Abstract
Hepatic epithelioid angiomyolipoma (EAML) is an uncommon mesenchymal tumor with malignant potential, which is clinically susceptible to being misdiagnosed as hepatocellular carcinoma. Therefore, accurate diagnosis of hepatic EAML and treatment is necessary. We report two cases of hepatic EAML that were identified by abdominal computed tomography (CT). The first case presented in a 37-year-old woman and was an oval-shaped liver mass, measuring 4.5×4.2×4.9 cm. The second case presented in a 51-year-old woman and was a round-shaped mass measuring 4×3.5×3.7 cm. Both patients underwent laparoscopic resection. Microscopically, we detected epithelioid and spindle-shaped cells with adipocytes. After the analysis of biomarkers, we found that both cases were positive for HMB45 and Melan-A, which helped to confirm the diagnosis. Hepatic EAML is a rare clinical tumor, which has a high rate of misdiagnosis and the final diagnosis depends on histopathologic and immunohistochemical features. Laparoscopic resection remains the recommended choice for hepatic EAML. Partial hepatic EAML has a tendency become malignant and thus long-term follow-up is needed.
Keywords: Hepatic epithelioid angiomyolipoma, laparoscopic resection, immunohistochemical staining
Introduction
Hepatic EAML is an uncommon mesenchymal tumor, which originated from the mesoderm. It has been reported that hepatic EAML predominantly affect women aged 30-50 years old [1]. Angiomyolipomas are grossly heterogeneous and composed of abnormal blood vessels, smooth muscle cells of different differentiation stages, and a small amount of adipose tissue. In immunohistochemistry, HMB45 and Melan-A simultaneously can be positive for diagnosis for this tumor. Hepatic EAML is often misdiagnosed as liver cancer and other malignant tumors, with a misdiagnosis rate up to 70.4% [2]. Previously, tumor resection use more open surgery, and patients suffer major trauma. In this report, we present two cases of resected hepatic EAML and discuss the characteristics of hepatic EAML.
Case report
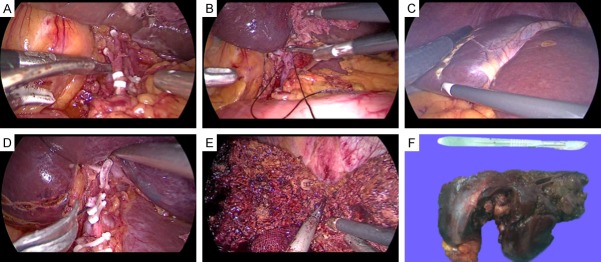
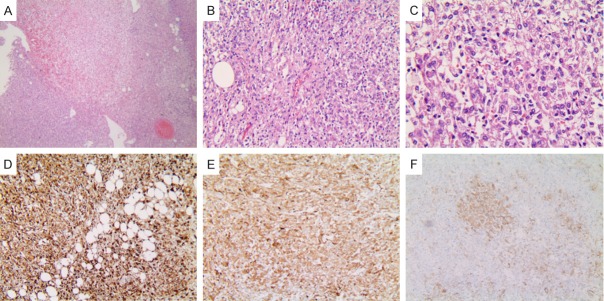
Case 1: A 37-year-old woman was referred to our hospital due to a mass in the left lateral lobe of the liver detected on ultrasonography (US) during a routine health checkup. She did not have hepatitis and denied the history of taking contraceptives. No abnormality was found in physical examination. The initial laboratory findings showed normal liver function tests (Table 1). Tumor marker tests for carbohydrate antigen 19-9, carbohydrate antigen 125, carcinoembryonic antigen, alpha-fetoprotein were all within the reference range (Table 1). Tests for hepatitis B and C virus were negative (Table 1). A computed tomography scan was performed, which showed the mass measured 4.5×4.2 cm at segment 2 and 3 of the liver (Figure 1A), was enhanced on arterial phase (Figure 1B), and washed out on portal period and delayed phase (Figure 1C), suggestive of hepatocellular carcinoma. The impression for the mass was hepatocellular carcinoma, so the patient underwent laparoscopic left lateral lobectomy of liver. At surgery, a 4.5×3.8 cm sized tumor arising from the left lateral lobe of liver with no capsule was found (Figure 2A). The mass was successfully excised (Figure 2B and 2C), and the patient’s postoperative course was uneventful. Histologically, the mass, measuring 4.5×3.8×4.7 cm, was well circumscribed with surrounding normal liver tissue (Figure 2D). Immunohistochemistry was performed using a panel of antibodies to tumor markers, which showed that the tumor cells were positive for HMB45 (Figure 4D), Melan-A (Figure 4E), smooth muscle actin (SMA) (Figure 4F) and negative for AE1/AE3, Hepatocyte, TFE3. The cell proliferation marker, Ki67 showed a cell proliferation rate of <5%. These findings confirmed a diagnosis of hepatic EAML.
