Abstract
Background: The zinc-finger transcription factor Sal-like protein 4 (SALL4) plays a pivotal role in tumor invasion and metastasis. Here, we investigated the clinicopathological significance of SALL4 overexpression in cervical squamous cell carcinoma (SCC) of the uterine cervix. Methods: SALL4 immunohistochemical staining was performed on cervical SCC specimens from 129 patients, as well as 98 cases of cervical squamous intraepithelial lesion (LSIL and HSIL) and 29 normal cervix samples. SALL4 immunofluorescence staining was performed in CaSki and SiHa cervical cancer cells. Statistical analyses were applied to evaluate correlations between SALL4 overexpression and clinicopathological features of SCC patients. Survival rates were calculated using the Kaplan-Meier method, and relationships between prognostic factors and patient survival were analyzed using Cox proportional hazard models. Results: SALL4 protein showed mainly nuclear staining in cervical cancer cells. Strong diffuse SALL4 staining was frequently seen in cervical cancer compared with normal tissues. SALL4 expression was significantly higher in cervical cancers than in LSIL, HSIL, and normal cervical epithelia. SALL4 overexpression was positively correlated with poor differentiation, late-stage, and lymph node metastasis. Moreover, the 5-year survival rate of early-stage cervical cancer patients with high SALL4 expression was significantly lower than patients with low SALL4 expression. Multivariate analysis suggested that SALL4 protein expression is an independent risk factor for survival in SCC. Conclusions: SALL4 plays an important role in SCC progression. High-level SALL4 expression is an independent prognostic factor of poor outcomes in SCC.
Keywords: SALL4, immunohistochemistry, prognosis, cervical cancer
Introduction
Cervical cancer is one of the most common cancers, ranking third amongst all gynecological cancers [1]. Annually, cervical cancer has been estimated to affect 500,000 women and cause 270,000 deaths worldwide [2]. Epidemiological studies have indicated that diverse risk factors are relevant to cervical tumorigenesis, including human papilloma virus (HPV) infection, smoking, and sexual behavior [3]. High-quality screening with cytology has markedly reduced the mortality of cervical squamous cell carcinoma (SCC), which is the most frequent type of neoplasia involving the cervix [4,5]. Surgery combined with adjuvant chemotherapy and radiotherapy are the main methods for curing this disease. Despite numerous achievements in cervical cancer treatment, it still has poor prognoses and high mortality rates following lymph node or distant organ metastasis. Thus, understanding the molecular events and mechanisms underlying tumor initiation and progression could contribute to early detection, which would facilitate cervical cancer prevention and treatment.
Sal-like protein 4 (SALL4) is a member of the SALL gene family. SALL4 is a zinc-finger transcription factor that plays an essential role in early embryonic development [6]. SALL4 was originally identified as a homeotic gene essential for head and tail determination in Drosophila and encodes a protein characterized by multiple double C2H2 zinc-finger motifs [7,8]. SALL4 maintains pluripotency-related transcription by regulating stem cell markers and interacts with NANOG, OCT4, and SOX2, which are essential for the induction and maintenance of pluripotency and renewal in embryonic stem cells [9]. SALL4 is also involved in the maintenance, proliferation/stabilization and cell fate decision of human adult stem cells [10]. SALL4 is involved in normal development, as well as tumorigenesis. Recent studies have revealed that SALL4 is the key transcriptional regulator in normal human hematopoiesis, and its expression correlates with disease progression in human myelodysplastic syndromes and acute myeloid leukemia [11-13], indicating that SALL4 plays a major role in leukemogenesis. SALL4 is expressed in germ cells and is an excellent marker for malignant germ cell tumors, in that most of these tumors (seminomas, embryonal carcinomas and yolk sac tumors) are strongly and uniformly positive for SALL4 [14]. Additionally, SALL4 overexpression has been observed in different non germ-cell carcinomas, such as carcinomas of the endometrium [15], ovary [16], lung [17], and digestive system [18-24]. Together, these studies indicate that SALL4 is a novel stem cell marker that is involved in embryogenesis, organogenesis and carcinogenesis.
The level of SALL4 protein expression and its correlation with the clinicopathological characteristics of SCC and squamous intraepithelial lesions (low-grade intraepithelial lesions (LSILs) and high-grade intraepithelial lesions (HSILs)) have not been adequately addressed. Herein, we investigated the clinicopathological significance of SALL4 protein overexpression in cervical SCC and demonstrate its prognostic value for SCC patients.
