of the gallbladder to the fundus of the gallbladder, and these alterations may affect gallbladder ICC function.
Keywords: Interstitial cells of Cajal, cholelithiasis, c-Kit, cell distribution
Introduction
Cholelithiasis is formation of gallstone, and most of that are cholesterol gallstones. Many factors are involved in the pathogenesis of cholelithiasis, such as hypersaturation of biliary cholesterol, changes of bile salts pool in bile juice, and motility disorders and so on [1,2]. Most cases of cholelithiasis are accompanied by gallbladder motility disorder and biliary dyskinesia, however, the mechanism is still unknown [2-4]. A recent study suggested that interstitial cells of Cajal (ICCs) play an important role in this disease [5].
ICCs function in the digestive tract smooth muscle by promoting gastrointestinal electrical activity and regulating gastrointestinal tract neurotransmitters [6-11]. In recent studies, ICCs have also been shown to be distributed in the biliary system [12,13]. Gallbladder motility involves various regulatory mechanisms. Indeed, recent studies have confirmed that ICCs in the biliary system are closely related to the production and spread of the gallbladder spontaneous rhythm and to adjusting gallbladder contraction and gallbladder movement [14]. Additionally, gallbladder ICCs may also have dramatic effects on various biliary system diseases, such as cholelithiasis and acute cholecystitis [15-17]. However, no studies have evaluated changes in the distributions of gallbladder ICCs during the process of cholesterol gallstone formation. Accordingly, in this study, we characterized gallbladder ICCs in order to evaluate changes in the distribution of these cells during cholesterol gallstone formation.
Materials and methods
Animals and animal experiments
Thirty guinea pigs (male and female, weighing 100-120 g) were obtained from Wuhan Institute of Biological Products Co., Ltd. (China). Animals were maintained under standard laboratory conditions (22±2°C with a 12-h light/12-h dark cycle and a relative humidity of 40-60%). All the guinea pigs were allowed free access to food and water. Animal experiments were approved by the Institutional Animal Care and Use Committee of Wuhan University.
Animals were randomly divided into 3 groups, there were 10 guinea pigs in each group. High cholesterol diet (HCD) 4 weeks group and HCD 8 weeks group were the study groups, and each was given the HCD (2% cholesterol) for 4 weeks or 8 weeks before experiments, and the control group was given a standard diet for 8 weeks.
Gallbladder specimen preparation and serum lipid test
Guinea pigs underwent laparotomy and cholecystectomy. Blood was aspirated from the heart and spun at 12,000 rpm for 5 minutes to separate serum, serum total cholesterol (TC), low density lipoprotein cholesterol (LDL), high density lipoprotein cholesterol (HDL), and triglyceride (TG) concentrations were assessed.
Immunohistochemistry (IHC)
IHC was performed on paraffin-embedded sections using a microwave-based antigen retrieval technique. Gallbladder mucosa and submucosa were peeled away, and the muscular layer was reserved. Gallbladder specimens were fixed in 4% paraformaldehyde solution and embedded in paraffin. Sections were then cut to 5-μm thickness and mounted on positively charged slides. The sections were then identified using rat monoclonal antibodies against CD117/c-Kit (eBioscience, San Diego, CA, USA), and the specimens were incubated at room temperature for 24 h, followed by appropriate incubation with secondary antibodies. Sections were then stained with diaminobenzidine and counterstained with hematoxylin. The stained samples were observed under a light microscope (Olympus BX53, Tokyo, Japan).
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assays for detection of apoptotic ICCs
TUNEL assays were performed using an In Situ Cell Death Detection Kit, Fluorescein (Roche Applied Science, Mannheim, Germany) to assess gallbladder ICC death in both the control group and study groups according to the manufacturer’s instructions. Images were obtained using a laser confocal microscope (Olympus FV1000).
Measurement of the numbers of total and apoptotic ICCs
Images of CD117/c-Kit-positive cells and CD117/c-Kit apoptosis-positive cells were taken in three randomly chosen fields (400× magnification) in each section from the neck of the gallbladder (upper part) to the fundus of the gallbladder (lower part) in all groups. Numbers of CD117/c-Kit-positive cells and CD117/c-Kit apoptosis-positive cells were assessed using Image-Pro plus 6.0 software (Media Cybernetics, Bethesda, MD, USA).
