Abstract
Carcinosarcoma of the pancreas is a very rare tumor with distinct malignant epithelial and mesenchymal components. The pathogenesis, however, remains to be further clarified. This is a report on a 44-year old Chinese woman with a solid tumor in the head of pancreas analyzed on immunohistochemistry, K-ras, and p53 sequence. On gross inspection, the tumor was grey-white and poorly circumscribed with a bone-like texture component. In the histology, it was also noteworthy that the heterogeneous features of the tumor were mixed in the same field, which consisted of adenocarcinoma, sarcoma, and osteosarcoma. Immunohistochemical results have confirmed that these are three histological components with different phenotypes. Gene mutation analyses confirmed the heterogeneity, in which the adenocarcinoma component and the sarcoma component did not burden the same K-ras mutation and p53 mutations as that of the osteosarcoma component. It is gratifying that the woman is still alive 48 months after surgery. Based on these findings, this case was the first case reported to date of pancreatic adenocarcinoma combing with sarcoma and osteosarcoma in the same tumor. Furthermore, this case was a unique pancreatic tumor composed of carcinosarcoma and extraskeletal osteosarcomas. The morphological, immunohistochemical, and genetic findings suggested that the pancreatic tumor was of a multiple clonal origins.
Keywords: Pancreas, carcinosarcoma, osteosarcoma, gene mutation, tumorigenesis
Introduction
Carcinosarcoma of the pancreas is a very rare tumor which is defined as a tumor consisting of two malignant components: epithelial and mesenchymal cells. Each of these elements shows distinct immunohistochemical and ultrastructural characteristics and the histogenesis of this tumor is still unclear. Up to now, 28 cases of pancreatic carcinosarcoma have been reported [1-25] and only three of them have shown heterologous mesenchymal components [1-2,9]. Moreover, there has been only one case reported as pancreatic adenocarcinoma with osteosarcoma component [9]. In addition, there was an extraskeletal osteosarcoma of pancreas recently reported [26]. There have been only two documented cases of osteosarcoma arising in the pancreas as of now. Herein, this report presents a unique case of pancreatic ductal adenocarcinoma combining with a sarcomatous component and heterogeneous osteosarcoma. The immune-phenotype and genotype were clearly different. Further study of associated morphological characteristics, immunohistochemical results, and genetic mutations were analyzed. The relevant cases in literature were reviewed in the discussion.
Case presentation
Clinical history
The patient was a 44 years old woman and came to our hospital in May 2013. She complained of discomfort in the upper abdomen. She showed progressively exacerbated jaundice which manifested icteric skin and sclera, dark urine, and pale stool. Laboratory tests showed the following results: alanine aminotransferase (ALT) was 482.1 U/L (0-40 U/L), aspartate aminotransferase (AST) was 345.6 U/L (13-35 U/L), total bilirubin was 184.2 mmol/L (3.4-17.1 mmol/L), direct bilirubin was 149.6 mmol/L (0-6.8 mmol/L), and CA-199>1200 kU/L (<37kU/L). Amylase, lipase levels, and other tests were within the normal range. The physical examination did not find any palpable abdominal mass. Doppler examination indicated a hypoechoic 2.9 cm×1.6 cm lesion in the lower segment of the common bile duct and dilation of hepatic bile ducts. Magnetic resonance imaging (MRI) and Magnetic Resonance Cholangiopancreatography (MRCP) confirmed the nodular lesion in the head of pancreas. Contrast-enhanced computed tomography (CT) showed that the nodular lesion was inhomogeneous with a bone like high-density lesion (Figure 1A).
Figure 1.
