Abstract
Objectives: To explore the expression and pathologic significance of renalase in tumor tissues of different molecular subtypes of breast cancer. Design: Immunofluorescence methods and laser confocal scanning microscope observations were used to detect expression of renalase, estrogen receptor (ER), phospho-extracellular signal-regulated kinase 1 and 2 (p-ERK1/2), and phospho-signal transducer and activator of transcription (p-STAT3) in 58 cases of breast cancer tissue, 11 normal tissues, and 14 benign fibroadenomas. Statistical analysis of its expression in different molecular subtypes of breast cancer was employed. Results: Compared with control tissue (benign lesions and normal breast tissue), renalase was highly expressed in invasive breast cancer and the difference was significant (P<0.0001). Renalase was also expressed significantly higher in ER-positive breast cancer, compared with control tissue (P<0.0001). There was a positive correlation between renalase and ER expression in breast cancer tissues (R=0.7246, P<0.0001) and a positive correlation between renalase and p-ERK 1/2 expression (R=0.6599, P<0.0001). Renalase had no significant correlation with p-STAT3 protein expression. Conclusion: Renalase is a new molecular marker for ER-positive breast cancer and may become a potential therapeutic target for the ER-positive/HER2-negative subtype breast cancer. Renalase may promote high ER expression and breast cancer cell proliferation and growth through the p-ERK1/2 pathway.
Keywords: Breast cancer, estrogen receptor, renalase
Introduction
Breast cancer is the most common malignancy in Chinese women. Its morbidity and mortality is increasing, and the average age of onset is getting younger [1-3]. Histopathologically, breast cancer can be divided into different subtypes [4]. In the past, immunohistochemical methods rather than molecular classification have been widely used to identify different subtypes of breast cancer [5-7], including the luminal A (ER-positive, PR (progesterone receptor, PR)-positive more than 20%, HER2-negative, Ki67 less than 20%), luminal B1 (ER-positive, PR-positive less than 20%, HER2-negative, Ki67 more than 20%), luminal B2 (ER-positive and/or PR-positive, HER2-positive), HER2-like (HER2-positive, ER-negative, PR-negative and Ki67 any value), and basal-like types (HER2-negative, ER-negative, PR-negative, CK5/6-positive and/or EGFR-positive).
Sixty to seventy percent of all breast cancers are ER-positive [8,9]. Existing endocrine therapy can reduce metastasis and the recurrence rate of breast cancer and prolong life in ER-positive breast cancer. However, about one-third of ER positive breast cancer patients do not respond to endocrine therapy [10,11]. Therefore, research on other methods of ER-positive breast cancer treatment, for example targeting other markers and pathways, is necessary.
Recent research shows that renalase is a flavin adenine dinucleotide-dependent amine oxidase secreted by the kidney [12-14] that can protect cells by ameliorating ischemia and decreasing cytotoxic effects through the PI3K-AKT and mitogen-activated protein kinase (MAPK) pathways [15]. One study found that renalase functions as a growth factor for pancreatic cancer cells, and that its expression is negatively correlated with the prognosis [16]. This study also confirmed that the expression level of renalase mRNA in various malignancies, including breast cancer, is significantly higher than in normal tissues. However, the expression of renalase protein in breast cancer cells has not been reported and important questions remain to be answered, including the following: (a) is renalase highly expressed in breast cancer cells as well; (b) what are its expressions levels in breast cancer cells; and (c) is there a connection between renalase and ER? Experimental research on the expression of renalase in breast cancer tissues and on relevant protein expression pathways is necessary to establish whether renalase could be used as a new breast cancer tumor marker and as a target in future therapies.
Materials and methods
Patients
The complete data, including age, tumor size, lymph node metastasis, tumor grade, tumor stage, of 58 breast cancer patients were obtained from the Department of Pathology of Shenzhen Traditional Chinese Medicine Hospital. All of the patients were female, with no prior chemotherapy, endocrine, or immunotherapy treatment. The tumor was graded according to the 2012 WHO breast tumor classification standard and staged according to the AJCC2017 standard [17]. Twenty-five patients with benign breast lesions and normal breast tissue were randomly selected as control group (including 11 normal tissues and 14 benign fibroadenomas). The protocol of the present study was reviewed and approved by the local ethics committee.