Statistical analysis
All statistical analyses were carried out using SPSS for Windows version 17.0 (SPSS, Chicago, IL, USA). Continuous variables were presented as the mean ± standard deviation (SD). Comparison of continuous variables was carried out using t tests. Comparison of categorical variables was carried out using analysis of variance. A two-sided P value of less than 0.05 was regarded as statistically significant.
Results
Evaluation of animals
No guinea pigs died both in the control groups and HCD groups. In the control group, gallbladders were normal, with clear, brightly colored bile juice. Gallbladders of guinea pigs in HCD 4 week group were swollen, with granular stones. Gallbladders of HCD 8 week group were very swollen, with thick bile juice and obvious granular, yellow, single or multiple stones.
In the control group, TC, LDL, HDL and TG were significantly lower than the HCD groups, and the HCD 4 week group was much lower than the HCD 8 week group, (all P<0.01, respectively). These data are presented in Table 1.
Table 1.
Serum Lipid Test during cholesterol gallstone formation
| TC* | LDL* | HDL* | TG* | |
|---|---|---|---|---|
| Control Group | 1.0250±0.38891 | 0.7200±0.31113 | 0.1600±0.04243 | 0.6767±0.6658 |
| HCD 4 week Group | 1.3650±0.12021 | 0.7950±0.10607 | 0.1300±0.02828 | 0.8533±0.01528 |
| HCD 8 week Group | 2.0400±0.32249 | 1.6625±0.10813 | 0.0670±0.02082 | 0.9700±0.04000 |
F-values respectively were 8.079, 28.710 and 45.735, P-values were 0.027, 0.002, 0.000 and 0.001, respectively.
These findings suggest that a high cholesterol diet could induce cholesterol gallstone formation.
IHC analysis
We used anti-CD117/c-Kit antibodies to detect CD117/c-Kit protein for identification of ICCs by IHC. After staining, the surface of ICCs was positive for c-Kit. Moreover, when observed under a light microscope, ICCs were fusiform, astrocyte-like, or fusiformis in shape, with 2-5 slender cynapses, and their nuclei were much larger, orbicular or ovate, with little cytoplasm in all groups. We also found that ICCs were mainly located in the muscular layer, with most within the muscularis propria, located primarily parallel to the smooth muscle cells. The ICCs typically appeared individually or in small clusters of 2-3 cells that were connected with each other to form a net-like structure. Mast cells were also immunolabeled positively for c-Kit, had round shapes, and were present in the mucosa of the gallbladders (Figure 1).
Figure 1.
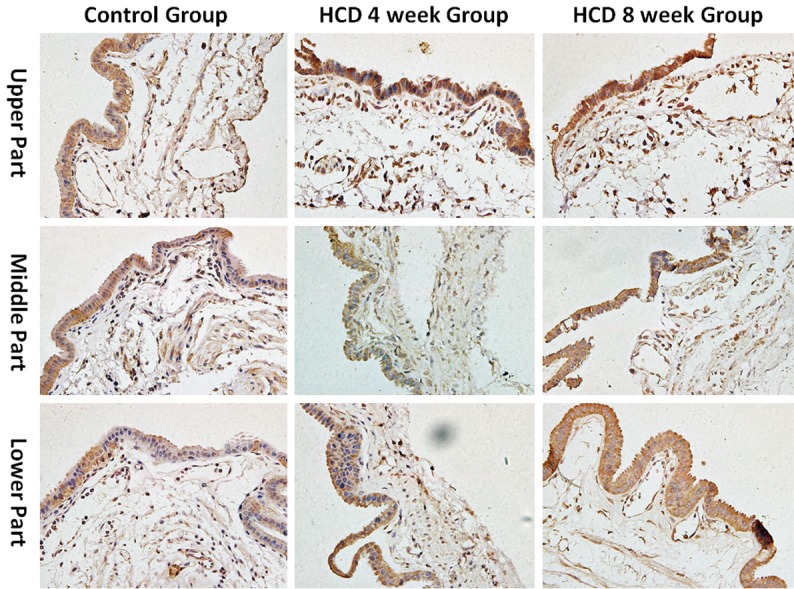
Density of ICCs during cholesterol gallstone formation (400×). The distribution of gallbladder ICCs densities decreased from the neck of the gallbladder to the fundus of the gallbladder and the densities of gallbladder ICCs decreased gradually during cholesterol gallstone formation (all P<0.01).