Materials and methods
Clinical samples
Routinely processed and diagnosed uterine cervical lesions, including 29 non-neoplastic cervical tissues, 32 LISL tissues (CIN-1), 66 HSIL tissues (CIN-2, n=35; CIN-3, n=31) and 129 SCC tissues, were collected from the Department of Pathology, the First Hospital of Zibo for SALL4 immunohistochemistry (IHC). All cervical tissue specimens were obtained from punch biopsy, LEEP or hysterectomy, and the 29 non-neoplastic cervical tissues were obtained from leiomyoma of uterus patients who underwent hysterectomy. The cancer patients were 31-79-years-old. All SCC tumor specimens were obtained from pretreatment surgical resections, and clinical data were retrieved from the patients’ operative and pathological reports, while follow-up data were obtained by phone and from the outpatient clinical database. Staging was performed according to the TNM and FIGO classification of carcinomas of the uterine cervix; 77 tumors were FIGO stage I-IIA (early-stage) and 52 were stage IIB-IV (advanced-stage) according to the International Union against Cancer (UICC) criteria 7th Edition and WHO classification [25,26]. All 129 SCC patients had follow-up records for more than 5 years, and the follow-up deadline was December 2015. Survival time was counted from the date of surgery to the follow-up deadline or date of death (usually the result of cancer recurrence or metastasis). Two experienced pathologists reviewed the hematoxylin and eosin-stained slides.
IHC
Tissue sections (4-μm thick) were deparaffinized in xylene and rehydrated. Antigen retrieval was performed at 95°C for 20 min by placing the slides in EDTA buffer, cooling to 30°C and washing with phosphate-buffered saline (PBS). Slides were incubated with primary antibodies for SALL4 (6E3, Zhongshan Goldenbridge Biotechnology, Beijing, China) at 4°C overnight and washed with PBS. After incubation with biotinylated secondary antibodies at room temperature for 30 min, the sections were incubated with diaminobenzidine and counterstained with hematoxylin. Appropriate negative and positive controls were added in each run.
All tissue sections were analyzed and scored independently by two investigators (Zhou P & Di C) that had no knowledge of clinical data. In cases of discrepancy, a final score was mutually agreed upon by reassessment using a double-headed microscope. SALL4 expression was semi-quantitatively analyzed and scored as ‘-’ (negative, no or <5% positive cells), ‘+’ (5%-25% positive cells), ‘++’ (26%-50% positive cells), and ‘+++’ (>50% positive cells). Strong nuclear SALL4 staining was regarded as positive staining, and ‘++’ or ‘+++’ scored samples were considered strongly positive. For survival analyses, ‘++’ or ‘+++’ scored samples were considered high-level SALL4 expression, while ‘-’ or ‘+’ scored samples were considered low-level.
Immunofluorescence (IF)
CaSki and SiHa cells were grown on coverslips to 70% confluence, fixed with 4% paraformaldehyde for 10 min and permeabilized with 0.5% TritonX-100 for 10 min. Blocking was performed with 3% Albumin Bovine V (Solarbio, Beijing, China) for 1 h at room temperature. After washing with PBS, cells were incubated with anti-rabbit SALL4 (Zhongshan Golden Bridge Biotechnology, Beijing, China) at 4°C overnight, followed by incubation with Alexa Fluor®488 goat anti-rabbit IgG (H+C) (1:1000, Invitrogen, Carlsbad, CA, USA) for 1 h at room temperature. After washing with PBS, the cells were counterstained with 49-6-diamidino-2-phenylindole (DAPI) (Beyotime, Haimen, China), and the coverslips were mounted with Antifade Mounting Medium (Beyotime). IF signals were visualized and recorded using a Leica SP5II confocal microscope (Leica, Wetzlar, Germany).
Statistical analysis
All analyses were performed using SPSS 17.0 statistical package (SPSS Inc., Chicago, IL, USA). The univariate association of SALL4 protein expression and clinicopathological parameters was analyzed using the Chi-square test. Survival curves were calculated using the Kaplan-Meier method, and differences were analyzed using the log-rank test. Multivariate survival analysis was performed on all significant characteristics measured using univariate survival analysis through the Cox proportional hazard regression model. A probability value less than 0.05 was considered statistically significant.
