Abstract
Background: Diabetic retinopathy (DR), a well-known serious complication of diabetes mellitus, can eventually advance to end-stage blindness. Aim: To investigate the role of profilin-1 (PFN1) in microvascular endothelial dysfunction (MVED) triggered by DR. Methods: We assessed the expression of PFN1 and hypoxia inducible factor-1 alpha (HIF-1α) in cultured human retinal microvascular endothelial cells (HRMECs) treated with high glucose and in 6 month-old Sprague-Dawley (SD) rats with DR. We also investigated the function of metformin in PFN1-mediated MVED. Results: High glucose upregulated PFN1 and HIF-1α expression levels. These changes were associated with increased permeability, apoptosis, and angiogenesis in vivo and in vitro. Metformin prevented high glucose or hyperglycemia-induced MVED by inhibition of HIF-1α/PFN1 signaling in cultured HRMECs and in SD rats with DR. Conclusion: Our results indicate that activation of HIF-1α/PFN1 by high glucose mediates permeability, apoptosis, and angiogenesis and that metformin alleviates MVED by suppressing HIF-1α/PFN1 signaling during DR. These results suggest a potential therapeutic strategy for preventing the onset of PFN1 in early-stage of DR.
Keywords: Profilin-1, HIF-1α, metformin, diabetic retinopathy
Introduction
Diabetic retinopathy (DR), especially proliferative diabetic retinopathy (PDR), is a major cause of blindness in the working-age population in developed countries. The search for effective treatment and prevention measures has long been a foci of study [1-3]. As an early pathophysiological precursor, microvascular endothelial dysfunction (MVED) is simultaneously a prevalent complication of both type 1 and type 2 diabetes, including primary hypertonicity, subsequent apoptosis, and angiogenesis, etc. MVED underlies and precedes the vascular barrier destruction of DR, whereas the underlying mechanism(s) remains an enigma. Due to the complex nature of MVED in DR, diverse (and poorly understood) signal pathways (e.g., hypoxia inducible factor-1 alpha [HIF-1α] and profilin-1, etc) are in persistent exploration day after day [4,5].
Profilin-1 (PFN1), aubiquitously expressed actin-binding protein that plays an important role in the regulation of actin polymerization and cytoskeleton remodeling, is required for migration, invasion, and capillary morphogenesis of vascular endothelial cells by regulating its biological function and facilitating microvascular cord regeneration [6-8]. Previous studies have provided ample evidence for the critical role of PFN1 in endothelial cell injuries, and blockade of PFN1-mediated biological effects may contribute to the prevention of endothelial abnormalities and vascular diseases due to hyperglycemia [1,9]. Of note, accumulating evidence hasunderscored that profilin-1 signaling providesmultiple and critical functionsin facilitating inflammation [10], apoptosis [11], and angiogenesis [12]. Meanwhile, our previous research also indicated the significance of HIF-1α/PFN1 signaling during DR [4]. Nonetheless, the specific mechanism and contribution of PFN1 to MVED in retinal endothelium due to DR has not been sufficiently elucidated yet.
Thus, the present study aimed to elucidate the role of PFN1 in MVED due to DR. Here, we tested whether the expression levels of PFN1 and HIF-1α were significantly increased under high glucose (HG) conditions in vivo and in vitro. Then, we set out to address that PFN1 knockdown by short hair RNA (shRNA) significantly suppressed the MVED induced by HG. Furthermore, our results indicate that metformin alleviates MVED by suppressing HIF-1α/PFN1 signaling during DR. These results suggest a potential therapeutic strategy for preventing the onset of PFN1 in early-stage of DR.
Materials and methods
Animals
All experiments were conducted in accordance with the requirements of the Association for Research in Vision and Ophthalmology, as outlined in the Statement for the Use of Animals in Ophthalmic and Vision Research. Male Sprague-Dawley (SD) rats, weighing 100-120 g, were purchased from Shanghai Laboratory Animal Center of the Chinese Academy of Sciences and housed five per cage in a room under controlled temperature, humidity, and lighting conditions, with food and water provided ad libitum. Rats were assigned randomly into groups receiving 60 mg/kg streptozotocin (STZ, Sigma-Aldrich, St. Louis, MI, USA) intraperitoneally or citrate buffer alone as previously described [13]. Briefly, diabetic rats were categorized as diabetic when the blood glucose exceeded 16.7 mmol/l at 48 h after STZ administration. One week after the injection of STZ, diabetic rats were randomly assigned to the following 4 groups for duration of the 24 weeks experiment: (1) normal (non-diabetic) rats; (2) diabetic rats; (3) diabetic rats treated with intravitreal injection of recombinant adenovirus encoding shRNA for PFN1; (4) diabetic rats treated with insulin plus metformin by oral route (100 mg/kg body weight/day) during the last 8 weeks. Diabetic rats received subcutaneous insulin (Humulin-N; Eli Lilly & Co., Indianapolis, IN) twice a week to maintain body weight and maximize survival rate (0-4 units).