Sample preparation
Paraffin-embedded samples were processed for immunohistochemistry and immunofluorescent staining. The specimens were fixed in 10% neutral-buffered formalin and embedded in paraffin and cut into 4-μm serial sections. The paraffin-embedded tissues were then dewaxed in xylene, rehydrated in a graded ethanol series of decreasing ethanol concentrations, and rinsed in PBS. Finally, the relevant antigens were retrieved with EDTA (for HER2, CK5/6, p-ERK1/2, p-STAT3, and renalase) and citric acid (for ER, PR and Ki67).
Immunohistochemistry
For immunohistochemical staining, the sections were permeabilized with 0.04% Triton X-100, blocked with 10% normal goat serum and 0.5% bovine serum albumin in PBS for 1 hour, and treated overnight at 4°C with the following primary antibodies: rabbit polyclonal antibody against ER (1:50 dilution; clone: SP1, Labvision), rabbit polyclonal antibody against PR (1:100 dilution; clone: SP2, Labvision), rabbit polyclonal antibody against HER2 (1:100 dilution; clone: EP3, Labvision), mouse monoclonal antibody against Ki67 (1:100 dilution; clone: MIB-1, Labvision) or mouse monoclonal antibody against CK5/6 (1:100 dilution; clone: D5/16B4, Labvision). The sections were washed 3 times in PBS for 10 minutes each and incubated in biotinylated secondary antiserum (Vectastain ABC kit, Vector Lab) for 30 minutes at room temperature. The sections were rinsed and incubated in ABC reagents (Vectastain ABC kit) for 30 minutes and then washed thoroughly and incubated in 0.05% diaminobenzidine (DAB) and 0.03% H2O2 for 3-5 minutes until brown reaction product was observed.
Immunohistochemical staining and manual scoring for ER, PR, HER2, Ki67 and CK5/6 were performed by a trained pathologist without knowledge of case outcomes. Table 1 summarizes the criteria of molecular classification of invasive breast cancer. Samples were scored as positive for ER or PR when 10% or more of tumor cell nuclei showed positive staining for ER and PR, respectively. For HER2, the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guideline and a membrane-staining score ranging from 0 to 3+ were used (a score of zero means no staining; 1+ means that 10% of cells or less show faint, barely perceptible, incomplete cell membrane staining; 2+ means that at least 10% of cells show staining with complete, weak to moderate cell membrane staining; and 3+ means that at least 10% of cells show circumferential, complete, and intense membrane staining). HER2 was considered positive when the score was 3+. CK5/6 was considered to be positive when more than 10% of tumor cells membranes or cytoplasms showed staining.
Table 1.
Criteria of molecular classification of invasive breast cancer
| Subtype | Markers | ||||
|---|---|---|---|---|---|
|
| |||||
| ER | PR | HER2 | Ki67 | CK5/6 | |
| Luminal A | >10%+ | >10%+ | - | <20%+ | - |
| Luminal B (HER2-) | >10%+ | any | - | >20%+ | - |
| Luminal B (HER2+) | >10%+ | any | + | any | - |
| HER2-Overexpression | - | - | + | any | - |
| BLBC | - | - | - | any | >10%+ |
Abbreviation: IHC: immunohistochemical, FISH: fluorescence in situ hybridization, BLBC: basal-like breast cancer.
Confocal microscopy
For immunofluorescent staining, sections were blocked and incubated overnight at 4°C with primary antibodies as mentioned above. The dilution of the primary antibodies against renalase (clone: ERP4212, Abcam, Cambridge, UK), p-ERK1/2 (clone: Thr202/Tyr204, Cell Signaling Technology Inc., Danvers, MA, USA) and p-STAT3 (clone: Tyr705/M9C6, Cell Signaling Technology Inc., Danvers, MA, USA) was 1:70, 1:50, and 1:50, respectively. After washes in PBS, sections were incubated with fluorescence-conjugated secondary antibodies (1:200 dilution, Alexa Fluor 568, Thermo fisher, USA) for 1 hour at room temperature. Sections were washed and counterstained with nuclear dye 4,6-diamino-2-phenylindole (DAPI). The resulting images were visualized and captured on a confocal microscope (LSM710, Carl Zeiss, Germany). A total of 3-5 photographs were randomly selected in each breast section to calculate the renalase fluorescence intensity, ER and p-ERK1/2 positive areas.