Results
SALL4 protein expression in cervical lesions
We first performed IF to determine the subcellular localization of SALL4 protein in CaSki and SiHa SCC cells. The results reveal that SALL4 protein is primarily nuclear localized (Figure 1). By IHC, we found positive SALL4 protein expression in only 17.2% (5/29) of normal cervical tissues, but higher rates in LSIL (CIN-1, 65.6%), HSIL (CIN-2, 71.4%; CIN-3, 74.2%) and SCC (79.1%, 102/129) (P<0.01). Moreover, high-level SALL4 protein expression was observed in significantly more SCC cases (59.7%, 77/129) than normal cervical epithelia (0%, 0/29), LSIL (CIN1, 12.5%), and HSIL (CIN2, 14.3%; CIN3, 22.6%) (Figure 2; Table 1).
Figure 1.
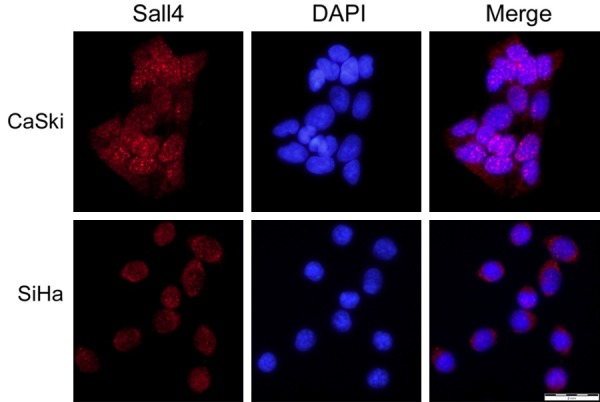
Immunofluorescence staining of SALL4 protein in CaSki and SiHa cervical SCC cells. Red indicates SALL4 staining, blue indicates DAPI.
Figure 2.
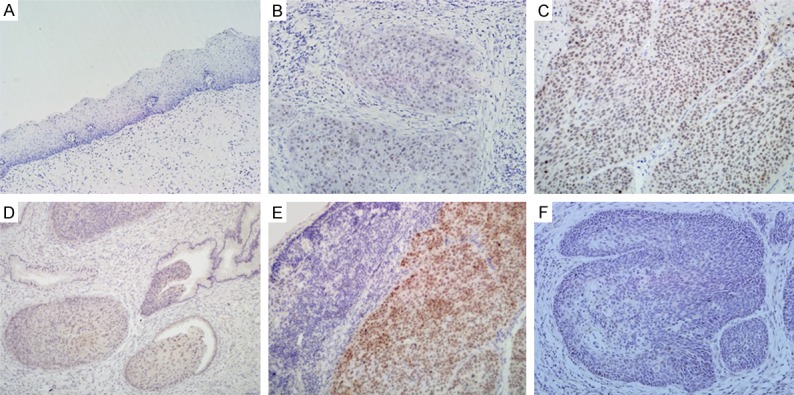
SALL4 protein expression and localization in uterine cervical lesions. (A) Sall4 protein was not expressed in normal cervical squamous epithelia. (B) Sall4 protein was weakly positive in cervical SCC. (C) Sall4 protein was strongly expressed in the nucleus of cervical SCC. (D) SALL4 was expressed in the atypical cells of CIN-3. (E) Sall4 positive staining was observed in the nodal metastasis of cervical SCC. (F) Negative SALL4 protein staining in cervical SCC without metastasis. (original magnification, 200× in A-F).
Table 1.
SALL4 protein expression in cervical lesions
| Diagnosis | Case n. | Sall4 | Positive rate (%) | Strongly positive rate (%) | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| - | + | ++ | +++ | ||||
| Normal cervix | 29 | 24 | 5 | 0 | 0 | 17.2% | 0 |
| LSIL (CIN-1) | 32 | 11 | 17 | 4 | 0 | 65.6%** | 12.5% |
| HSIL (CIN-2) | 35 | 10 | 20 | 5 | 0 | 71.4%** | 14.3% |
| HSIL (CIN-3) | 31 | 8 | 16 | 7 | 0 | 74.2%** | 22.6% |
| SCC | 129 | 27 | 25 | 49 | 28 | 79.1%** | 59.7% |
LSIL: low-grade intraepithelial lesion; HSIL: high-grade intraepithelial lesion; CIN: cervical intraepithelial neoplasia; SCC: squamous cell carcinoma.
P<0.01 compared with the normal cervix.