Table 1.
Clinical features of the 2 patients with hepatic EAML
| Case | Patient 1 | Patient 2 |
|---|---|---|
| Age (year) | 37 | 51 |
| Gender | F | F |
| Lesion Location | left lateral lobe | left lobe |
| Lesion amount | 1 | 1 |
| Size (cm) | 4.5×4.2×4.9 | 4×3.5×3.7 |
| Viral hepatitis | N | N |
| ALT (u/l) | 8 | 39 |
| AST (u/l) | 15 | 26 |
| γ-GT (u/l) | 9 | 32 |
| ALP (u/l) | 71 | 64 |
| TBIL (μmol/l) | 5.2 | 4.8 |
| IBIL (μmol/l) | 1.9 | 1.7 |
| AFP (ng/ml) | 3.29 | 2.75 |
| CEA (ng/ml) | 1.01 | 0.76 |
| CA19-9 (U/ml) | 11.31 | 4.83 |
| CA125 (U/ml) | 15.25 | 7.72 |
F, female; N, no; ALT, alanine aminotransferase; AST, aspartate aminotransferase; γ-GT, γ-glutamyl transpeptidase; ALP, alkaline phosphatase; TBIL, total bilirubin; IBIL, indirect bilirubin; AFP, alpha fetoprotein; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; CA125, carbohydrate antigen 125.
Figure 1.
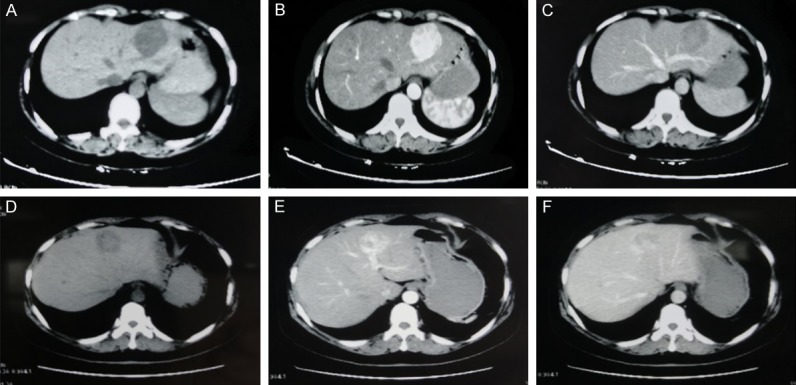
Imaging characteristics of hepatic EAML. A 37-year-old woman with hepatic EAML in left lateral lobe of liver. (patient 1, A. Non-enhanced CT scan shows hypo-attenuating lesion in segment. B. Contrast-enhanced CT scan shows obviously enhanced lesion in the arterial phase. C. The lesion is hypo-attenuating in the portal venous phase). A 51-year-old woman with hepatic EAML in left lobe of liver. (patient 2, D. Non-enhanced CT scan shows hypo-attenuating lesion in segment. E. Contrast-enhanced CT scan shows of the homogeneous enhanced lesion with thickly distorted vessels in the arterial phase. F. The lesion shows partly prolonged hyper-attenuating in the portal venous phase).
Figure 2.