Statistical analysis
Renalase fluorescence intensity is expressed as mean ± SD. Statistical differences between two groups were analyzed using unpaired Student’s t tests. Differences among multiple groups were analyzed using one-way ANOVA. Bivariate correlation analysis was performed to elucidate the associations between the fluorescence intensity of renalase and ER or p-ERK1/2 positive area ratio, and the statistical significance was evaluated by the Pearson’s correlation test. Statistical analyses were performed using SPSS statistical software, version 16.0; P<0.05 was considered statistically significant.
Results
Clinical characteristics
Fifty-eight breast cancer patients, aged 29-82, were examined. The median age was 46 years. There were 4 grade I, 15 grade II, and 39 grade III cases and 11 stage I, 38 stage II, and 9 stage III cases. There were 36 cases of positive breast cancer-16 luminal A and 20 luminal B cases (3 of the luminal B cases were HER2 positive). There were 18 cases of axillary lymph node metastasis (Table 2).
Table 2.
Clinical characteristics of patients with invasive breast cancer
| Characteristics | Subcategories | Total samples |
|---|---|---|
| Age (years) | 46.40±11.29 | 58 |
| Tumor size (cm) | ≤2 | 20 |
| 2-5 | 33 | |
| >5 | 5 | |
| Lymph node metastasis | Yes | 18 |
| No | 40 | |
| Grade | I | 4 |
| II | 15 | |
| III | 39 | |
| Stage | I | 11 |
| II | 38 | |
| III | 9 | |
| Subtypes | Luminal A | 16 |
| Luminal B | 20 | |
| BLBC | 13 | |
| HER2+ | 9 |
Pathological classification
Using immunohistochemical-rather than the commonly employed molecular classification-method to detect ER, PR, Ki67, CK5/6 and HER2, we found that of the 58 cases of breast cancer, 36 cases were ER positive, and 16 luminal A and 20 luminal B cases (3 of the 20 luminal B cases were HER2 positive). There were 13 cases of basal type breast cancer, and 9 cases of HER2-overexpression breast cancer (Figure 1).
Figure 1.
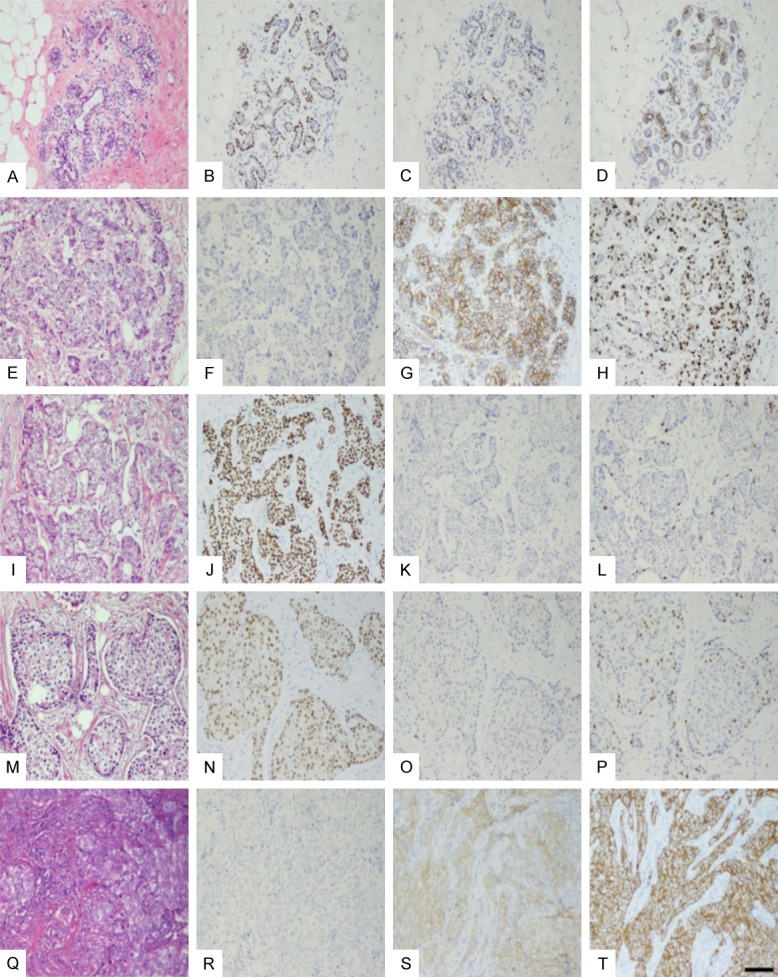
Pathological characteristics of normal breast and IBC tissue. A-D. Normal breast tissue. A. Terminal ductal lobular structure (HE staining). B. ER expression in epithelial cells with strong and weak heterogeneity in the small lobule. C. p63 was positive in normal breast myoepithelial cells. D. CK5/6 showed a positive expression in normal breast glandular epithelium. E-H. HER2- overexpression breast cancer. E. Morphological features of infiltrating carcinoma (HE staining), cancer cells in invasive growth pattern. F. Cancer cells are ER negative. G. Immunohistochemical