Abstract
Periostin (PN), originally named osteoblast-specific factor-2 (OSF-2), is a multifunctional glycoprotein which can significantly promote EMT (epithelial-mesenchymal transition). Recently, many studies have shown that high-level expression of PN is correlated significantly with tumor angiogenesis and prognosis in many kinds of human cancer. In previous experiments, we screened PN from prostate cancer through iTRAQ technology and found that PN affects occurrence and development of prostate cancer (PCa). However, whether and how periostin expression influences tumor angiogenesis in prostate cancer remains unknown. Our study aimed to examine expression of PN in patients with PCa and explored the relationship of PN expression with clinicopathologic factors and tumor angiogenesis. Immunohistochemistry was performed to determine expression of PN in PCa and benign prostate hyperplasia (BPH). Vascular endothelial growth factor (VEGF) and CD31 (used to mark tumor angiogenesis) were also examined in tissues from the PCa patients and hyperplasia patients mentioned above. The results showed that PN expression was significantly (P<0.001) higher in PCa (58%) than in BPH (18.8%) and VEGF expression was significantly (P=0.003) higher in PCa (55%) than in BPH (24.5%). Increased PN protein expression was associated with Gleason score (P=0.005) but there was no correlation with age (P=0.548), PSA (P=0.343) or clinic tumor staging (P=0.049). The results also showed that high expression of PN correlated with VEGF expression (P<0.001) and that tumors with PN-positive expression had significantly higher microvessel density (38.7±14.4 vs. 29.7±10.5; P=0.026) compared to those with PN-negative. In conclusion, our findings suggest that PN may have an important role in tumor progression and may impact tumor angiogenesis in prostate cancer.
Keywords: Periostin, angiogenesis, correlation, prostate cancer
Introduction
Prostate cancer is one of the most common malignancies of the urinary system and is mostly found in the prostate epithelium. According to data of Cancer Statistics 2017, there were 161,360 new cases of prostate cancer in the United States, about 19% of male malignant tumors. Deaths by prostate cancer were 26,730, about 8% of cancer deaths in males [1]. Although slightly lower, it is still one of the most malignant tumors in American males. In another report [2], morbidity and mortality of prostate cancer are increasing in China and had reached to 60.3/100000 and 26.6/100000 in 2015. In addition, elderly men are the crowd of peak incidence of prostate cancer. As the population ages, morbidity and mortality are expected to increase further. Because of the trend of rapid growth of prostate cancer, we have struggled to explore relevant mechanisms in the occurrence, development, and metastasis of prostate cancer and look for new diagnosis and treatment targets of prostate cancer.
Periostin (PN), also named osteoblast-specific factor-2 (OSF-2), was originally identified in 1993 as a 90 kDa protein acid which was secreted from the mouse osteoblastic cell line MC3T3-E1 [3]. This protein appears homologous with an insect cell adhesion protein named fasciclin I (FAS I) protein family, members of which are involved in many biologic processes such as cell motility, adhesion, metastatic growth, and angiogenesis [4]. Recently, accumulating evidence has revealed that high-level expression of PN is correlated significantly with various human cancers including colon, breast, head and neck, liver, and esophagus, etc. [5-9].
In previous studies, we have proven high expression of PN in prostate stroma through the Western blot technique and animal experiments. We have demonstrated that silencing PN could not only inhibit migration of LNCap cells but also promote the apoptosis of the LNCap in vitro [10]. We have also found that PN mediates TGF-β-Induced Epithelial Mesenchymal Transition (EMT) in Prostate Cancer Cells [11].
PN plays an important role in the process of cell migration and adhesion, especially in the process of EMT in tumors [12]. Maybe this characteristic and function also makes it play an important role in tumor angiogenesis. Furthermore, emerging evidence has suggested that high expression of PN protein is closely correlated with tumor angiogenesis in some types of human cancer [13-18].
Although in prostate cancer, the role of the periostin in tumor angiogenesis is still unclear. In our present study, we used immunohistochemistry to examine the expression of PN, vascular endothelial growth factor (VEGF), and microvessel density (MVD) in PCa tissues. The objective of this study was to elucidate expression of PN in PCa and to examine its correlation with tumor angiogenesis and clinicopathological characteristics.
Materials and methods
Patients and specimens
Fifty-three patients with prostate cancer were selected at the Department of Urology between 2013 and 2015. The 53 cases were diagnosed with benign prostatic hyperplasia (BPH) in Huashan Hospital of Fudan university. The Pathology Department took paraffin block and sliced. This study was approved by the Institutional Review Board of the Huashan Hospital affiliated to Fudan University. All of the patients involved in this study signed the informed consent. The study protocol conformed to the ethical guide lines of the Declaration of Helsinki.