A 37-year-old woman with hepatic EAML undergoing laparoscopic hepatectomy (patient 1). A. The mass in the left lateral lobe of the liver could be seen under laparoscopy. B. Resection of the left lateral liver lobe. C. Complete resection of the left lateral lobe of the liver. D. The excised section of the tumor shows a solid component, without a cystic component or apparent capsule.
Figure 4.
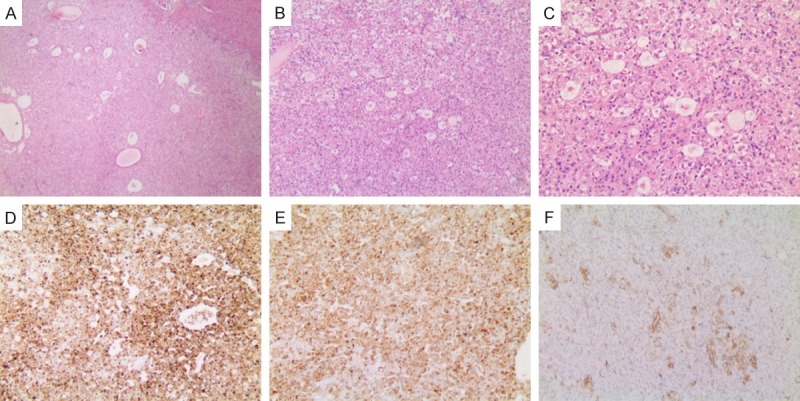
Histopathology of hepatic EAML (patient 1). (A) The tumor cells were arranged radial around blood vessels. Microscopic features signifying aggressive behavior were observed (H&E stain, ×40). (B) Eosinophilic epithelioid tumor cells can be seen. Stromal fibrosis or necrosis is not observed (H&E stain, ×100). (C) Epithelioid nuclei enlarged and nucleoli visible (H&E stain, ×200). Immunohistochemical analysis: The majority of the tumor cells were positive staining for HMB45 (D) and Melan-A (E), inconspicuous for smooth muscle α-actin (F) (original magnification ×200).
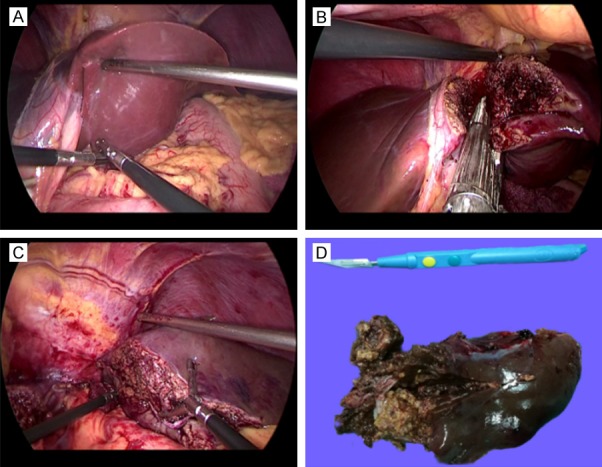
Case 2: A 51-year-old woman who had no remarkable medical history had an abdominal sonography at local hospital for regular checkup. The scan revealed a 4.6×4 cm sized mass in the left hepatic lobe. On attending at the hospital, the patient denied having a history of hepatitis and taking contraceptives. No abnormality was found in physical examination. She was advised to have a CT scan of the abdomen, which showed a well-defined, round mass in the left hepatic lobe measuring 4×3.5×3.7 cm (Figure 1D). After contrast administration, on arterial phase, there was significant enhancement on CT in the most areas of the tumor (Figure 1E). On portal period, there was no clear enhancement observed in most areas of the tumor and still some slight enhancement in small areas of the tumor (Figure 1F). Laboratory findings, including liver function tests, tumor marker tests (CA19-9, CA125, CEA, AFP), and tests for hepatitis B and C virus were normal (Table 1). The clinical diagnosis was a liver occupying lesion. The patient underwent laparoscopic left lobectomy of liver (Figure 3A-E). The mass was successfully excised, and the patient’s postoperative course was uneventful. Immunohistochemical staining for HMB45 (Figure 5D), MelanA (Figure 5E), and SMA (Figure 5F) showed diffuse strong positive staining and Ki67 showed a cell proliferation rate of <5%, supporting a diagnosis of hepatic EAML.