staining reveals HER2 positive cells (3+). H. High level of Ki67 expression with more than 50% nuclear positive cells. I-L. Luminal A type breast cancer. I. Morphological features of invasive carcinoma with no special type (HE staining). J. Immunohistochemically strong positive for ER with high level of expression (more than 90% nuclear positive cells), K. HER2 negative, L. Low expression of Ki67 with less than 5% nuclear positive cells. M-P. Luminal B type breast cancer. M. Morphological features of invasive carcinoma with no special type (HE staining), N. High expression for ER with more than 90% the nuclear positive cells. O. HER2-negative breast cancer. P. High level of Ki67 expression with more than 40% nuclear positive cells. Q-T. BLBC type breast cancer. Q. Morphological features of invasive carcinoma with no special type (HE staining). R. IHC, ER negative. S. IHC revealed EGFR positive cells (3+). T. IHC, Cytoplasmic positive (3+) for CK5/6. Scale bar, 100 um.
Renalase expression in IBC
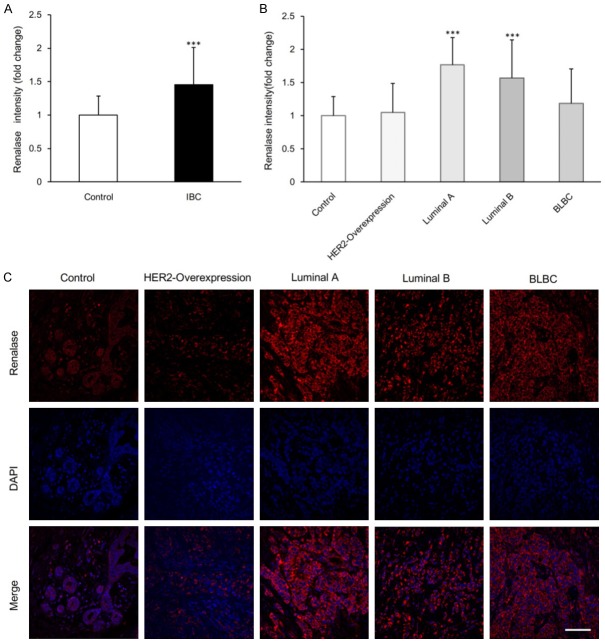
Laser confocal microscopy was used to detect expression of renalase in tumor tissues and statistical analysis of renalase expression for different types of breast cancer was performed. We found that the renalase fluorescence level in the 58 examined cases of invasive breast cancer was significantly stronger than that in the normal control group with a significant difference (P=0.0002) (Figure 2A). There was a statistically significant difference between the control group and the 16 cases of luminal A breast cancer (P<0.0001) and between the control group and the 20 cases of luminal B breast cancer (P<0.0001). However, there was no statistically significant difference between the control group and the 9 cases of HER2-overexpression breast cancer (P=0.7131) or between the control group and the BLBC type of breast cancer cases (P=0.1659) (Figure 2B).
Figure 2.
Renalase expression in normal breast tissue and all subtypes of IBC tissue. A. Comparison of renalase fluorescence intensity in normal control group (n=25) and IBC group (n=58). Data are mean ± SD. ***P<0.001 when compared to control group. B. Comparison of renalase fluorescence intensity in normal control (n=25), HER2-Overexpression (n=9), luminal A (n=16), luminal B (n=20) and BLBC (n=13) group. Data are mean ± SD. ***P<0.001 when compared to control group. C. Representative confocal immunofluorescence images of renalase staining. Red, renalase staining. Blue, DAPI staining of the nucleus. Scale bar, 100 um.