Association between SALL4 overexpression and clinicopathological features of cervical SCCs
SALL4 overexpression was significantly correlated with differentiation stage and lymph node metastasis. High-level SALL4 protein expression was more frequently found in poorly (74.3%, 26/35) and moderately (69.6%, 39/56) differentiated cervical SCC compared with well differentiated cervical SCC (31.6%, 12/38) (P<0.05). SALL4 protein expression was significantly higher in metastatic cervical SCC (80.3%, 57/71) than in non-metastatic cases (34.5%, 20/58) (P<0.01). High-level SALL4 protein expression was observed in significantly more advanced-stage cervical SCC (IIV-IV) patients (88.5%, 46/52) compared with early-stage patients (I-II) (40.3%, 31/77) (P<0.01). However, no statistical difference was found among keratinizing (56.3%, 27/48) and non-keratinizing (61.7%, 50/81) cervical SCC (P>0.05) (Table 2; Figure 3).
Table 2.
Sall4 overexpression and clinicopathological features of cervical cancers
| Clinical features | Case n. | Strongly positive cases n. (%) | P value |
|---|---|---|---|
| Histological types | 129 | ||
| Keratinizing | 48 | 27 (56.3%) | NS |
| Non-keratinizing | 81 | 50 (61.7%) | |
| Differentiation | 129 | ||
| Poorly diff. | 35 | 26 (74.3%)* | <0.05a |
| Moderately diff. | 56 | 39 (69.6%)* | |
| Well diff. | 38 | 12 (31.6%) | |
| Staging | 129 | ||
| Early (I-IIA) | 77 | 31 (40.3%) | <0.01b |
| Late (IIB-IV) | 52 | 46 (88.5%)** | |
| LN Metastasis | 129 | ||
| Positive | 71 | 57 (80.3%)** | <0.01 |
| Negative | 58 | 20 (34.5%) |
Diff.: differentiation; NS: not significant.
P<0.05;
P<0.01.
Poorly and moderately differentiated vs. well differentiated;
Early stage (Stage-IA + Stage-IB + Stage IIA) vs. Late stage (Stage-IIB + Stage-III + Stage-IV).
Figure 3.
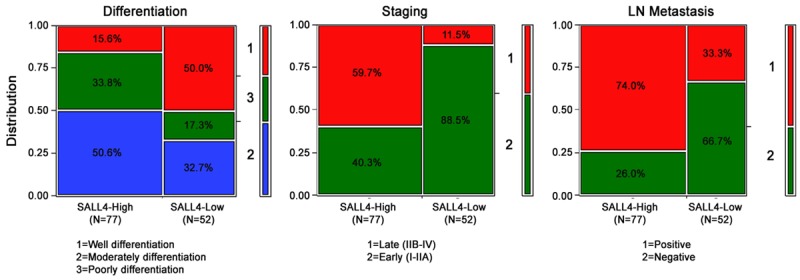
Relationship between SALL4 overexpression and clinicopathological features of cervical cancer.
SALL4 expression is an independent prognostic factor for SCC
All 129 SCC patients with complete survival data were identified for prognostic evaluation. Among them, 68 (52.7%) died from SCC, while 61 (47.3%) remained alive. We analyzed the prognostic value of SALL4 expression in selective patient subgroups stratified according to pTNM. Survival analyses demonstrated that high-level SALL4 expression was associated with a lower 5-year survival rate for early-stage (I-II) patients (log-rank P<0.001), but there was no association in advanced-stage (III-IV) patients (data not shown, log-rank P=0.071) (Figure 4), indicating that SALL4 protein expression might be a useful biomarker for the prognostic evaluation of cervical SCC.
Figure 4.
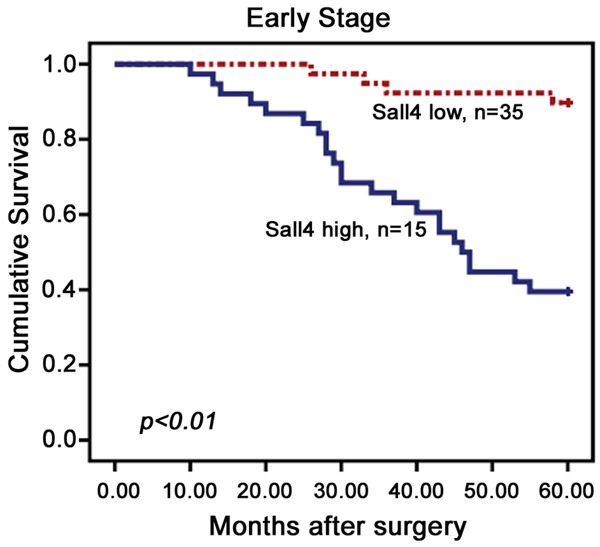
Kaplan-Meier survival curves illustrating the significance of SALL4 expression in uterine cervical cancers. In early-stage (n=50) patients with high SALL4 expression had significantly reduced 5-year survival rates relative to those with low SALL4 expression (P<0.001).