Measurement of the density of ICCs
The densities of gallbladder ICCs were decreased from the neck of the gallbladder (upper part) to the fundus of the gallbladder (lower part) in all groups. Additionally, the densities of gallbladder ICCs in the neck (upper part), body (middle part), and fundus (lower part) of the gallbladder were all significantly lower in the HCD groups than that in the control group. Additionally, the density of ICCs in different parts of the gallbladder in the HCD 8 week group was lower than that in the HCD 4 week group (all P<0.05, respectively). These data are presented in Figure 1 and Table 2.
Table 2.
Density of ICCs during cholesterol gallstone formation
| Group | Part of Gallbladder | ||
|---|---|---|---|
|
| |||
| Upper Part | Middle Part | Lower Part | |
| Control Group* | 76.3333±5.85947 | 54.6667±1.52753 | 46.3333±3.78594 |
| HCD 4 week Group* | 59.3333±3.51188 | 48.0000±2.00000 | 43.0000±1.73205 |
| HCD 8 week Group* | 37.6667±4.93288 | 29.3333±5.13160 | 24.3333±3.21455 |
F-values respectively were 52.005, 47.510 and 45.735, P-values were 0.000, 0.000 and 0.000, respectively.
The distributions of gallbladder ICC densities decreased from the neck of the gallbladder to the fundus of the gallbladder. Additionally, the structures of gallbladder ICCs were damaged in cholelithiasis and the densities of gallbladder ICCs decreased gradually during cholesterol gallstone formation. These changes may affect the function of gallbladder ICCs.
TUNEL assays for analysis of apoptosis
The numbers of apoptotic gallbladder ICCs increased from the neck of the gallbladder to the fundus of the gallbladder in all groups. Additionally, the numbers of apoptotic gallbladder ICCs were significantly higher in the HCD groups than in the control group for all portions of the gallbladder, and the numbers of apoptotic ICCs in individual parts of the gallbladder were higher in the HCD 8 week group than in the HCD 4 week group (all P<0.01, respectively). These data are summarized in Figure 2 and Table 3.
Figure 2.
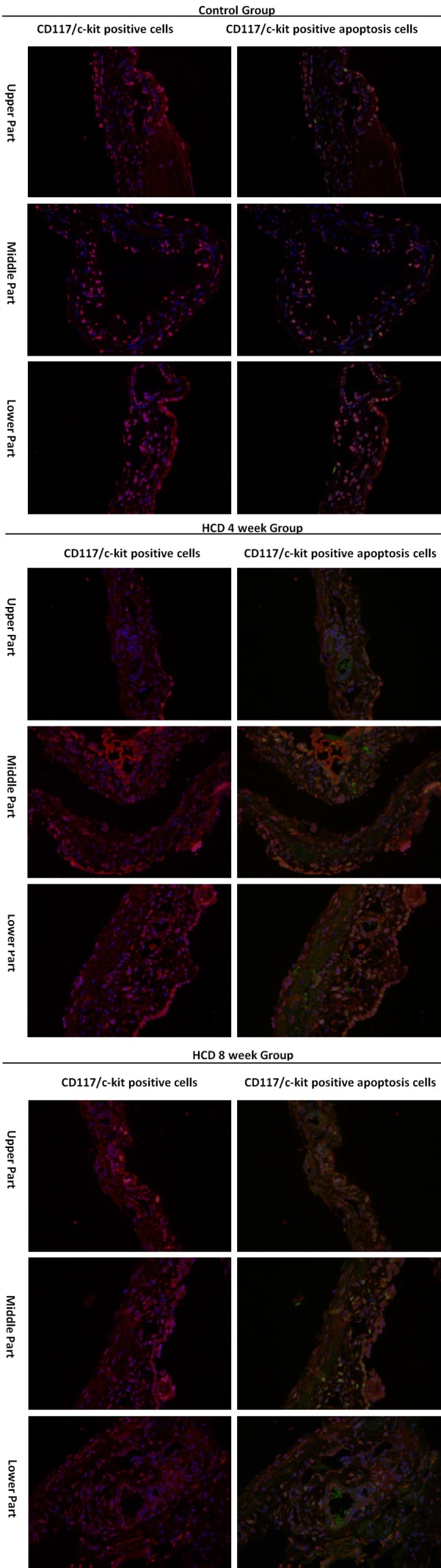
TUNEL assays for analysis of apoptosis ICCs (400×). The distribution of apoptotic gallbladder ICCs increased from the neck of the gallbladder to the fundus of the gallbladder, and the density of apoptotic gallbladder ICCs was increased gradually during cholesterol gallstone formation (all P<0.01).