The red fluorescence signal from renalase was significantly stronger in luminal A breast cancer compared with the other subtypes of breast cancer. The intensity of the renalase fluorescence signal of luminal B breast cancer was also stronger. On the other hand, the intensity of the renalase fluorescence signal of both HER2-overexpression and BLBC subtype breast cancer was weak. Weak renalase fluorescence signals emitted by epithelial cells in the terminal ductal-lobular unit (TDLU) were also found in the control group. In addition, the fluorescence signals emitted by mesenchymal cells in normal breast tissue were weak and significantly different from the fluorescence signal intensity of renalase in invasive carcinoma (Figure 2C).
Renalase expression in ER-positive IBC
The finding that the renalase expression is significantly higher in both luminal A as well as luminal B breast cancer than that of the normal group suggests that renalase expression is high in ER positive breast cancer. We compared renalase expression of all the ER positive breast cancer cases with normal breast tissue. Renalase expression in the 36 cases of ER positive breast cancer (16 cases of luminal A and 20 cases luminal B) was significantly higher than that of 25 cases of normal breast tissue (P<0.0001) (Figure 3A).
Figure 3.
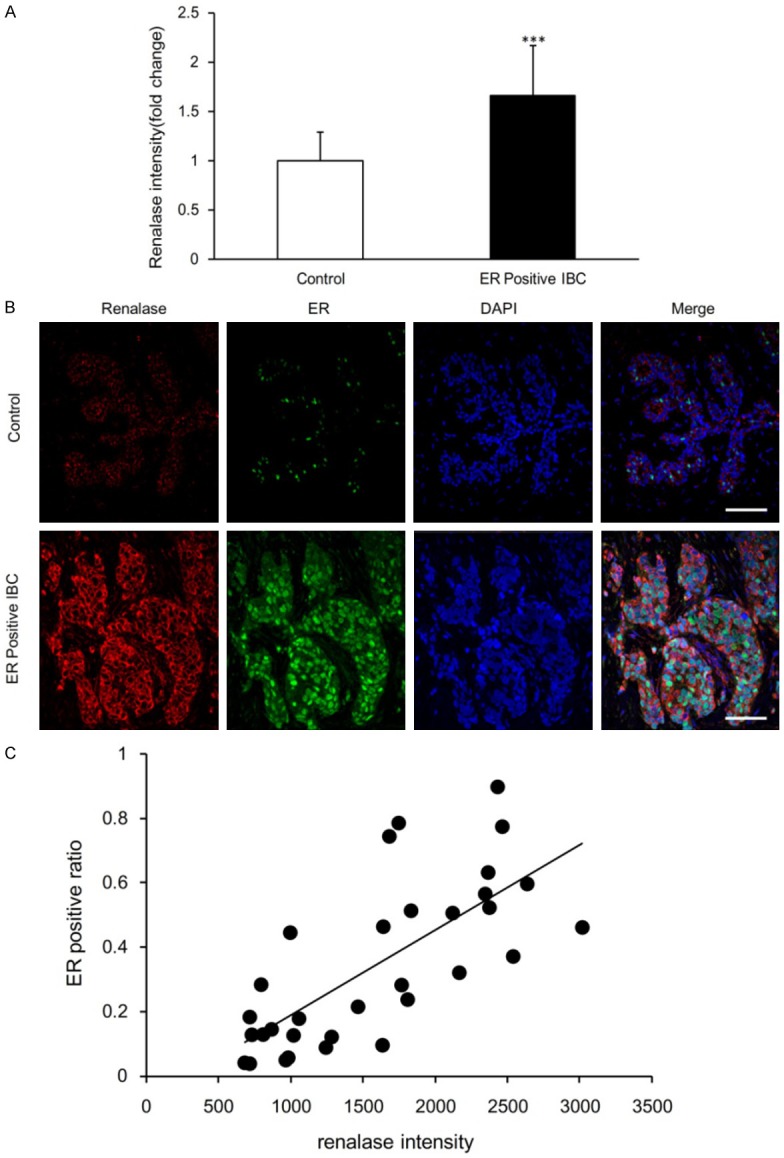
Renalase expression in normal breast tissue and ER positive IBC tissue. A. Comparison of renalase fluorescence intensity in normal control group (n=25) and ER positive IBC group (n=36). Data are mean ± SD. ***P<0.001 when compared to control group. B. Co-immunostaining to show the localization of renalase (red) and ER (green). Blue, DAPI staining of the nucleus. Scale bar, 100 um. C. Correlation between renalase fluorescence intensity and ER positive ratio in normal control (n=15) and ER positive IBC (n=17) sections. The correlation coefficient (R) was 0.7246 and the statistical significance (P) less than 0.0001.