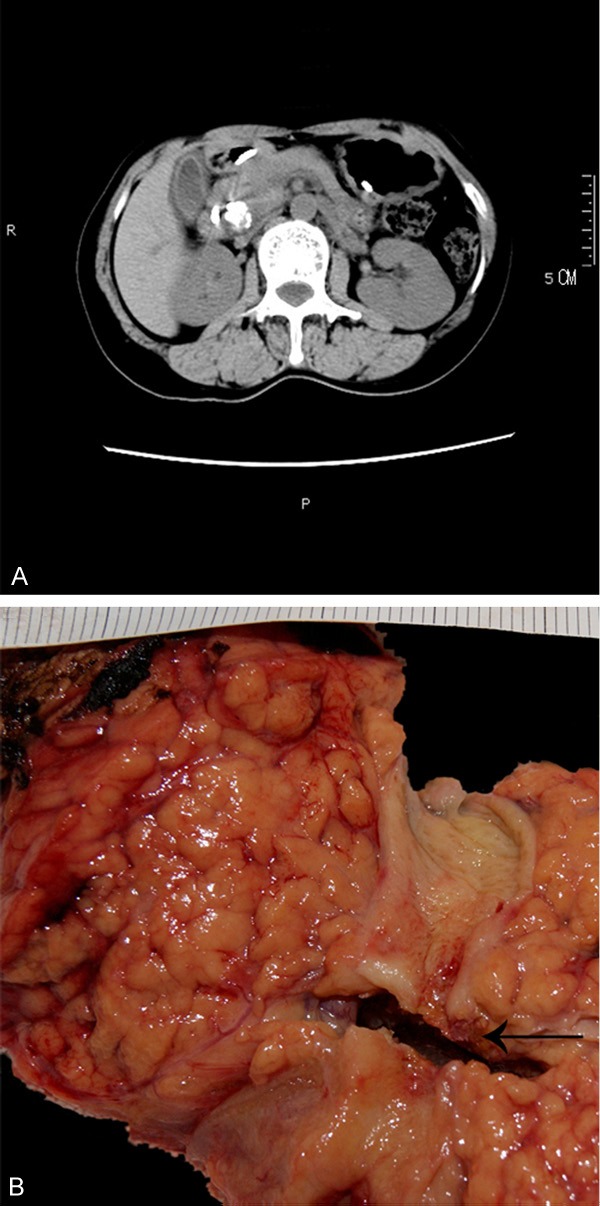
A. Abdominal CT image showing a 3 cm high density nodositas at the back of pancreatic head. B. Fresh cut view of the body and head of the pancreas.
The patient had no history of smoking or alcoholism. She underwent the appendectomy 4 years ago and the epiglottic cystectomy 2 years ago. No explicit familial history of cancer was found. Subsequently, a radical Whipple pancreatico-duodenectomy was performed and the specimen was sent to our department. The follow up revealed that the patient was still alive 48 months after of the operation, which was relatively better than mean survival time in patients with pancreatic adenocarcinoma.
Pathological and genetic findings
The resection specimen was composed of partial pancreas, stomach, duodenum, and the distal common bile duct. In gross examination, a gray-white irregular tumor was measured as 3.0 cm×2.0 cm×1.6 cm in the head of the pancreas. The tumor was inhomogenous and some portions were as hard as cancellous bone (Figure 1B). The tumor was poorly circumscribed and infiltrated the distal common bile duct cross pancreas and invaded into the peripancreatic adipose tissue.
Histologically, it was noteworthy that triphase histological features were found in the lesion (Figure 2A), which consisted of ductal adenocarcinoma, sarcoma, and osteosarcoma (Figure 2B-D). The first component was a moderately differentiated ductal adenocarcinoma which invaded into peripancreatic adipose tissue and the common bile duct wall. The second component mainly exhibited a fascicular and storiform growth pattern, which was composed of pleomorphic spindle cells and was presented as predominantly with highly cellular areas containing abundant cytoplasm, hyperchromatic nuclei, and prominent nucleoli. Abnormal cells with hyperchromatic, poly lobated nuclei, or multinucleated giant cells were also present occasionally. The pathological mitotic figures were about 4-6/10 HPF. The sarcoma component was morphologically compatible with a high-grade sarcoma. The third component was characterized by a dense proliferation of malignant short spindle-shaped, polygon-shaped, and small round cells that had atypical and abnormal nuclei and formed tumor osteoid and bone matrix. There was no vascular invasion and the specimen margins and adjacent organs were free of tumor but 3 of 18 peripancreatic lymph nodes were positive for metastatic cancer, the invasive component originated from adenocarcinoma.
Figure 2.