Figure 3.
A 51-year-old woman with hepatic EAML undergoing laparoscopic hepatectomy (patient 2). A. Occlusion of left hepatic artery and branches. B. Ligation of left branch of portal vein. C. The margin line between left and right liver appears. Intraoperative ultrasound confirmed the location of the tumor and the middle hepatic vein, the range of liver resection was marked. D. Transection of the left branch of portal vein. E. Resection of the left liver lobe. F. The cut surface shows a spherical, well demarcated tumor consisting of yellowish-brown tissue and areas of hemorrhage.
Figure 5.
Histopathology of hepatic EAML (patient 2). (A) Tumors mainly consisted of epithelioid cells that comprised approximately 95% of the total neoplastic mass (H&E stain, ×40). (B) The tumor was comprised of sheets of large polygonal cells with abundant granular eosinophilic cytoplasm (H&E stain, ×200). (C) Atypical epithelioid cells contain eosinophilic granular cytoplasm and pleomorphic nuclei (H&E stain, ×400). Immunohistochemical staining was positive for HMB-45 (D), Melan-A (E) and smooth muscle α-actin (F) (original magnification ×200).
Discussion
Angiomyolipoma (AML), derived from mesoderm, is a rare and malignant tumor. Apart from angiomyolipoma, lymphangiomyomatosis, clear cell “sugar” tumor of the lung and a group of rare, morphologically and immunophenotypically similar lesions arising at a variety of visceral and soft tissue sites, nearly all show immunoreactivity for both melanocytic (HMB-45 and/or melan-A) and smooth muscle (actin and/or desmin) markers. These tumors all share a distinctive cell type, the perivascular epithelioid cell or ‘PEC’. Therefore, those tumors are classified as perivascular epithelioid cell tumors [3]. Epithelioid angiomyolipoma (EAML) is a rare subtype of AML. It is commonly seen in the kidney and is rare in the liver.
Hepatic EAML comprises three tissue elements: blood vessels, epithelia, or spindle-shaped smooth muscle cells, and mature adipose tissue. According to the tissue components, hepatic EAML can be divided into four subtypes: myoma type (adipose tissue <10%), hemangioma type, lipoma type (adipose tissue >70%) and hybrid type (smooth muscle, blood vessels and fat accounted for a certain proportion). For both of the two cases in this report, the tumor belonged to a typical hybrid type.
Most of the patients with hepatic EAML have no symptoms and their lesions are detected incidentally during routine medical examination. Few patients suffer from liver abdominal discomfort or pain due to a larger tumor, and few patients are treated by spontaneous rupture of the tumor [4]. The ultrasonography findings are also non-specific, with a definite diagnosis rate of <1% [5].