Additionally, differentiation, FIGO stage, LN metastasis, and SALL4 expression were also associated with the 5-year survival rate of cervical SCC patients with high SALL4 expression by univariate analysis (Table 3). These data suggest that SALL4 could also be a valuable prognostic factor for cervical SCC. Therefore, further multivariate analysis was performed using the Cox proportional hazards model for all significant variables from the univariate analysis. We found that FIGO stage (95% confidence interval (CI): 1.107-2.419; P=0.014) and LN metastasis (95% CI: 1.050-2.295; P=0.027) were independent prognostic factors for survival in cervical SCC. Importantly, SALL4 overexpression also emerged as a significant independent prognostic factor for cervical SCC (95% CI: 1.101-2.444; P=0.015) (Table 3).
Table 3.
Univariate and multivariate analyses of clinicopathological factors used to determine overall survival rates for 129 patients with cervical SCC
| Factors | B | SE | Wald | Exp(B) | 95% CI for Exp(B) | P value | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lower Upper | |||||||
| Univariate analysis | |||||||
| Histological types | 0.131 | 0.182 | 0.519 | 1.140 | 0.798 | 1.630 | 0.471 |
| Differentiation | 0.589 | 0.195 | 9.137 | 1.802 | 1.230 | 2.640 | 0.003** |
| Staging | 0.730 | 0.183 | 15.896 | 2.076 | 1.450 | 2.973 | 0.000** |
| LN Metastasis | 0.761 | 0.181 | 17.699 | 2.141 | 1.502 | 3.053 | 0.000** |
| SALL4 | 0.790 | 0.184 | 18.474 | 2.204 | 1.537 | 3.159 | 0.000** |
| Multivariate analysis | |||||||
| Differentiation | 0.349 | 0.210 | 2.759 | 1.418 | 0.939 | 2.141 | 0.097 |
| Staging | 0.493 | 0.199 | 6.102 | 1.636 | 1.107 | 2.419 | 0.014* |
| LN Metastasis | 0.440 | 0.199 | 4.860 | 1.552 | 1.050 | 2.295 | 0.027* |
| SALL4 expression | 0.495 | 0.203 | 5.926 | 1.641 | 1.101 | 2.444 | 0.015* |
P<0.05;
P<0.01.
Discussion
SALL4 was first isolated from Drosophila and encodes a protein characterized by multiple double C2H2 zinc-finger motifs [8]. In humans, SALL4 is involved in normal development, as well as tumorigenesis. There is also research indicating that SALL4 is involved in hematopoiesis and leukemogenesis, while other studies have demonstrated that abnormal SALL4 expression is tightly linked with acute myeloid leukemia [13,25]. SALL4 is also dysregulated and aberrantly expressed in several cancers. In germ cell tumors, such as seminomas, embryonal carcinomas and yolk sac tumors, practically uniform high-level nuclear SALL4 expression has been observed by tissue IHC, but this expression pattern is less frequently observed in teratomas and clear cell carcinomas of the ovary and endometrium [15,19,26].
In line with these findings, SALL4 protein was highly expressed and positively associated with advanced FIGO stage, high histological grade, lymph node involvement, distant metastasis, and recurrence in serous ovarian carcinomas [16]. Survival analyses showed that high-level SALL4 expression is associated with poorer overall and progression-free survival. Moreover, univariate and multivariate analyses showed that SALL4 is an independent prognostic marker for cervical SCC.
Li et al. [15] found that SALL4 was aberrantly expressed in primary human endometrial cancer tissues compared with normal or hyperplastic endometrial tissues. More importantly, SALL4 expression was negatively correlated with patient survival and positively associated with aggressive features such as metastasis in endometrial carcinoma. Furthermore, downregulation of SALL4 significantly inhibited migration and invasion properties of endometrial cancer cells in vitro, as well as their metastatic potential in vivo. Moreover, manipulating SALL4 expression affected the carboplatin sensitivity of endometrial cancer cells.
High-level SALL4 expression has been observed in human liver cancers [23], including hepatocellular carcinomas (HCCs), cholangiocarcinomas (CCs), combined hepatocellular and CCs, and fibrolamellar HCCs. Bioinformatics analyses have indicated that elevated SALL4 expression in HCC patients is associated with poor survival. Yin et al. [24] and Deng et al. [27] also reported high-level SALL4 overexpression in HCC and CC tissue specimens, respectively. Importantly, patients with higher SALL4 expression levels had worse prognoses.