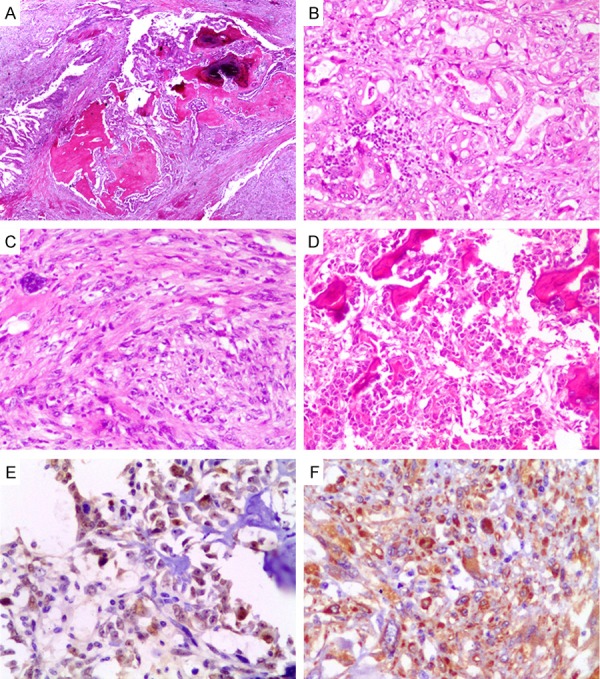
Histopathological and Immunohistochemical features. (A) A very-low-power view of the polymorphy lesion (H&E ×40). (B-D) Mid-power view of the triphase histological features (H&E ×200). (B) Adenocarcinoma. (C) Sarcoma. (D) Osteosarcoma. (E, F) Immunohistochemical expression of p53. (E) and osteonectin (F) in the osteosarcoma area (Envision ×400).
An antibody panel (Table 1) included a serial of epithelial markers and a serial of mesenchymal markers used to analyze the phenotype. Immunohistochemically (Figure 2E, 2F), the carcinomatous cells were strongly and diffusely reactive for antibodies to cytokeratin (AE1/AE3), cytokeratin7, mucin1, and Epithelial Membrane Antigen (EMA) but were negative for vimentin, CD1a, CD10, CD34, CD117, SMA, desmin, S-100, cytokeratin20, and mucin2, which was consisted with the adenocarcinoma originated from pancreatic duct or bile duct. The spindle cells of the sarcomatous component, on the other hand, showed strong reactivity for antibody to vimentin, and weakly positive to osteopontin and osteonectin but no reactivity for other antibodies. The osteosarcoma cells were only reactive for vimentin, osteopontin, and osteonectin. All three components showed positive for p53 but the range and intensity of the expression of the three components were different. The Ki-67 proliferation index in the carcinomatous, sarcomatous, and osteosarcoma components were 38%, 24%, and 30%, respectively.
Table 1.
Results of immunohistochemical findings
| Arcinosarcoma | Osteosarcoma | ||
|---|---|---|---|
|
|
|||
| Marker | Adenocarcinoma | Sarcoma | |
| CK AE1/3 | positive | negative | negative |
| Vimentin | negative | positive | positive |
| EMA | positive | negative | negative |
| p53 | weakly positive | weakly positive | positive |
| CK7 | positive | negative | negative |
| CK20 | negative | negative | negative |
| mucin1 | positive | negative | negative |
| mucin2 | negative | negative | negative |
| EGFR | negative | negative | negative |
| SMA | negative | negative | negative |
| CD1a | negative | negative | negative |
| CD117 | negative | negative | negative |
| CD10 | negative | negative | negative |
| Desmin | negative | negative | negative |
| S-100 | negative | negative | negative |
| CD34 | negative | negative | negative |
| Osteopontin | negative | weakly positive | positive |
| Osteonectin | negative | weakly positive | positive |
| Ki-67 | 38% | 24% | 30% |
As to the genotype, three distinct tumor components (adenocarcinoma, sarcoma, and osteosarcoma) and control samples (non-neoplastic pancreatic parenchyma adjacent to the tumor) were archived separately by laser micro-dissected method and sent to be analyzed for K-ras and p53 mutations. DNA samples extracted from the micro-dissected tissues were subjected to PCR with a pair of specific primers (Table 2) to amplify exon 1-2 of K-ras and exon 4-9 of p53 and isolated PCR products were sequenced bidirectionally. The analysis revealed some identical K-ras (mutation in exon 1) and p53 (mutation in exon 4 and 8) mutations only in osteosarcoma examined (Table 3). The same gene mutations were not detected in adenocarcinoma, sarcoma components, and control samples.
Table 2.