Ethical approval
Ethical approval for the use of human subjects was obtained from the Research Ethics Committee of Fudan University.
Histopathological and immunohistochemical analyses
The histopathological and immunohistochemical analyses were performed. The expressions of PN, VEGF, and CD31 were detected by immunohistochemistry using a two-step method according to the manufacturer’s instructions. Semi-quantitative estimation was made to interpret the results of immunohistochemistry according to the percentage of staining cells per 100 cells in 10 microscopic fields with high-power (200×) microscope, as follows: 0-10%, negative (-); 10-30%, weak positive (+); >30%, strong positive (++). MVD was quantified in five fields in which there was high expression using high-power lens (200×) and values were expressed by average measurements.
Statistical analysis
All statistical analyses were performed using state 14.0. Corrections between PN expression and clinicopathological parameters were assessed by Chi-square test, Fisher’s exact test and Logistic regression analysis. P<0.05 was considered statistically significant.
Results
Expression of PN in prostate cancer and benign prostatic hyperplasia tissues
As shown in Table 1, the positive rate of PN expression was 58.4% (31/53) in PCa and 18.8% (10/53) in BPH samples. The protein expression level of PN was significantly higher in PCa tissues than in BPH tissues (P<0.05). The distribution of positive expression area of PN was mainly interstitial expression, some tumor cells stained strongly while others exhibited slight or no staining at all (Figure 1).
Table 1.
Differential expression of periostin between PCa tissues and BPH tissues (cases)
| Tissues | Case number | Positive | Negative | Positive rate (%) |
|---|---|---|---|---|
| PCa | 53 | 31 | 22 | 58.40% |
| BPH | 53 | 10 | 43 | 18.80% |
Figure 1.
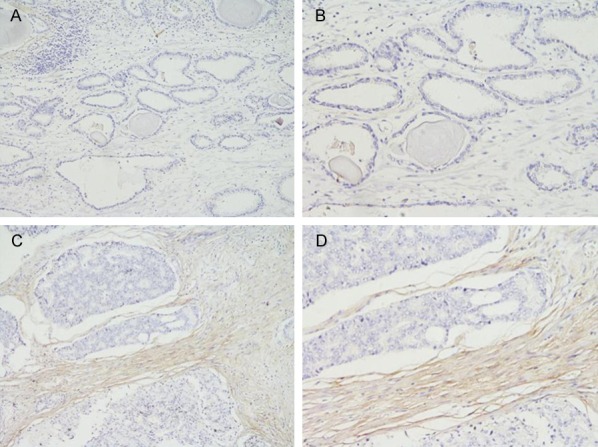
In BPH tissues, the Periostin expression is weak or not expressed: (A) HE staining of BPH, ×100; (B) HE staining of BPH, ×200. In PCa, the positive Periostin expression: (C) HE staining of BPH, ×100; (D) HE staining of BPH, ×200.
Expression of PN in prostate cancer with different clinic data
As shown in Table 2, fifty-three patients with prostate cancer were mean age of 67.7±6.3 years old and mean PSA of 19.3±25.6 ng/mL. They were grouped by age, PSA, Gleason score, and clinic tumor stage. To elucidate its clinical significance, we also assessed correlation between PN expression and clinicopathological parameters available for the patients (Table 2). Positive expression of PN in PCa was significantly correlated with Gleason score (P=0.005). However, there was no significant correlation with age, PSA, and T staging (P=0.548, P=0.343, P=0.049).
Table 2.
Periostin expression status in relation to selected clinicopathologic features in 53 prostate cancer patients (cases)
| Clinicopathologic data | Case number | Positive | Negative | v2 | P value |
|---|---|---|---|---|---|
| Age at diagnosis (year) | |||||
| 67.7±6.3 (50-80) | 0.36 | 0.548 | |||
| <70 | 34 | 20 | 14 | ||
| ≥70 | 19 | 11 | 8 | ||
| Pre-operative PSA (ng/ml) | |||||
| 19.3±6.3 (0.93-158.3) | 0.9 | 0.343 | |||
| <10 | 21 | 11 | 10 | ||
| 10-20 | 21 | 12 | 9 | ||
| >20 | 11 | 8 | 3 | ||
| Gleason score | 7.87 | 0.005 | |||
| 2-4 | 1 | 0 | 1 | ||
| 5-6 | 10 | 4 | 6 | ||
| 7 | 20 | 9 | 11 | ||
| 8-10 | 22 | 18 | 4 | ||
| T staging | 3.85 | 0.049 | |||
| T1-T2 | 41 | 23 | 18 | ||
| T3 | 12 | 8 | 4 |
Correlation of periostin and VEGF in prostate cancer
Expression of VEGF and MVD in prostate cancer tissues and its correlation with PN
The positive rate of VEGF expression was 54.71% (29/53) and the distribution of positive expression area was mainly localized in the cytoplasm (Figure 2). The relationship between expression of PN and VEGF was calculated and is outlined in Table 3. The result also shows that high expression of PN correlates with VEGF expression (r=0.707; P<0.001; Table 3).