Table 3.
Density of apoptotic ICCs during cholesterol gallstone formation
| Group | Part of Gallbladder | ||
|---|---|---|---|
|
| |||
| Upper Part | Middle Part | Lower Part | |
| Control Group* | 5.6667±2.51661 | 16.3333±2.08167 | 19.6667±1.15470 |
| HCD 4 week Group* | 9.6667±1.52753 | 18.6667±3.21455 | 26.0000±5.29150 |
| HCD 8 week Group* | 22.6667±0.57735 | 25.6667±1.52753 | 36.3333±0.57735 |
F-values respectively were 7.000, 14.811 and 154.778, P-values were 0.027, 0.005 and 0.000, respectively.
The distributions of apoptotic gallbladder ICCs increased from the neck of the gallbladder to the fundus of the gallbladder. In cholelithiasis, gallbladder ICCs were lost, and the density of apoptotic gallbladder ICCs was increased gradually during the process of cholesterol gallstone formation.
Taken together, the results from TUNEL assays support the findings of IHC analysis and demonstrate that during cholesterol gallstone formation, the density of gallbladder ICCs was reduced, and gallbladder ICC death is increased from the neck of the gallbladder to the fundus of the gallbladder.
Discussion
Cholesterol gallstone formation is a complicated process and many factors, such as hyper-saturation of biliary cholesterol, changes of bile salts pool in bile juice, and motility disorders, are involved [1,2]. Surgery is usually required for the treatment of cholelithiasis and acute cholecystitis [18]. Moreover, most cases of cholelithiasis are complicated by biliary dyskinesia [2-4].
Gallbladder motility is involved in various regulatory mechanisms, including gallbladder smooth muscle and nervous circuit activity, and gallbladder ICCs play an important role in this process [19]. ICCs were first identified in the gastrointestinal tract in 1893 [20-23]. More recent studies have shown that ICCs are also distributed in the gallbladder and extrahepatic biliary duct both guinea pigs and humans [24-26]. Moreover, previous studies have shown that the specific marker CD117/c-Kit is expressed on the surfaces of ICCs [14]. Thus, in this study, we found that after IHC, the surfaces of gallbladder ICCs were positive for c-Kit, and gallbladder ICCs were distributed throughout the muscular layer of the gallbladder, most of which were arranged parallel to smooth muscle cells. Gallbladder ICCs typically appeared individually or in small clusters of 2-3 cells that were connected with each other to form a net-like structure. In contrast, mast cells were also positive for c-Kit and showed round shapes. These cells were located in the mucosa of the gallbladders, but were difficult to identify.
ICCs in the biliary system may have functions as smooth muscle pacemakers to produce and maintain the spontaneous rhythm of the gallbladder, adjusting the contraction and movement of the gallbladder [6-12]. Recent studies have confirmed that ICCs in the biliary system are involved in initiating pacemaker activity to adjust gallbladder movement, and decreases in the number of gallbladder ICCs are related to gallbladder motility disorders [27]. Additionally, gallbladder ICCs may have close relationships with various biliary system diseases, such as acute cholecystitis and cholelithiasis [28]. A previous study confirmed that gallbladder ICC numbers were decreased in cholelithiasis [15]. Additionally, in this study, we found that, during cholesterol gallstone formation, the number of gallbladder ICCs was significant lower, whereas the number of apoptotic gallbladder ICCs was increased.
The physiology of gallbladder motility involves three phases: a slow emptying that occurs immediately after ingestion of food, followed by refilling and then rapid emptying [29]. Gallbladder contraction and emptying occurs from the fundus to the body and neck of the gallbladder [30]. Physiologically, the densities of gallbladder ICCs were decreased from the neck of the gallbladder to the body and fundus [14]. Moreover, during cholesterol gallstone formation, the densities of gallbladder ICCs were also decreased from the upper part of the gallbladder to the body of the gallbladder and finally the lower part of the gallbladder. Thus, these data indicate that changes in the distribution of gallbladder ICCs may be closely related to gallbladder motility disorder.
In summary, our study indicates that cholesterol gallstone formation causes structural damage to gallbladder ICCs, reduces the density of gallbladder ICCs, and increases apoptosis in gallbladder ICCs from the neck to the fundus of the gallbladder. Thus, these changes may affect the function of gallbladder ICCs and contribute to gallbladder motility disorder.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81170351) and Xi’an Medical University Doctor Scientific Research Foundation project (No. 2017DOC04).