Specific primer sequences of KRAS and p53
| Gene/EXON | Specific primer sequences |
|---|---|
| p53-E4 | F: TGCTCTTTTCACCCATCTAC |
| R: GCCAGGCATTGAAGTCTCAT | |
| p53-E5 | F: GACTTTCAACTCTGTCTCCT |
| R: ATCAGTGAGGAATCAGAGGC | |
| p53-E6 | F: GCCTCTGATTCCTCACTGAT |
| R: CACTGACAACCACCCTTAAC | |
| p53-E7 | F: CCTCATCTTGGGCCTGTGTT |
| R: CAGTGTGCAGGGTGGCAAGT | |
| p53-E8 | F: GGACCTGATTTCCTTACTGC |
| R: GCTTCTTGTCCTGCTTGCTT | |
| p53-E9 | F: CAAGAAGCGGTGGAGGAGAC |
| R: ACGGCATTTTGAGTGTTAGA | |
| KRAS-E1 (G12-G13) | F: TGTATTAACCTTATGTGTGACA |
| R: GTCCTGCACCAGTAATATG | |
| KRAS-E2 (Q61) | F: TGAAGTAAAAGGTGCACTGT |
| R: TCAATTTAAACCCACCTATAATG |
Table 3.
Mutations of KRAS and TP53 genes in each tumor component
| Sample | Exon | Alleles | Flanking Sequence | Comments |
|---|---|---|---|---|
| Control | KRAS (G12+G13) | no mutation | ||
| Control | p53 EXON4~9 | no mutation | ||
| Adenocarcinoma | KRAS (G12+G13) | no mutation | ||
| Adenocarcinoma | p53 EXON4~9 | no mutation | ||
| Sarcoma | KRAS (G12+G13) | no mutation | ||
| Sarcoma | p53 EXON4~9 | no mutation | ||
| Osteosarcoma | p53 EXON4 | C/G | AATGCCAGAGGCTGCTCCCC (C/G) CGTGGCCCCTGCACCAGCAG | c.215C>G, p.P54R |
| Osteosarcoma | p53 EXON8 | A/AG | GAGACCGGCGCACAGAGGAAG (A/AG) GAATCTCCGCAAGAAAGGGG | c.860A>AG, p.E287EG |
| Osteosarcoma | KRAS (G12+G13) | G/AG | TAAACTTGTGGTAGTTGGAGCT (G/AG) GTGGCGTAGGCAAGAGTGCCTT | c.34G>AG, p.G12GS |
Discussion
Carcinosarcoma is a rare neoplasm when in the pancreas or other organs. By definition, it is a biphasic tumor consisting of an intimate admixture of malignant epithelial and mesenchymal components identifiable on the basis of their morphological, immunohistochemical, and ultrastructural features. There are two main hypotheses for the genesis of carcinosarcoma. Collision theory implies development of two independent elements intermingled. Monoclonal hypothesis, more widely accepted, implies a common precursor in the form of dedifferentiated or pluripotent cells that undergo divergent differentiation.
Carcinosarcoma rarely occurs in the pancreas and about 28 cases of carcinosarcoma of the pancreas have been reported (Table 4) in publications, which includes 7 cases in Chinese papers. The major cases were of malignant mesenchymal component composed of malignant spindle-shaped cells. Besides, it also included leiomyosarcoma [1,2,10,23], malignant peripheral nerve sheath tumor (MPNST) [2], malignant fibrous histiocytoma (MFH) [5,13,15,21], and similar to giant cell carcinoma (GCT) of bone differentiation [3,4,14]. Among these cases, only three cases showed heterologous mesenchymal components [1,2,9], namely, chondrosarcoma and rhabdomyosarcoma [1], chondroid [2], or osteosarcoma [9] features.
Table 4.