ER negative cells showed weak expression of renalase. In ER positive breast cancer, renalase positive red fluorescence was observed in the cancer cell cytoplasm, while ER showed a strong positive green fluorescence in the nucleus. In normal breast tissue, the cytoplasm of the epithelial cells in TDLU emitted a uniform weak red fluorescence, whereas ER emitted an uneven green fluorescence (Figure 3B). In ER positive breast cancer, the intensity of renalase fluorescence was significantly correlated with the expression level of ER. There was a statistically significant correlation between renalase expression and ER expression ratio in ER positive breast cancer (R=0.7246, P<0.0001) (Figure 3C).
The relation of renalase and p-ERK1/2 expression in ER-positive IBC
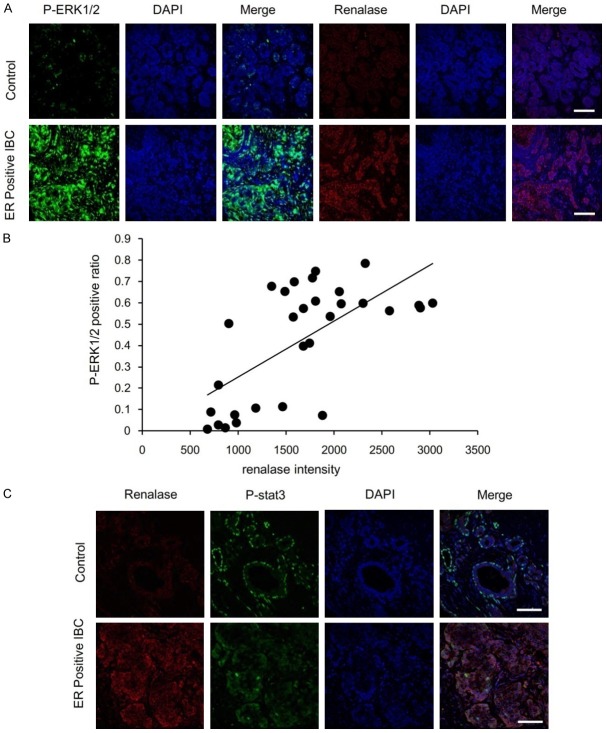
In ER positive breast cancer, the fluorescence intensity of renalase was significantly correlated with the fluorescence intensity of p-ERK1/2. In the tumor tissue, p-ERK1/2 showed strong green fluorescence and renalase showed significant red fluorescence. In normal breast tissue, the p-ERK1/2 in the epithelial cells of TDLU showed a faint green fluorescence and the same breast tissue showed a faint red fluorescence in different sections (Figure 4A). Expression of renalase was positively correlated with p-ERK1/2 expression (R=0.6599, P<0.0001) (Figure 4B).
Figure 4.
The relation of renalase and p-ERK1/2 expression in ER-positive IBC. A. Colocalization of renalase with p-ERK1/2, as stained in serial tissue section images. Red for renalase staining, green for p-ERK1/2.Blue for DAPI staining of the nucleus. Scale bar, 100 um. B. Correlation between renalase fluorescence intensity and p-ERK1/2 positive ratio in normal control (n=11) and ER positive IBC (n=19) sections. R=0.6599, P<0.0001. C. P-STAT3 (green) and renalase (red) expression in control and ER-positive IBC. p-STAT3 was expressed in the normal breast tissue of the epithelial and myoepithelial cells, in the myoepithelial cells it was expressed in the nuclei of the cells. The positive expression rate of p-STAT3 in ER positive breast cancer was low. p-STAT3 was expressed in the cytoplasm and the nucleus of tumor cells. Blue, DAPI staining of the nucleus. Scale bar, 100 um.
p-STAT3 expressed in normal breast tissue of the epithelial cells and myoepithelial cells was more highly localized to the nucleus of myoepithelial cells. The positive expression rate of p-STAT3 in ER positive breast cancer was low. Expression of p-STAT3 was located in the cytoplasm and the nucleus of tumor cells (Figure 4C). Fluorescence staining showed that p-STAT3 and renalase were not correlated, therefore no correlation analysis was performed.