Disclosure of conflict of interest
None.
References
- 1.Fan Y, Wu SD, Fu BB, Weng C, Wang XP. Decreased number of interstitial cells of Cajal play an important role in the declined intestinal transit during cholesterol gallstone formation in guinea pigs fed on high cholesterol diet. Int J Clin Exp Med. 2014;7:1262–1268. [PMC free article] [PubMed] [Google Scholar]
- 2.Kimura Y, Takada T, Kawarada Y, Nimura Y, Hirata K, Sekimoto M, Yoshida M, Mayumi T, Wada K, Miura F, Yasuda H, Yamashita Y, Nagino M, Hirota M, Tanaka A, Tsuyuguchi T, Strasberg SM, Gadacz TR. Definitions, pathophysiology, and epidemiology of acute cholangitis and cholecystitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg. 2007;14:15–26. doi: 10.1007/s00534-006-1152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rong ZH, Chen HY, Wang XX, Wang ZY, Xian GZ, Ma BZ, Qin CK, Zhang ZH. Effects of sphincter of Oddi motility on the formation of cholesterol gallstones. World J Gastroenterol. 2016;22:5540–5547. doi: 10.3748/wjg.v22.i24.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goussous N, Kowdley GC, Sardana N, Spiegler E, Cunningham SC. Gallbladder dysfunction: how much longer will it be controversial? Digestion. 2014;90:147–154. doi: 10.1159/000365844. [DOI] [PubMed] [Google Scholar]
- 5.Pasternak A, Matyja A, Gil K, Gajda M, Tomaszewski KA, Gajda M, Tomaszewski KA, Matyja M, Walocha JA, Kulig J. Interstitial cajal-like cells and bile lithogenicity in the pathogenesis of gall-stone disease. Pol Przegl Chir. 2013;85:311–316. doi: 10.2478/pjs-2013-0046. [DOI] [PubMed] [Google Scholar]
- 6.Al-Shboul OA. The importance of interstitial cells of cajal in the gastrointestinal tract. Saudi J Gastroenterol. 2013;19:3–15. doi: 10.4103/1319-3767.105909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev. 2014;94:859–907. doi: 10.1152/physrev.00037.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abramovic M, Radenkovic G, Velickov A. Appearance of interstitial cells of Cajal in the human midgut. Cell Tissue Res. 2014;356:9–14. doi: 10.1007/s00441-013-1772-x. [DOI] [PubMed] [Google Scholar]
- 9.Lies B, Gil V, Groneberg D, Seidler B, Saur D, Wischmeyer E, Jiménez M, Friebe A. Interstitial cells of Cajal mediate nitrergic inhibitory neurotransmission in the murine gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2014;307:G98–G106. doi: 10.1152/ajpgi.00082.2014. [DOI] [PubMed] [Google Scholar]
- 10.Huizinga JD, Chen JH, Mikkelsen HB. Interstitial cells of Cajal, from structure to function. Front Neurosci. 2013;7:43. doi: 10.3389/fnins.2013.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huizinga JD, Chen JH. Interstitial cells of Cajal: update on basic and clinical science. Curr Gastroenterol Rep. 2014;16:363. doi: 10.1007/s11894-013-0363-z. [DOI] [PubMed] [Google Scholar]
- 12.Lavoie B, Balemba OB, Nelson MT, Ward SM, Mawe GM. Morphological and physiological evidence for interstitial cell of Cajal-like cells in the guinea pig gallbladder. J Physiol. 2007;579:487–501. doi: 10.1113/jphysiol.2006.122861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasternak A, Szura M, Mazur M, Mróz I, Matyja M, Matyja A. Number and distribution of interstitial cells of Cajal in human gallbladder. Folia Med Cracov. 2014;54:71–77. [PubMed] [Google Scholar]
- 14.Huang Y, Mei F, Yu B, Zhang HJ, Han J, Jiang ZY, Zhou DS. Distribution of the interstitial Cajallike cells in the gallbladder and extrahepatic biliary duct of the guinea-pig. Acta Histochemica. 2009;111:157–165. doi: 10.1016/j.acthis.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Huang ZP, Qiu H, Yang Y, Zhang L, Yang B, Lin MJ, Yu BP. The role of interstitial cells of Cajal in acute cholecystitis in guinea pig gallbladder. Cell Physiol Biochem. 2016;38:1775–1784. doi: 10.1159/000443116. [DOI] [PubMed] [Google Scholar]