The imaging characteristics of hepatic EAML are correlated with its histological components. Thickly distorted vessels and adipose tissue are observed in these lesions as an important feature of diagnosis. But most hepatic EAML has less or no adipose tissue, little or no fatty attenuation or tense was observed on CT or MRI images, which makes preoperative diagnosis difficult. In the plain phase, the CT scan of a lipoma type shows mass similar to fat, and the density of soft tissue which is composed of a small amount of malformed vessels and spindle smooth muscle cells can be observed in the lesion. Contrast-enhanced CT scan showed that there was no enhancement in fat components and visible enhancement in soft tissue of cords, which could be distinguished from liparomphalus. Hemangioma type had little or no adipose tissue. Non-enhanced CT scan showed a low density shadow, and inside the mass, there was a strip of vascular calcification. Contrast-enhanced CT scan showed tumors were evenly enhanced in the arterial phase, with most lesions decreased in the portal venous phase but the density was still higher than that of liver parenchyma. The myoma type and hybrid type were often found to be solid masses cause of the smooth muscle content. The low density or mixed density masses in the liver were found in the CT scan. All the tumors were markedly enhanced in the arterial phase with vascular malformations with obvious enhancement were seen at the center or margin of the tumor. Some tumors showed a false capsule formed by the compressed liver parenchyma and sparse fibrosis tissues with small vessels, which led to lesions with small or no vessels that continued enhanced in the portal venous/delayed phase [6]. But there were a few hepatic EAML cases, such as case 1, which had neither deformed thick blood vessels, but had the “fast forward and fast out” mode, and could easily be misdiagnosed as hepatocellular carcinoma. The difference between the two lies in that not only the enhancement of hepatocellular carcinoma in the arterial phase was weaker than that in hepatic EAML, and the time of enhancement was earlier than that of hepatic EAML, but also the margins of hepatocellular carcinoma were more clear than those of hepatic EAML in the portal venous phase [7]. In addition, hepatocellular carcinoma usually occurs on the basis of viral hepatitis or cirrhosis. Although the enhancement pattern of case 1 is similar with hepatocellular carcinoma, the boundary of the tumor in the portal venous phase was coarse, and the patient whose tumor marker was normal had no history of viral hepatitis or cirrhosis. Two cases in this report had a lack of preoperative MR. If the flow like blood vessels within the tumor could be seen on MR scan and enhanced gross vessels could be seen after enhanced scab, it could be further diagnosed as hepatic EAML [8].
The final diagnosis of hepatic EAML depends on histopathologic and immunohistochemical features. Hepatic EAML which dominated by expansive growth is usually a single solid mass with no capsule. The reported diameter of the tumor varies from 5.5 to 16 cm. Gross findings of hepatic EAMLs are usually a brown nodule with variegated appearance cause of intra-tumoral hemorrhage or necrosis. Microscopically, the tumor is mainly composed of large polygonal and spider reticular epithelioid cells, arranged in an irregular trabecular structure and separated by a large sinusoidal vascular network with abundant sinusoids. The tumor cells have two kinds of forms: a cell cytoplasm was translucent, surrounding the vacuole formation, central eosinophilic fine particles aggregate into blocks and another had an eosinophilic cytoplasm that was full of fine particles, with cells surrounding the deep staining, central light staining, similar to the signet ring cell. Microscopic findings of hepatic EAML are characterized by epithelioid tumor cells with plump eosinophilic granular cytoplasm arranged around blood vessels. Immunohistochemical staining showed that the tumor cells expressed not only the markers of melanocytes but also the smooth muscle source markers in different degrees. HMB45 was the most sensitive marker in melanocytes (100%), followed by Melan-A (89%) and Mitf (50%~60%), meanwhile, smooth muscle cell markers SMA, and MSA were positive [9]. Hepatic EAML is the only tumor in liver that expresses HMB45 and Melan-A at present. Therefore, HMB45 and Melan-A are positive, which is of great significance in the diagnosis of hepatic EAML. SMA, Vim, and CD34 can be used as auxiliary diagnostic indexes. In these cases, HMB45 and Melan-A were positive and SMA was partly positive, which were in accordance with the typical EAML phenotype. Under electron microscope, organelles such as microfilaments, mitochondria, glycogen, rough endoplasmic reticulum, and many electron dense granules were found in the cytoplasm. At high magnification, the dense particles were surrounded by a unit membrane, and the crystal structure consisted of filaments with a diameter of 50×10-10~60×10-10, arranged periodically every 100×10-10, and this crystal structure had diagnostic significance.