Figure 2.
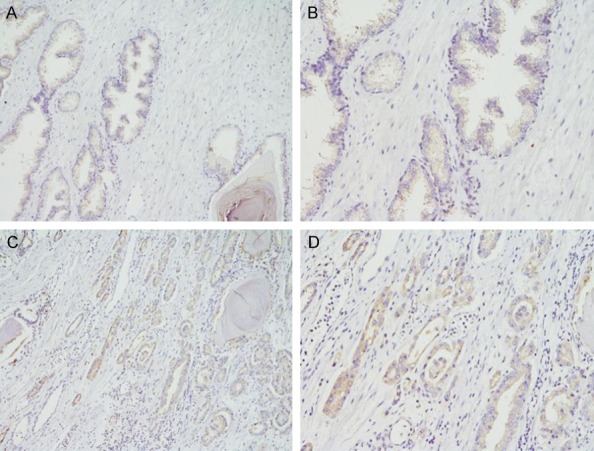
In BPH tissues, the VEGF expression is weak or not expressed: (A) HE staining of BPH, ×100; (B) HE staining of BPH, ×200. In PCa, the positive VEGF expression (C) HE staining of BPH, ×100; (D) HE staining of BPH, ×200.
Table 3.
The expression correlation between vascular endothelial growth factor (VEGF) and periostin (cases)
| Stain | Periostin | ||||
|---|---|---|---|---|---|
|
|
|||||
| Positive | Negative | r | P value | ||
| VEGF | Positive | 26 | 3 | 0.707 | <0.001 |
| Negative | 5 | 19 | |||
Correlation of periostin and MVD in prostate cancer
To further evaluate the association between PN and angiogenesis, we detected MVD by expression of CD31 in the PCa and the BPH tissue using an antibody against CD31 (Figure 3). The results indicated that tumors with PN-positive expression had significantly higher MVD (38.7±14.4 vs. 29.7±10.5; P=0.026) compared to tumors with PN-negative. (Graph 1).
Figure 3.
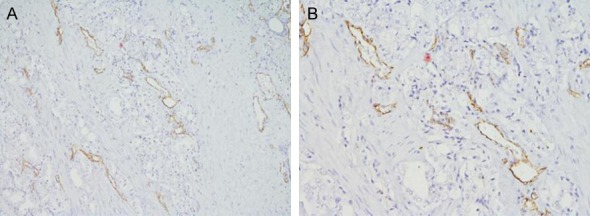
Immunohistochemical staining of CD31 in prostate cancer tissues. A: HE staining, ×100; B: HE staining, ×200.
Graph 1.
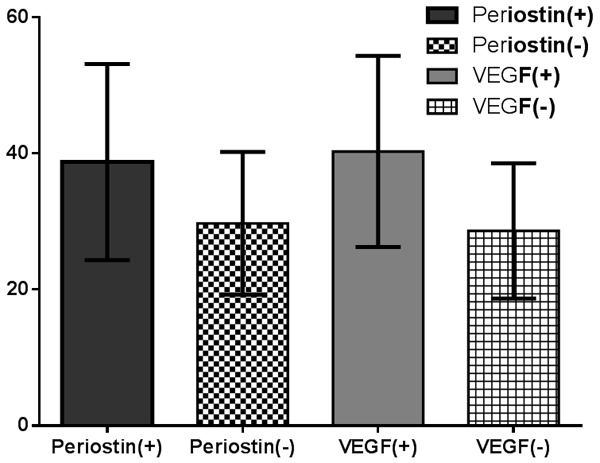
Expression of MVD in prostate cancer with different expression of PN and VEGF.
Discussion
PN, a stroma-associated protein, has previously been found to be involved in formation and maintenance of the normal structure of bone and teeth and the development of the heart [19,20]. Recently, it has been frequently reported that PN is overexpressed in various types of human malignant tumors. Moreover, accumulating evidence has revealed that PN was correlated significantly with tumor invasiveness and progression in nasopharyngeal carcinoma and can regulate epithelial-to-mesenchymal transition (EMT) and cell invasiveness in prostate and bladder cancer cells as well as mediate proangiogenic activity in oophoroma [21].