Zhang et al. demonstrated that aberrant SALL4 expression was highly correlated with lymph node metastasis, and that SALL4 plays oncogenic roles in gastric cancer by modulating EMT and cell stemness [20]. Furthermore, SALL4 has been used as a marker for AFP-producing gastric carcinoma [28]. SALL4 expression is significantly higher in hepatoid adenocarcinoma than in non-hepatoid adenocarcinoma, and the combination of PLUNC, HepPar-1, and SALL4 could be a reliable prognostic indicator for gastric hepatoid adenocarcinoma. The study by Takashi et al. suggested that SALL4 is useful for diagnosing a series of gastric carcinoma variants with retro differentiation such as gastric adenocarcinoma with enteroblastic differentiation [29].
Additionally, SALL4 has been reported to be abnormally expressed in liver cancer [23,24], lung cancer [17,30], breast cancer [31], and esophageal SCC [18,19,32], indicating a direct correlation between SALL4 protein expression and the invasiveness/metastasis potential of these diseases. However, more comprehensive and systematic studies on the functional role of SALL4 in human cervical cancer are needed.
In this study, we performed IHC to analyze SALL4 protein expression in 129 cervical SCC samples, 98 precancerous samples and 9 non-neoplastic cervical tissues. Our data reveal that SALL4 protein expression is significantly higher in SCC cases, and that higher SALL4 expression is positively correlated with poor differentiation, tumor stage, and lymph node metastasis. We also found that early-stage (I-II) SCC patients with high-level SALL4 expression had lower 5-year survival rates. Univariate and multivariate analyses both showed that SALL4 overexpression is a significant independent prognostic factor in SCC. These results indicated that SALL4 is a potential predictor of poor prognosis, similar to studies focused on other solid tumors [15,16]. However, a recent study by Hao et al. [22] showed that SALL4 expression is significantly lower in colorectal cancer than in atypical hyperplasia and normal colorectal tissue. Furthermore, well-differentiated colorectal cancers expressed higher SALL4 levels than moderately- and poorly-differentiated cancers. However, SALL4 expression positively correlated with lymph node metastasis and TNM and Dukes stages. Additionally, Kilic et al. demonstrated that SALL4 expression was not found in breast tumors, serous ovarian carcinomas, or ovarian carcinomas of endometrioid or mucinous type. SCCs have been mostly negative for SALL4, with the exceptions of lung, esophagus, and anus carcinomas. In cervical cancers, adenocarcinomas of the cervix showed SALL4 positivity in 2.08% of the cases, and all showed weak nuclear staining, while SCCs and adenosquamous carcinomas were all negative for SALL4 [19]. We reviewed the study and findings, and speculate that since the previous study did not contain clinicopathological information of cases, FIGO stage, or histological grade, and a sampling error may be possible.
In conclusion, our results suggest that SALL4 is significantly overexpressed in cervical SCC tissues, and that staining intensity correlates with adverse SCC progression and prognostic markers. However, the molecular mechanisms of SALL4 that are responsible for cervical tumor progression remain to be elucidated, and additional studies are warranted regarding the role of SALL4 in cervical cancer progression.