Summary of reported carcinosarcoma of the pancreas
| Reference | Race | Age (years) | Sex | Max size (cm) | Carcinoma | Sarcoma | Heterologous differentiation | Haematogenous metastases | Lymph node metastases | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Millis [1] | White | 50 | F | 8.8 | AC | Leiomyosarcoma | Chondrosarcoma | - | - | +3 |
| Rhabdomyosarcoma | ||||||||||
| Wenig [2] | White | 67 | M | 19 | MCAC | MPNST | Chondroid | - | unknown | +15 |
| Polynesian | 48 | F | unknown | MCAC | Leiomyosarcoma | - | - | unknown | 12 | |
| Black | 65 | F | 30 | MCAC | Leiomyosarcoma | - | Omentum | unknown | +9 | |
| Watanabe [3] | Japanese | 76 | M | 5 | AC | Similar to GCT of bone | - | - | unknown | +3 |
| Fischer [4] | German | 73 | F | 10 | AC | Similar to GCT of bone | - | - | - | +2 |
| Darvishian [5] | White | 74 | M | 4 | AC | MFH | - | - | unknown | +4 |
| Yamazaki [6] | Japanese | 90 | M | 18 | AC | Small round cells; | - | Left adrenal gland; Liver; | Left neck; | +1 |
| Undifferentiated short cells of spindle shaped | Lung; Peritoneal membrane | Hepatic hilum | ||||||||
| Barkatullah [7] | White | 67 | F | 2.5 | AC | High grade spindle cell | - | - | - | +9 |
| Nakano [8] | Japanese | 82 | F | 18 | AC | Polygonal or short spindle shaped cells | - | - | unknown | +13 days* |
| Okamura [9] | Japanese | 64 | F | 3.5 | IPMC | Malignant spindle shaped and pleomorphic cells | Osteosarcoma | - | - | 12 |
| Chmiel [10] | Polish | 47 | M | unknown | AC | Leiomyosarcoma | - | unknown | unknown | unknown |
| Bloomston [11] | White | 67 | F | 4 | MCAC | Malignant spindle shaped and pleomorphic cells | - | Liver Omentum | - | +4 |
| Gelos [12] | White | 61 | F | 7 | AC | Spindle cells | - | - | Peripancreatic | +11 |
| Shen [13] | Chinese | 72 | F | 5 | AC | MFH | - | Duodenum | - | +2 |
| Kim [14] | Korea | 48 | M | 3.5 | MCAC | Similar to GCT of bone | - | Liver; Omentum | Peripancreatic | +4 |
| Oymaci [15] | Turkey | 66 | M | 3.5 | AC | MFH | - | - | Peripancreatic | +20 days& |
| Kim [16] | Korea | 77 | M | 2.2 | AC | spindle cells | - | Liver | unknown | unknown |
| Lee [17] | Korea | 24 | F | 4.7 | AC | oval to spindle-shaped cells | - | - | Peripancreatic | unknown |
| Shi [18] | Chinese | 74 | F | 5 | MCAC | Spindle cells | - | - | - | 6 |
| Mszyco [19] | White | 85 | M | 12 | AC | Spindle cells | - | Liver | unknown | 2 |
| Wu [20]** | Chinese | 66 | F | 26 | MCAC | Spindle cells | - | Liver; Omentum | unknown | +2 |
| Shao [21] | Chinese | 55 | F | 5 | MCAC | Spindle cells | - | - | unknown | +6 |
| Chinese | 52 | M | 6 | AC | MFH | - | - | Peripancreatic | +14 | |
| Yin [22] | Chinese | 49 | F | 8 | AC | Spindle cells | - | - | unknown | 10 |
| Zhu [23] | Chinese | 53 | F | 4 | AC | Leiomyosarcoma | - | Duodenum | Peripancreatic | 20 |
| Xiao [24] | Chinese | 74 | F | 5 | AC | Spindle cells | - | - | unknown | 1 |
| Jiang [25]** | Chinese | 74 | M | 8 | unknow | Spindle cells | - | - | unknown | unknown |
| Our case | Chinese | 44 | F | 3 | AC | Malignant spindle shaped and pleomorphic cells | Osteosarcoma | - | Peripancreatic | 48 |
Abbreviations: AC: adenocarcinoma. MCAC: mucinous cystadenocarcinoma. IPMC: intraductal papillary-mucinous carcinoma. MPNST: malignant peripheral nerve sheath tumor. MFH: malignant fibrous histiocytoma. GCT: giant cell tumor. Max Size: maximal size. F: female. M: male. -: negative. +: Death.
Died on postoperative day 13 due to disseminated intravascular coagulation.
Due to postoperative complication.
Only histological diagnosis.
The combination of osteoclastic giant cell tumor of the pancreas (OGTP) with carcinoma was rare. Watanabe et al. [3] reported an OGTP associated with a pleomorphic-type giant cell carcinoma and an ordinary adenocarcinoma. The OGTP element was reactive for antibodies to vimentin, matrix metalloproteinase type 3, and CD68 while the pleomorphic-type giant cell carcinoma element was reactive only for epithelial markers, including cytokeratin, EMA, and etc. The presence of abundant mitochondria and lack of desmosomes in the cells from the OGTP component further emphasized the mesenchymal nature of these cells. Similarly, Fischer et al. [4] reported 2 cases in which the pancreatic neoplasms were composed of an OGTP component and a glandular component with moderate pleomorphism. However, they proved the divergent nature of these 2 components in only 1 case by immunohistochemistry.