Discussion
Renalase was first found in kidney tissue [18]. It is a novel flavin adenine dinucleotide-dependent amine oxidase secreted by the kidney and is mainly expressed in the renal proximal tubule. Desir [19] recently found that renalase may be a cytokine that plays a protective role in cells by activating the MAPK signaling pathway. Currently, research on renalase has focused on the kidneys and the cardiovascular system. Renalase is also expressed in myocardial, liver, skeletal muscle, peripheral nerve, adrenal, and adipose tissue cells [20], indicating that it is widely available in the human body. Our study found that in normal breast tissue, renalase shows constant expression with weak fluorescence in adenoepithelial, myoepithelial, and interstitial fibroblast cells in the TDLU. This finding suggests that renalase may be a normal enzyme in mammary epithelial and stromal cells that maintains the normal growth of tissue cells.
A positive correlation between renalase and tumors was found in pancreatic cancer [16], and the study found that renalase mRNA was highly expressed in pancreatic cancer cells. Other studies have found that renalase protein is highly expressed in malignant melanoma and is negatively correlated with its prognosis [21]. So far, the expression and significance of renalase protein in breast tumors has not been reported. Our study found that expression of renalase is statistically significantly higher in breast cancer cells than in benign lesions and normal tissues of the breast. We found that different molecular subtypes of breast cancer express different concentrations of renalase. ER-positive breast cancer (luminal A and luminal B) had high renalase expression, while HER2-positive breast cancer, and basal-type breast cancer both had low renalase expression. Among them, renalase expression in luminal A breast cancer was the highest with the average intensity of the fluorescence signal being the strongest. Statistical analysis showed that renalase has obvious correlation with ER in breast cancer tissue, suggesting a connection between renalase and ER and this correlation may be associated with signaling pathways.
The study found that the mechanism of action of renalase is similar to that of cytokines. Extracellular renalase in combination with plasma membrane calcium ATPase isoform 4b (PMCA4b) receptors on the cell membrane activate STAT3. This activates the mitogen-activated protein kinase (MAPK) pathway and promotes cell survival [20,21]. The MAPK signaling pathway is one of the most important signal transduction systems in the body and can transduce extracellular signals into the cell mainly through the following four ways: STAT3, ERK, p38 mitogen-activated kinase (p38) and protein kinase B (AKT). This also includes the ERK1/2 signal transduction pathways which regulate cell growth and differentiation in cell division, migration, and survival and has an important role in regulating aggression. The study also found that the reduction of renalase expression and signaling pathway by RNAi or renalase monoclonal antibody m28-rnls could reduce the proliferation of melanoma cells and increase the apoptosis of tumor cells. This study demonstrates that renalase is important in the growth and evolution of tumor cells. Our results showed that the myoepithelial cells expressed STAT3 in normal breast tissue. In breast cancer tissues, STAT3 protein was either not expressed at all or expressed only in a small number of cells. This might suggest that the STAT3 pathway may not be the main pathway to promote growth of breast cancer cells. It is known that ERK1/2 functions in regulating cell growth and differentiation. We found that the high expression of p-ERK1/2 in ER positive breast cancer was positively correlated with ER expression, suggesting an inner relationship between p-ERK1/2 and ER, a relationship which promotes expression of p-ERK1/2, which, in turn, influences expression of ER. P-ERK1/2 and ER promotes the growth of tumor cells, not the STAT3 pathway. Other data in this study supported this assumption from another point of view.
Our study showed that renalase widely exists in various mammary gland cells, is a type of cytokine that maintains normal cell growth, and by activating MAPK signaling pathways, plays a role in cell protection. However, abnormal upregulation of the renalase signaling pathway may activate p-ERK1/2 and thus promote high ER expression and breast cancer cell proliferation and growth. Therefore, we believe that renalase can be used as an indicator of ER positive/HER2-negative subtype of breast cancer. Inhibiting the renalase signaling pathway may also be an alternative to ER positive breast cancer treatment.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (81673794, 81202818) and the Shenzhen Science and Technology Project (JCYJ20150401163247233, JCYJ20160330171116798).