Laparoscopic resection is the first choice for the treatment of hepatic EAML, which has advantages of less trauma and quicker recovery. There is no definite conclusion in academic therapy. Local resection was the main treatment, including the whole tumor and the surrounding normal liver tissue. Because the tumor is small and located in the liver parenchyma, it is necessary to prepare intraoperative ultrasound to determine the surgical margin according to the location. About 9.3%~10% of the patients experienced postoperative metastasis or recurrence [10,11]. Metastatic lesions have been reported in the lung, liver, diaphragm, and mesentery [12]. The literature shows that for biopsy proven hepatic EAML, if the diameter is <5 cm, the liver function is normal and the serum hepatitis virus marker is negative, the patient can be treated conservatively, but close ultrasound or MR follow-up is necessary [13]. Considering that China has a high incidence rate of hepatitis, hepatic EAML should be differentiated from hepatocellular carcinoma first, so conservative treatment should be very cautious. Although hepatic EAML is generally managed as a benign tumor in clinical settings, it is becoming increasingly clear that hepatic EAML should be regarded as tumors of uncertain malignant potential. Eight cases of malignant hepatic EAML have been reported [12,14-20]. Strict criteria for a diagnosis of malignant hepatic EAML have not been established universally, relevant literature suggests that significant cellular polymorphism, vascular invasion, portal vein thrombosis, mitotic activity, mutation of p53, loss of CD117 expression, and metastasis appear to be associated with malignant transformation of hepatic EAML [21-23]. The incidence of malignant EAML accumulation in the liver is about 4.1%, so it is necessary to follow up after operation [13]. The 2 patients in this report were cured after six days and eight days, no complications occurred, and the long-term follow-up is ongoing.
Disclosure of conflict of interest
None.
References
- 1.Zeng JP, Dong JH, Zhang WZ, Wang J, Pang XP. Hepatic angiomyolipoma: a clinical experience in diagnosis and treatment. Dig Dis Sci. 2010;55:3235–3240. doi: 10.1007/s10620-010-1144-2. [DOI] [PubMed] [Google Scholar]
- 2.Yu F, Wang K, Yan ZL, Zhang XF, Li J, Dong H, Cong WM, Shi LH, Shen F, Wu MC. Clinical study of 169 patients with hepatic angiomyolipoma. Chin J Surg. 2010;48:1621–1624. [PubMed] [Google Scholar]
- 3.Hornick JL, Fletcher CD. PEComa: what do we know so far? Histopathology. 2006;48:75–82. doi: 10.1111/j.1365-2559.2005.02316.x. [DOI] [PubMed] [Google Scholar]
- 4.Tajima S, Suzuki A, Suzumura K. Ruptured hepatic epithelioid angiomyolipoma: a case report and literature review. Case Rep Oncol. 2014;7:369–375. doi: 10.1159/000363690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X, Li A, Wu M. Hepatic angiomyolipoma: clinical, imaging and pathological features in 178 cases. Med Oncol. 2013;30:416. doi: 10.1007/s12032-012-0416-4. [DOI] [PubMed] [Google Scholar]
- 6.Ji JS, Lu CY, Wang ZF, Xu M, Song JJ. Epithelioid angiomyolipoma of the liver: CT and MRI features. Abdom Imaging. 2013;38:309–314. doi: 10.1007/s00261-012-9911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan Y, Xie X, Lin Y, Huang T, Huang G. Hepatic epithelioid angiomyolipoma: clinical features and imaging findings of contrast-enhanced ultrasound and CT. Clin Radiol. 2017;72:339.e1–339.e6. doi: 10.1016/j.crad.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Högemann D, Flemming P, Kreipe H, Galanski M. Correlation of MRI and CT findings with histopathology in hepatic angiomyolipoma. Eur Radiol. 2001;11:1389–1395. doi: 10.1007/s003300000750. [DOI] [PubMed] [Google Scholar]