Acknowledgements
This study was funded by Medical and Health Science Technology Development Plan of Shandong (grant number 2016WS0738).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Khenchouche A, Sadouki N, Boudriche A, Houali K, Graba A, Ooka T, Bouguermouh A. Human papillomavirus and Epstein-Barr virus co-infection in cervical carcinoma in Algerian women. Virol J. 2013;10:340. doi: 10.1186/1743-422X-10-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acladious NN, Sutton C, Mandal D, Hopkins R, Zaklama M, Kitchener H. Persistent human papillomavirus infection and smoking increase risk of failure of treatment of cervical intraepithelial neoplasia (CIN) Int J Cancer. 2002;98:435–439. doi: 10.1002/ijc.10080. [DOI] [PubMed] [Google Scholar]
- 4.Gustafsson L, Ponten J, Bergstrom R, Adami HO. International incidence rates of invasive cervical cancer before cytological screening. Int J Cancer. 1997;71:159–165. doi: 10.1002/(sici)1097-0215(19970410)71:2<159::aid-ijc6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Gustafsson L, Ponten J, Zack M, Adami HO. International incidence rates of invasive cervical cancer after introduction of cytological screening. Cancer Causes Control. 1997;8:755–763. doi: 10.1023/a:1018435522475. [DOI] [PubMed] [Google Scholar]
- 6.Sweetman D, Munsterberg A. The vertebrate spalt genes in development and disease. Dev Biol. 2006;293:285–293. doi: 10.1016/j.ydbio.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Jurgens G. Head and tail development of the Drosophila embryo involves spalt, a novel homeotic gene. EMBO J. 1988;7:189–196. doi: 10.1002/j.1460-2075.1988.tb02799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakaki-Yumoto M, Kobayashi C, Sato A, Fujimura S, Matsumoto Y, Takasato M, Kodama T, Aburatani H, Asashima M, Yoshida N, Nishinakamura R. The murine homolog of SALL4, a causative gene in Okihiro syndrome, is essential for embryonic stem cell proliferation, and cooperates with Sall1 in anorectal, heart, brain and kidney development. Development. 2006;133:3005–3013. doi: 10.1242/dev.02457. [DOI] [PubMed] [Google Scholar]
- 9.Yang J, Chai L, Fowles TC, Alipio Z, Xu D, Fink LM, Ward DC, Ma Y. Genome-wide analysis reveals Sall4 to be a major regulator of pluripotency in murine-embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105:19756–19761. doi: 10.1073/pnas.0809321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J, Gao C, Chai L, Ma Y. A novel SALL4/OCT4 transcriptional feedback network for pluripotency of embryonic stem cells. PLoS One. 2010;5:e10766. doi: 10.1371/journal.pone.0010766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao C, Kong NR, Li A, Tatetu H, Ueno S, Yang Y, He J, Yang J, Ma Y, Kao GS, Tenen DG, Chai L. SALL4 is a key transcription regulator in normal human hematopoiesis. Transfusion. 2013;53:1037–1049. doi: 10.1111/j.1537-2995.2012.03888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang F, Guo Y, Chen Q, Yang Z, Ning N, Zhang Y, Xu Y, Xu X, Tong C, Chai L, Cui W. Stem cell factor SALL4, a potential prognostic marker for myelodysplastic syndromes. J Hematol Oncol. 2013;6:73. doi: 10.1186/1756-8722-6-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Y, Cui W, Yang J, Qu J, Di C, Amin HM, Lai R, Ritz J, Krause DS, Chai L. SALL4, a novel oncogene, is constitutively expressed in human acute myeloid leukemia (AML) and induces AML in transgenic mice. Blood. 2006;108:2726–2735. doi: 10.1182/blood-2006-02-001594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miettinen M, Wang Z, McCue PA, Sarlomo-Rikala M, Rys J, Biernat W, Lasota J, Lee YS. SALL4 expression in germ cell and non-germ cell tumors: a systematic immunohistochemical study of 3215 cases. Am J Surg Pathol. 2014;38:410–420. doi: 10.1097/PAS.0000000000000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li A, Jiao Y, Yong KJ, Wang F, Gao C, Yan B, Srivastava S, Lim GS, Tang P, Yang H, Tenen DG, Chai L. SALL4 is a new target in endometrial cancer. Oncogene. 2015;34:63–72. doi: 10.1038/onc.2013.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang M, Xie X, Ding Y. SALL4 is a marker of poor prognosis in serous ovarian carcinoma promoting invasion and metastasis. Oncol Rep. 2016;35:1796–1806. doi: 10.3892/or.2016.4545. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi D, Kuribayashi K, Tanaka M, Watanabe N. Overexpression of SALL4 in lung cancer and its importance in cell proliferation. Oncol Rep. 2011;26:965–970. doi: 10.3892/or.2011.1374. [DOI] [PubMed] [Google Scholar]