Okamura et al. [9] reported a case of carcinosarcoma with a heterologous mesenchymal component (osteosarcoma) originating in an intra-ductal papillary-mucinous carcinoma (IPMC). It was the first report of the heterologous pancreatic tumor. Immunohistochemical and gene mutation analyses revealed that both the adenocarcinoma and osteosarcoma expressed TP53 and shared similar mutation sites of K-ras and p53 genes as well as the IPMC. Their results indicated that two different neoplastic components in the same pancreatic tumor had common origin, which was same as the IPMC. This case report provided new insight supporting that carcinosarcoma, even with a heterologous mesenchymal component, was consistent with the monoclonal hypothesis. In addition, Resch et al. [26] reported the first case of an extraskeletal osteosarcomas (ESOS) arising from the pancreas. The patient was a 64-year-old man with an approximately 8 cm partially cystic and partially solid mass in the head of the pancreas.
Although our case was the third reported osteosarcoma occurring in the pancreas in literature, it was unique in genotype from the other 2 cases above. Immunohistochemical results had confirmed that the three histological components with different phenotypes respectively which were epithelial, mesenchymal, and osteosarcoma origin. The differential diagnosis of our case included sarcomatoid adenocarcinoma, metastatic osteosarcomas, and undifferentiated carcinoma with osteoclast-like giant cells. The immunohistochemical results argued against a sarcomatoid carcinoma. Extensive clinical investigation of the patient, including PET-CT (positron emission tomography computerized tomography) scan, ruled out the possibility of metastatic osteosarcomas remotely from other organ. The typical histology morphology, especially malignant osteoid production, would not see an undifferentiated carcinoma with osteoclast-like giant cells.
The exact cell of origin of carcinosarcoma remains unknown. Two possible tumorigenic mechanisms were proposed. One involves 2 separate and independent clones, proliferating and merging as a single tumor. The second tumorigenic mechanism involves a single cell of origin with subsequent diversion and differentiation into carcinomatous and sarcomatous pathways. The second mechanism was supported by Millis [1], Nakano [8], Okamura [9], Kim [16], and van den Berg [27]. Papers from Millis, Okamura, and Kim have shown that different areas of tumor all revealed the same genetic mutations. The K-ras sequence was analyzed in samples taken from the center of the tumor (sarcoma-dominant area) and the duodenum sample was taken as control. Point mutations were detected at codon 12 and codon 34 on exon 2 in the tumor sample while no point mutation was observed in the duodenum sample. Nakano et al. speculated that because the K-ras point mutation was not observed in the normal duodenum sample, one possibility is that the codon 12 mutation induced the adenocarcinoma to develop while the mutation at codon 34 caused the additional sarcomatous change. Van den Berg et al. also demonstrated identical genetic alterations in 6 chromosomal loci in the carcinomatous and sarcomatous components in 2 of the 3 cases they studied. In their third case, 5 of 6 chromosomal loci showed identical genetic alterations in the carcinomatous and sarcomatous components. Interestingly, the analysis of gene mutation in our case showed that epithelial and mesenchymal components did not undergo K-ras and p53 mutations, only the osteosarcoma component had K-ras and p53 mutations (Table 3). The result is different from the above. The case was, so far, the only report of a multi clonal nature of pancreatic carcinosarcoma, which also verifies the first hypothesis. Therefore our data are more likely to confirm it a collision tumor, i.e., pancreatic sarcoma with ESOS.
Based on the limited literature, we’d like to summarize these cases of pancreatic carcinosarcoma. Female and male incidence ratio was 1.64:1 (18:11) and a median age of onset was 66 years old (range from 24 to 90 years old). The mean diameter of tumor was 8.5 cm (range from 2.2 cm to 30 cm). The tumor was located at pancreatic head (55.2%, 16/29) and tail. Usually the head of the pancreas were solid lesions (81.3%, 13/16) and the body and tail of the pancreas were mostly cystic lesions (69.2%, 9/13). The prognosis of carcinosarcoma of the pancreas is dismal. Patients in 17 of the 29 cases that we compiled, died of the disease with a median postoperative survival time of 6 months (range from 0.5 month to 48 month). Our patient, who has enjoyed a rare long survival time, is alive and well 48 months after surgery. Follow ups are ongoing.