- 16.Villanacci V, Del Sordo R, Salemme M, Cadei M, Sidoni A, Bassotti G. The enteric nervous system in patients with calculous and acalculous gallbladder. Dig Liver Dis. 2016;48:792–795. doi: 10.1016/j.dld.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Hu WM, Luo HS, Ding XW, Wang L. Expression of C-kit messenger ribonucleic acid and C-kit protein in the gallbladders in guinea pigs of high cholesterol diet. Dig Dis Sci. 2009;54:1651–1655. doi: 10.1007/s10620-008-0552-z. [DOI] [PubMed] [Google Scholar]
- 18.Ambe PC, Papadakis M, Zirngibl H. A proposal for a preoperative clinical scoring system for acute cholecystitis. J Surg Res. 2016;200:473–479. doi: 10.1016/j.jss.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Pasternak A, Gajda M, Gil K, Matyja A, Tomaszewski KA, Walocha JA, Kulig J, Thor P. Evidence of interstitial Cajal-like cells in human gallbladder. Folia Histochem Cytobiol. 2012;50:581–585. doi: 10.5603/19673. [DOI] [PubMed] [Google Scholar]
- 20.Sanders KM, Ward SM. Interstitial cells of Cajal: a new perspective on smooth muscle function. J Physiol. 2006;576:721–726. doi: 10.1113/jphysiol.2006.115279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thuneberg L. One hundred years of interstitial cells of Cajal. Microsc Res Tech. 1999;47:223–238. doi: 10.1002/(SICI)1097-0029(19991115)47:4<223::AID-JEMT2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 22.Yin J, Chen JD. Roles of interstitial cells of Cajal in regulating gastrointestinal motility: in vitro versus in vivo studies. J Cell Mol Med. 2008;12:1118–1129. doi: 10.1111/j.1582-4934.2008.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Lopez P, Garcia-Marin V, Martínez-Murillo R, Freire M. Updating old ideas and recent advances regarding the interstitial cells of Cajal. Brain Res Rev. 2009;61:154–169. doi: 10.1016/j.brainresrev.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Hinescu ME, Ardeleanu C, Gherghiceanu M, Popescu LM. Interstitial Cajal-like cells in human gallbladder. J Mol Histol. 2007;38:275–284. doi: 10.1007/s10735-007-9099-0. [DOI] [PubMed] [Google Scholar]
- 25.Ahmadi O, Nicholson Mde L, Gould ML, Mitchell A, Stringer MD. Interstitial cells of Cajal are present in human extrahepatic bile ducts. J Gastroenterol Hepatol. 2010;25:277–285. doi: 10.1111/j.1440-1746.2009.05980.x. [DOI] [PubMed] [Google Scholar]
- 26.Sun X, Yu B, Xu L, Dong W, Luo H. Interstitial cells of Cajal in the murine gallbladder. Scand J Gastroenterol. 2006;41:1218–26. doi: 10.1080/00365520600708800. [DOI] [PubMed] [Google Scholar]
- 27.Fan Y, Wu S, Fu B, Weng C, Wang X. The role of interstitial Cajal-like cells in the formation of cholesterol stones in guinea pig gallbladder. Hepatol Int. 2015;9:612–620. doi: 10.1007/s12072-015-9623-3. [DOI] [PubMed] [Google Scholar]
- 28.Pasternak A, Gil K, Matyja A, Gajda M, Sztefko K, Walocha JA, Kulig J, Thor P. Loss of gallbladder interstitial Cajal-like cells in patients with cholelithiasis. Neurogastroenterol Motil. 2013;25:e17–e24. doi: 10.1111/nmo.12037. [DOI] [PubMed] [Google Scholar]
- 29.Mehra R, Sodhi KS, Saxena A, Thapa BR, Khandelwal N. Sonographic evaluation of gallbladder motility in children with chronic functional constipation. Gut Liver. 2015;9:388–394. doi: 10.5009/gnl13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkins T, Agabin E, Varghese J, Talukder A. Gallbladder dysfunction: cholecystitis, choledocholithiasis, cholangitis and biliary dyskinesia. Prim Care. 2017;44:575–597. doi: 10.1016/j.pop.2017.07.002. [DOI] [PubMed] [Google Scholar]