- 18.Forghanifard MM, Ardalan Khales S, Javdani-Mallak A, Rad A, Farshchian M, Abbaszadegan MR. Stemness state regulators SALL4 and SOX2 are involved in progression and invasiveness of esophageal squamous cell carcinoma. Med Oncol. 2014;31:922. doi: 10.1007/s12032-014-0922-7. [DOI] [PubMed] [Google Scholar]
- 19.Kilic E, Tennstedt P, Hogner A, Lebok P, Sauter G, Bokemeyer C, Izbicki JR, Wilczak W. The zinc-finger transcription factor SALL4 is frequently expressed in human cancers: association with clinical outcome in squamous cell carcinoma but not in adenocarcinoma of the esophagus. Virchows Arch. 2016;468:483–492. doi: 10.1007/s00428-016-1908-y. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Xu Z, Xu X, Zhang B, Wu H, Wang M, Zhang X, Yang T, Cai J, Yan Y, Mao F, Zhu W, Shao Q, Qian H, Xu W. SALL4, a novel marker for human gastric carcinogenesis and metastasis. Oncogene. 2014;33:5491–5500. doi: 10.1038/onc.2013.495. [DOI] [PubMed] [Google Scholar]
- 21.Forghanifard MM, Moghbeli M, Raeisossadati R, Tavassoli A, Mallak AJ, Boroumand-Noughabi S, Abbaszadegan MR. Role of SALL4 in the progression and metastasis of colorectal cancer. J Biomed Sci. 2013;20:6. doi: 10.1186/1423-0127-20-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hao L, Zhao Y, Wang Z, Yin H, Zhang X, He T, Song S, Sun S, Wang B, Li Z, Su Q. Expression and clinical significance of SALL4 and beta-catenin in colorectal cancer. J Mol Histol. 2016;47:117–128. doi: 10.1007/s10735-016-9656-5. [DOI] [PubMed] [Google Scholar]
- 23.Oikawa T, Kamiya A, Zeniya M, Chikada H, Hyuck AD, Yamazaki Y, Wauthier E, Tajiri H, Miller LD, Wang XW, Reid LM, Nakauchi H. Sal-like protein 4 (SALL4), a stem cell biomarker in liver cancers. Hepatology. 2013;57:1469–1483. doi: 10.1002/hep.26159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin F, Han X, Yao SK, Wang XL, Yang HC. Importance of SALL4 in the development and prognosis of hepatocellular carcinoma. World J Gastroenterol. 2016;22:2837–2843. doi: 10.3748/wjg.v22.i9.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li A, Yang Y, Gao C, Lu J, Jeong HW, Liu BH, Tang P, Yao X, Neuberg D, Huang G, Tenen DG, Chai L. A SALL4/MLL/HOXA9 pathway in murine and human myeloid leukemogenesis. J Clin Invest. 2013;123:4195–4207. doi: 10.1172/JCI62891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nogales FF, Prat J, Schuldt M, Cruz-Viruel N, Kau B, D’Angelo E, Matias-Guiu X, Vidal A, McCluggage WG, Oosterhuis JW. Germ cell tumour growth patterns originating from clear cell carcinomas of the ovary and endometrium: a comparative immunohistochemical study favouring their origin from somatic stem cells. Histopathology. 2018;72:634–647. doi: 10.1111/his.13426. [DOI] [PubMed] [Google Scholar]
- 27.Deng G, Zhu L, Huang F, Nie W, Huang W, Xu H, Zheng S, Yi Z, Wan T. SALL4 is a novel therapeutic target in intrahepatic cholangiocarcinoma. Oncotarget. 2015;6:27416–27426. doi: 10.18632/oncotarget.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osada M, Aishima S, Hirahashi M, Takizawa N, Takahashi S, Nakamura K, Tanaka M, Maehara Y, Takayanagi R, Oda Y. Combination of hepatocellular markers is useful for prognostication in gastric hepatoid adenocarcinoma. Hum Pathol. 2014;45:1243–1250. doi: 10.1016/j.humpath.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Murakami T, Yao T, Mitomi H, Morimoto T, Ueyama H, Matsumoto K, Saito T, Osada T, Nagahara A, Watanabe S. Clinicopathologic and immunohistochemical characteristics of gastric adenocarcinoma with enteroblastic differentiation: a study of 29 cases. Gastric Cancer. 2016;19:498–507. doi: 10.1007/s10120-015-0497-9. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez E, Chen L, Ao MH, Geddes S, Gabrielson E, Askin F, Zhang H, Li QK. Expression of transcript factors SALL4 and OCT4 in a subset of non-small cell lung carcinomas (NSCLC) Transl Respir Med. 2014;2:10. doi: 10.1186/s40247-014-0010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dirican E, Akkiprik M. Functional and clinical significance of SALL4 in breast cancer. Tumour Biol. 2016;37:11701–11709. doi: 10.1007/s13277-016-5150-7. [DOI] [PubMed] [Google Scholar]
- 32.He J, Zhou M, Chen X, Yue D, Yang L, Qin G, Zhang Z, Gao Q, Wang D, Zhang C, Huang L, Wang L, Zhang B, Yu J, Zhang Y. Inhibition of SALL4 reduces tumorigenicity involving epithelial-mesenchymal transition via Wnt/beta-catenin pathway in esophageal squamous cell carcinoma. J Exp Clin Cancer Res. 2016;35:98. doi: 10.1186/s13046-016-0378-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