Conclusion
To the best of our knowledge, this case was the first case reported to date of pancreatic adenocarcinoma combined with sarcoma and osteosarcoma in the same tumor. Although, in the recent classification of pancreatic tumors, carcinosarcoma has been replaced by the terminology of undifferentiated carcinoma. However, carcinosarcoma might really exit as an entity regarding this case. Furthermore, the case was a unique pancreatic tumor composed of carcinosarcoma and ESOS. The morphological, immunohistochemical, and genetic findings suggest that the pancreatic tumor was of multiple clonal origins.
Acknowledgements
The authors would like to thank Dr. Dengfeng Cao and Dr. Li Jiang for their constructive comments on this work.
Disclosure of conflict of interest
None.
References
- 1.Millis JM, Chang B, Zinner MJ, Barsky SH. Malignant mixed tumor (carcinosarcoma) of the pancreas: a case report supporting organinduced differentiation of malignancy. Surgery. 1994;115:132–137. [PubMed] [Google Scholar]
- 2.Wenig BM, Albores-Saavedra J, Buetow PC, Heffess CS. Pancreatic mucinous cystic neoplasm with sarcomatous stroma: a report of three cases. Am J Surg Pathol. 1997;21:70–80. doi: 10.1097/00000478-199701000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe M, Miura H, Inoue H, Uzuki M, Noda Y, Fujita N, Yamazaki T, Sawai T. Mixed osteoclastic/pleomorphic-type giant cell tumor of the pancreas with ductal adenocarcinoma: histochemical and immunohistochemical study with review of the literature. Pancreas. 1997;15:201–208. doi: 10.1097/00006676-199708000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Fischer HP, Altmannsberger M, Kracht J. Osteoclastic-type giant cell tumor of the pancreas. Virchows Archiv A Pathol Anat Histopathol. 1988;412:247–253. doi: 10.1007/BF00737149. [DOI] [PubMed] [Google Scholar]
- 5.Darvishian F, Sullivan J, Teichberg S, Basham K. Carcinosarcoma of the pancreas: a case report and review of the literature. Arch Pathol Lab Med. 2002;126:1114–1117. doi: 10.5858/2002-126-1114-COTP. [DOI] [PubMed] [Google Scholar]
- 6.Yamazaki K. A unique pancreatic ductal adenocarcinoma with carcinosarcomatous histology, immunohistochemical distribution of hCGbeta, and the elevation of serum alpha-fetoprotein. J Submicrosc Cytol Pathol. 2003;35:343–349. [PubMed] [Google Scholar]
- 7.Barkatullah SA, Deziel DJ, Jakate SM, Kluskens L, Komanduri S. Pancreatic carcinosarcoma with unique triphasic histological pattern. Pancreas. 2005;31:291–292. doi: 10.1097/01.mpa.0000178283.96276.8a. [DOI] [PubMed] [Google Scholar]
- 8.Nakano T, Sonobe H, Usui T, Yamanaka K, Ishizuka T, Nishimura E, Hanazaki K. Immunohistochemistry and K-ras sequence of pancreatic carcinosarcoma. Pathol Int. 2008;58:672–677. doi: 10.1111/j.1440-1827.2008.02289.x. [DOI] [PubMed] [Google Scholar]
- 9.Okamura J, Sekine S, Nara S, Ojima H, Shimada K, Kanai Y, Hiraoka N. Intraductal carcinosarcoma with a heterologous mesenchymal component originating in intraductal papillary-mucinous carcinoma (IPMC) of the pancreas with both carcinoma and osteosarcoma cells arising from IPMC cells. J Clin Pathol. 2010;63:266–269. doi: 10.1136/jcp.2009.071613. [DOI] [PubMed] [Google Scholar]
- 10.Chmiel B, Wodołazski A, Kozaczka A. Carcinosarcoma of the pancreas-case report and literature review. Wiad Lek. 2005;58:243–246. [PubMed] [Google Scholar]
- 11.Bloomston M, Chanona-Vilchis J, Ellison EC, Ramirez NC, Frankel WL. Carcinosarcoma of the pancreas arising in a mucinous cystic neoplasm. Am Surg. 2006;72:351–355. [PubMed] [Google Scholar]