In our study, overexpression of PN was detected in 31 of 53 (58.4%) tumor tissues. Furthermore, we found that PCa with PN-positive expression had higher Gleason scores than osteosarcoma with PN-negative expression. Therefore, we believe PN can affect invasiveness of prostate cancer. PCa often tends to develop distant metastasis, especially in bone, and results ultimately in death. There is plenty of evidence to support the significance of angiogenesis in initiation, development, and aggressiveness [22]. VEGF is considered a prime mediator for both physiological and pathological angiogenesis and has been implicated in carcinogenesis and metastasis. In normal prostate tissue, there is a small amount of expression or no expression of VEGF. In prostate cancer tissues we can detect high expression of VEGF [23]. Our experiments about VEGF have verified this point. In addition, in the serum and urine of patients with prostate cancer, we could significantly detect higher expression of VEGF and compared with the control group and non-metastasis group, VEGF levels of metastasis group was obviously higher [24]. These experiments suggest that VEGF plays a role in helping to determine tumor progression.
To explore the relationship between PN and tumor angiogenesis in our study, we quantified the levels of VEGF and MVD, the most widely accepted markers of tumor angiogenesis. Our results show that tumors with PN-positive group express higher VEGF and have higher MVD than those in PN-negative group. We analyzed the relation between VEGF and PN and found that there was a significant positive correlation between PN and VEGF. Taken together, these findings suggest that PN plays a crucial role in PCa tumorigenesis by inducing and/or promoting tumor angiogenesis.
PN plays a major role in stromal invasion and tumor adhesion. However, its exact mechanism of action remains unclear. Here, PN can act as an adhesion protein by facilitating interaction between the cancer stem cells and the niche. As mentioned above, PN can also promote angiogenesis in tumor metastases and facilitate survival and proliferation of tumor cells following their colonization of distant tissues and may be related to the role in the process of cell migration and adhesion, especially in the process of EMT in tumors. To demonstrate these hypotheses, further investigation is needed to explore possible molecular mechanisms.
There are several inherent limitations to this study, including small sample size and possible selection bias of the cohort. Thus, the current findings should be verified in a prospective, randomized, and multicenter study.
In conclusion, our study found that PN is overexpressed in human PCa compared with BPH tissues. Overexpression of PN has a close correlation with tumor invasiveness and angiogenesis and may be used as a prognostic biomarker and therapeutic target in patients with prostate cancer.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No.81372316) and Chenguang Program of Shanghai Municipal Education Commission (No.158554). In addition, we are grateful for the guidance of Professor Guowei Xia and the Department of Urology, Huashan hospital of fudan university, China.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics in China, 2015. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Takeshita S, Kikuno R, Tezuka K, Amann E. Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J. 1993;294:271–8. doi: 10.1042/bj2940271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Litvin J, Selim AH, Montgomery MO, Lehmann K, Rico MC, Devlin H, Bednarik DP, Safadi FF. Expression and function of periostin-isoforms in bone. J Cell Biochem. 2004;92:1044–61. doi: 10.1002/jcb.20115. [DOI] [PubMed] [Google Scholar]
- 5.Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, Shao R, Anderson RM, Rich JN, Wang XF. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004;5:329–39. doi: 10.1016/s1535-6108(04)00081-9. [DOI] [PubMed] [Google Scholar]
- 6.Shao R, Bao S, Bai X, Blanchette C, Anderson RM, Dang T, Gishizky ML, Marks JR, Wang XF. Acquired expression of periostin by human breast cancers promotes tumor angiogenesisthrough up-regulation of vascular endothelial growth factor receptor 2 expression. Mol Cell Biol. 2004;24:3992–4003. doi: 10.1128/MCB.24.9.3992-4003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kudo Y, Ogawa I, Kitajima S, Kitagawa M, Kawai H, Gaffney PM, Miyauchi M, Takata T. Periostin promotes invasion and anchorageindependent growth in the metastatic process of head and neck cancer. Cancer Res. 2006;66:6928–35. doi: 10.1158/0008-5472.CAN-05-4540. [DOI] [PubMed] [Google Scholar]