- 9.Shi QL, Hu Y, Xia H, et al. Clinicopathological diagnosis of hepatic epithelioid angiomyolipoma. Chin J Gen Surg. 2010;25:493–494. [Google Scholar]
- 10.Liu J, Zhang CW, Hong DF, Tao R, Chen Y, Shang MJ, Zhang YH. Primary hepatic epithelioid angiomyolipoma: a malignant potential tumor which should be recognized. World J Gastroenterol. 2016;22:4908–4917. doi: 10.3748/wjg.v22.i20.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SY, Kim B. Epithelioid angiomyolipoma of the liver: a case report. Clin Mol Hepatol. 2017;23:91–94. doi: 10.3350/cmh.2017.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuda Y, Omiya H, Takami K, Mori K, Kodama Y, Mano M, Nomura Y, Akiba J, Yano H, Nakashima O, Ogawara M, Mita E, Nakamori S, Sekimoto M. Malignant hepatic epithelioid angiomyolipoma with recurrence in the lung 7 years after hepatectomy: a case report and literature review. Surg Case Rep. 2016;2:31. doi: 10.1186/s40792-016-0158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klompenhouwer AJ, Verver D, Janki S, Bramer WM, Doukas M, Dwarkasing RS, de Man RA, IJzermans JNM. Management of hepatic angiomyolipoma: a systematic review. Liver Int. 2017;37:1272–1280. doi: 10.1111/liv.13381. [DOI] [PubMed] [Google Scholar]
- 14.Deng YF, Lin Q, Zhang SH, Ling YM, He JK, Chen XF. Malignant angiomyolipoma in the liver: a case report with pathological and molecular analysis. Pathol Res Pract. 2008;204:911–918. doi: 10.1016/j.prp.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Rouquie D, Eggenspieler P, Algayres JP, Béchade D, Camparo P, Baranger B. [Malignant-like angiomyolipoma of the liver: report of one case and review of the literature] . Ann Chir. 2006;131:338–341. doi: 10.1016/j.anchir.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen TT, Gorman B, Shields D, Goodman Z. Malignant hepatic angiomyolipoma: report of a case and review of literature. Am J Surg Pathol. 2008;32:793–8. doi: 10.1097/PAS.0b013e3181607349. [DOI] [PubMed] [Google Scholar]
- 17.Dalle I, Sciot R, de Vos R, Aerts R, van Damme B, Desmet V, Roskams T. Malignant angiomyolipoma of the liver: a hitherto unreported variant. Histopathology. 2000;36:443–50. doi: 10.1046/j.1365-2559.2000.00891.x. [DOI] [PubMed] [Google Scholar]
- 18.McKinney CA, Geiger JD, Castle VP, Ruiz RE, Strouse PJ. Aggressive hepatic angiomyolipoma in a child. Pediatr Hematol Oncol. 2005;22:17–24. doi: 10.1080/08880010590896206. [DOI] [PubMed] [Google Scholar]
- 19.Croquet V, Pilette C, Aube C, Bouju B, Oberti F, Cervi C, Arnaud JP, Rousselet MC, Boyer J, Calès P. Late recurrence of a hepatic angiomyolipoma. Eur J Gastroenterol Hepatol. 2000;12:579–582. doi: 10.1097/00042737-200012050-00018. [DOI] [PubMed] [Google Scholar]
- 20.Mizuguchi T, Katsuramaki T, Nobuoka T, Nishikage A, Oshima H, Kawasaki H, Kimura S, Satoh M, Hirata K. Growth of hepatic angiomyolipoma indicating malignant potential. J Gastroenterol Hepatol. 2004;19:1328–1330. doi: 10.1111/j.1440-1746.2004.03583.x. [DOI] [PubMed] [Google Scholar]
- 21.Kamimura K, Nomoto M, Aoyagi Y. Hepatic angiomyolipoma: diagnostic findings and management. Int J Hepatol. 2012;2012:410781. doi: 10.1155/2012/410781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamimura K, Oosaki A, Sugahara S, Mori S, Moroda T, Satoh O, Morita T, Kimura K, Kamura T, Nomoto M, Aoyagi Y. Malignant potential of hepatic angiomyolipoma: case report and literature review. Clin J Gastroenterol. 2010;3:104–110. doi: 10.1007/s12328-010-0136-2. [DOI] [PubMed] [Google Scholar]
- 23.Mete O, van der Kwast TH. Epithelioid angiomyolipoma: a morphologically distinct variant that mimics a variety of intra-abdominal neoplasms. Arch Pathol Lab Med. 2011;135:665–670. doi: 10.5858/2009-0637-RSR.1. [DOI] [PubMed] [Google Scholar]