- 12.Gelos M, Behringer D, Philippou S, Mann B. Pancreatic carcinosarcoma. Case report of multimodal therapy and review of the literature. JOP. 2008;9:50–55. [PubMed] [Google Scholar]
- 13.Shen ZL, Wang S, Ye YJ, Wang YL, Sun KK, Yang XD, Jiang KW. Carcinosarcoma of pancreas with liver metastasis combined with gastrointestinal stromal tumour of the stomach: is there a good prognosis with the complete resection? Eur J Cancer Care (Engl) 2010;19:118–123. doi: 10.1111/j.1365-2354.2008.00977.x. [DOI] [PubMed] [Google Scholar]
- 14.Kim HS, Joo SH, Yang DM, Lee SH, Choi SH, Lim SJ. Carcinosarcoma of the pancreas: a unique case with emphasis on metaplastic transformation and the presence of undifferentiated pleomorphic high-grade sarcoma. J Gastrointestin Liver Dis. 2011;20:197–200. [PubMed] [Google Scholar]
- 15.Oymaci E, Argon A, Coşkun A, Uçar AD, Carti E, Erkan N, Yildirim M. Pancreatic carcinosarcoma: case report of a rare type of pancreatic neoplasia. JOP. 2013;14:212–215. doi: 10.6092/1590-8577/1309. [DOI] [PubMed] [Google Scholar]
- 16.Kim HS, Kim JI, Jeong M, Seo JH, Kim IK, Cheung DY, Kim TJ, Kang CS. Pancreatic adenocarcinosarcoma of monoclonal origin: a case report. World J Gastroenterol. 2014;20:12682–12686. doi: 10.3748/wjg.v20.i35.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J, Hyun JJ, Lee HS. A rare cause of abdominal pain by pancreatic mass in a young female patient. Carcinosarcoma of the pancreas. Gastroenterology. 2015;149:e3–5. doi: 10.1053/j.gastro.2014.12.051. [DOI] [PubMed] [Google Scholar]
- 18.Shi HY, Xie J, Miao F. Pancreatic carcinosarcoma: first literature report on computed tomography imaging. World J Gastroenterol. 2015;21:1357–1361. doi: 10.3748/wjg.v21.i4.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mszyco S, Teng L, Annunziata J, Hartman MS. Pancreatic carcinosarcoma: a case report highlighting computed tomography characteristics. Curr Probl Diagn Radiol. 2017;46:342–345. doi: 10.1067/j.cpradiol.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Wu SJ, Lei SX. A case of huge pancreatic carcinosarcoma. Beijing Med J (Chinese) 2000;22:251. [Google Scholar]
- 21.Shao CH, Hu XG, Gao L, Tang Y, Liu R, Zhang YJ, Hu ZH, Jin J. Clinicopathologic analysis of pancreatic carcinosarcoma. Chin J Pancreatol (Chinese) 2005;5:21–23. [Google Scholar]
- 22.Yin XP, Li CY, Zhang D, Feng HL. A case of pancreatic carcinosarcoma. J Clin Radiol (Chinese) 2008;27:1612. [Google Scholar]
- 23.Zhu WY, Liu TG, Zhu H. Long-term recurrencefree survival in a patient with pancreatic carcinosarcoma: a case report with a literature review. Med Oncol. 2012;29:140–143. doi: 10.1007/s12032-010-9804-9. [DOI] [PubMed] [Google Scholar]
- 24.Xiao SJ, Zhang J, Qin LL. Pancreatic carcinosarcoma: a clinicopathologic analysis. J Diag Pathol (Chinese) 2013;20:760–762. [Google Scholar]
- 25.Jiang TT, Liu XH, Gu YJ, Peng WJ. A case of pancreatic carcinosarcoma. Oncoradiology (Chinese) 2014;23:50–51. [Google Scholar]
- 26.Resch TR, Hwang SS, Norris CE, Helmer SD, Osborne DL. Extraskeletal osteosarcoma of the pancreatic head. Am Surg. 2013;79:E281–283. [PubMed] [Google Scholar]
- 27.van den Berg W, Tascilar M, Offerhaus GJ, Albores-Saavedra J, Wenig BM, Hruban RH, Gabrielson E. Pancreatic mucinous cystic neoplasms with sarcomatous stroma: molecular evidence for monoclonal origin with subsequent divergence of the epithelial and sarcomatous components. Mod Pathol. 2000;13:86–91. doi: 10.1038/modpathol.3880013. [DOI] [PubMed] [Google Scholar]