Disclosure of conflict of interest
None.
References
- 1.Zeng H, Zheng R, Zhang S, Zou X, Chen W. Female breast cancer statistics of 2010 in China: estimates based on data from 145 population-based cancer registries. J Thorac Dis. 2014;6:466–470. doi: 10.3978/j.issn.2072-1439.2014.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azim HA, Ibrahim AS. Breast cancer in Egypt, China and Chinese: statistics and beyond. J Thorac Dis. 2014;6:864–866. doi: 10.3978/j.issn.2072-1439.2014.06.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xing L, He Q, Wang YY, Li HY, Ren GS. Advances in the surgical treatment of breast cancer. Chin Clin Oncol. 2016;5:34. doi: 10.21037/cco.2016.05.08. [DOI] [PubMed] [Google Scholar]
- 4.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, Akslen LA, Ragaz J, Gown AM, Gilks CB, van de Rijn M, Perou CM. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 6.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the Carolina breast cancer study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 7.The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, Senn HJ. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, Abraham M, Medeiros Alencar VH, Badran A, Bonfill X, Bradbury J, Clarke M, Collins R, Davis SR, Delmestri A, Forbes JF, Haddad P, Hou MF, Inbar M, Khaled H, Kielanowska J, Kwan WH, Mathew BS, Mittra I, Muller B, Nicolucci A, Peralta O, Pernas F, Petruzelka L, Pienkowski T, Radhika R, Rajan B, Rubach MT, Tort S, Urrutia G, Valentini M, Wang Y, Peto R. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li G, Xu J, Wang P, Velazquez H, Li Y, Wu Y, Desir GV. Catecholamines regulate the activity, secretion, and synthesis of renalase. Circulation. 2008;117:1277–1282. doi: 10.1161/CIRCULATIONAHA.107.732032. [DOI] [PubMed] [Google Scholar]
- 13.Sonawane PJ, Gupta V, Sasi BK, Kalyani A, Natarajan B, Khan AA, Sahu BS, Mahapatra NR. Transcriptional regulation of the novel monoamine oxidase renalase: crucial roles of transcription factors Sp1, STAT3, and ZBP89. Biochemistry. 2014;53:6878–6892. doi: 10.1021/bi500798n. [DOI] [PubMed] [Google Scholar]
- 14.Moran GR, Hoag MR. The enzyme: renalase. Arch Biochem Biophys. 2017;632:66–76. doi: 10.1016/j.abb.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Safirstein R, Velazquez H, Guo XJ, Hollander L, Chang J, Chen TM, Mu JJ, Desir GV. Extracellular renalase protects cells and organs by outside-in signalling. J Cell Mol Med. 2017;21:1260–1265. doi: 10.1111/jcmm.13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo X, Hollander L, MacPherson D, Wang L, Velazquez H, Chang J, Safirstein R, Cha C, Gorelick F, Desir GV. Inhibition of renalase expression and signaling has antitumor activity in pancreatic cancer. Sci Rep. 2016;6:22996. doi: 10.1038/srep22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Li G, Wang P, Velazquez H, Yao X, Li Y, Wu Y, Peixoto A, Crowley S, Desir GV. Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J Clin Invest. 2005;115:1275–1280. doi: 10.1172/JCI24066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desir GV, Peixoto AJ. Renalase in hypertension and kidney disease. Nephrol Dial Transplant. 2014;29:22–28. doi: 10.1093/ndt/gft083. [DOI] [PubMed] [Google Scholar]
- 20.Malyszko J, Bachorzewska-Gajewska H, Dobrzycki S. Renalase, kidney and cardiovascular disease: are they related or just coincidentally associated? Adv Med Sci. 2015;60:41–49. doi: 10.1016/j.advms.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Hollander L, Guo X, Velazquez H, Chang J, Safirstein R, Kluger H, Cha C, Desir GV. Renalase expression by melanoma and tumor-associated macrophages promotes tumor growth through a STAT3-mediated mechanism. Cancer Res. 2016;76:3884–3894. doi: 10.1158/0008-5472.CAN-15-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]