- 8.Lv Y, Wang W, Jia WD, Sun QK, Li JS, Ma JL, Liu WB, Zhou HC, Ge YS, Yu JH, Xia HH, Xu GL. High-level expression of periostin is closely related to metastatic potential and poor prognosis of hepatocellular carcinoma. Med Oncol. 2013;30:385. doi: 10.1007/s12032-012-0385-7. [DOI] [PubMed] [Google Scholar]
- 9.Wang W, Sun QK, He YF, Ma DC, Xie MR, Ji CS, Hu B. Overexpression of periostin is significantly correlated to the tumor angiogenesis and poor prognosis in patients with esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:593–601. [PMC free article] [PubMed] [Google Scholar]
- 10.Sun C, Zhao X, Xu K, Gong J, Liu W, Ding W, Gou Y, Xia G, Ding Q. Periostin: a promising target of therapeutical intervention for prostate cancer. J Transl Med. 2011;9:99. doi: 10.1186/1479-5876-9-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Q, Tong S, Zhao X, Ding W, Gou Y, Xu K, Sun C, Xia G. Periostin mediates TGF-β-induced epithelial mesenchymal transition in prostate cancer cells. Cell Physiol Biochem. 2015;36:799–809. doi: 10.1159/000430139. [DOI] [PubMed] [Google Scholar]
- 12.Morra L, Moch H. Periostin expression and epithelial-mesenchymal transition in cancer: a review and an update. Virchows Arch. 2011;459:465–475. doi: 10.1007/s00428-011-1151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siriwardena BS, Kudo Y, Ogawa I, Kitagawa M, Kitajima S, Hatano H, Tilakaratne WM, Miyauchi M, Takata T. Periostin is frequently overexpressed and enhances invasion and angiogenesis in oral cancer. Br J Cancer. 2006;95:1396–1403. doi: 10.1038/sj.bjc.6603431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tung KH, Lin CW, Kuo CC, Li LT, Kuo YH, Lin CW, Wu HC. CHC promotes tumor growth and angiogenesis through regulation of HIF-1α and VEGF signaling. Cancer Lett. 2013;331:58–67. doi: 10.1016/j.canlet.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Takanami I, Abiko T, Koizumi S. Expression of periostin in patients with non-small cell lung cancer: correlation with angiogenesis and lymphangiogenesis. Int J Biol Markers. 2008;23:182–186. doi: 10.1177/172460080802300308. [DOI] [PubMed] [Google Scholar]
- 16.Puglisi F, Puppin C, Pegolo E, Andreetta C, Pascoletti G, D’Aurizio F, Pandolfi M, Fasola G, Piga A, Damante G, Di Loreto C. Expression of periostin in human breast cancer. J Clin Pathol. 2008;61:494–498. doi: 10.1136/jcp.2007.052506. [DOI] [PubMed] [Google Scholar]
- 17.Siriwardena BS, Kudo Y, Ogawa I, Kitagawa M, Kitajima S, Hatano H, Tilakaratne WM, Miyauchi M, Takata T. Periostin is frequently overexpressed and enhances invasion and angiogenesis in oral cancer. Br J Cancer. 2006;95:1396–1403. doi: 10.1038/sj.bjc.6603431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takanami I, Abiko T, Koizumi S. Expression of periostin in patients with non-small cell lung cancer: correlation with angiogenesis and lymphangiogenesis. Int J Biol Markers. 2008;23:182–186. doi: 10.1177/172460080802300308. [DOI] [PubMed] [Google Scholar]
- 19.Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 20.Conway SJ, Molkentin JD. Periostin as a heterofunctional regulator of cardiac development and disease. Curr Genomics. 2008;9:548–555. doi: 10.2174/138920208786847917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ratajczak-Wielgomas K, Dziegiel P. The role of periostin inneoplastic processes. Folia Histochem Cytobiol. 2015;53:120–32. doi: 10.5603/FHC.a2015.0014. [DOI] [PubMed] [Google Scholar]
- 22.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 23.Bok RA, Halabi S, Fei DT, Rodriquez CR, Hayes DF, Vogelzang NJ, Kantoff P, Shuman MA, Small EJ. Vascular endothelial growth factor and basic fibroblast growth factor urine levels as predictors of outcome in hormone-refractory prostate cancer patients: a cancer and leukemia group B study. Cancer Res. 2001;61:2533–2536. [PubMed] [Google Scholar]
- 24.Ferrer FA, Miller LJ, Andrawis RI, Kurtzman SH, Albertsen PC, Laudone VP, Kreutzer DL. Vascular endothelial growth factor (VEGF) expression in human prostate cancer: in situ and in vitro expression of VEGF by human prostate cancer cells. J Urol. 1997;157:2329–2333. [PubMed] [Google Scholar]