Cell culture and reagents
Human retinal microvascular endothelial cells (HRMECs; cat. no. ACBRI 181) were purchased from Cell Systems (Kirkland, WA) and cells from passages 3-7 were used in the experiment. Cells were grown in M199 medium with 45 ng/mL bFGF and heparin and 20% fetal bovine serum. Confluent cells were switched to a serum-free medium for 24 h before treatment. Cells were exposed to normal glucose (NG, 5 mmol/L) or HG (30 mmol/L) with or without shRNA for PFN1. Primary antibodies against profilin-1, HIF-1α, and GAPDH were from R&D Systems. The shRNA for PFN1 (100 nmol/L) was from Genesil Biotechnology (Wuhan, China) and the PFN1-shRNA sequences were 5’-AGAAGGUGUCCACGGUGGUUU-3’ (forward) and 5’-ACCACCGUGGACACCUUCUUU-3’ (reverse). At 24 h after passage, at a 1:5 ratio, cells were transfected using Lipofectamine 2000 (0.15%, vol/vol) (Invitrogen, Carlsbad, CA) following the protocol provided by the manufacturer. Lipofectamine 2000 was removed by changing to fresh medium containing 10% FBS 5 h post-transfection, and the cells were analyzed 48 h after transfection.
Real-time PCR
The total RNA was extracted from RF/6A cells using TRIZOL reagent (Invitrogen Life Technologies, Gaithersburg, MD) and stored at -80°C. Theprimer sequences used were as follows: PFN1, 5’-ATCGACAACCTCATGGCGGACG-3’ (forward) and 5’-TTGCCAACCAGGACACCCACCT-3’ (reverse); GAPDH, 5’-CAAGGTCATCCATGACAACTTTG-3’ (forward) and 5’-GTCCACCACCCTGTTGCTGTAG-3’ (reverse). Relative quantification of the signals was performed by normalizing the signals of different genes to that of the GAPDH signal.
Western blot analysis
Adherent cells grown to sub-confluency on tissue culture plates and were then washed with phosphate-buffered saline (PBS) and lysed in 250 μl of 2× concentrated 12% SDS Lysis Buffer (BeyotimeBiotchnology, China). The samples collected were first boiled at 95°C for 5 min and then frozen in ice crush for 5 min in turn. After three times of successive manufacturing, cell lysates were electrophoresed in SDS-PAGE, and transferred onto nitrocellulose membrane. The membrane was blocked with 5% nonfat dried milk solution and subsequently incubated overnight with gentle agitation at 4°C with anti-PFN1. This was followed by incubation with corresponding horseradish peroxidase (HRP) conjugated secondary antibodies. GAPDH was used as internal control. Immunoreactive bands were identified using an enhanced chemiluminescence detection system (Pierce Biotechnology, Inc., Rockford, IL, USA).
Immunofluorescence microscopy
Paraffin wax-embedded sections of the retina were dehydrated and subsequently incubated with antibodies against PFN1 (Abcam, Cambridge, MA). Counterstaining of nuclei was performed with 4’, 6-diamidino-2-phenylindole (DAPI; Sigma). The staining was observed under an Axioplan 2 fluorescence microscope or a Cell Observer SD confocal laser scanning microscope (Carl Zeiss, Göttingen, Germany).
Clearance of bovine serum albumin (BSA)
For the detection of HRMEC permeability, albumin diffusion across endothelial monolayers was measured as described previously [14]. The permeability of the RF/6A cells monolayer was quantified as a percentage clearance of BSA from insert to lower well, as described by Quan and Godfrey [15] and Bonner and O’Sullivan [16]. Finally, the clearance rates of Trypan blue-labelled albumin through the cell monolayers were measured using a spectrophotometer.
Transendothelial electrical resistance (TEER)
TEER was determined as previously described [17]. We partly modified that method from Pavan B et al. [18]. RF/6A cells were seeded to form confluent monolayers to develop low electrical resistance on Transwell-permeable supports (area of 0.33 cm2) in 24-well plates [19]. TEER was measured using an epithelial voltmeter (WPI, Sarasota, FL).
Retinal permeability
Briefly, Evans blue dye was dissolved in normal saline (45 mg/mL). Then, under deep anesthesia, the dye (45 mg/kg) was injected into the jugular vein of each rat. Blood (200 µL) was withdrawn from the iliac artery 2 min after Evans blue injection and then every 30 min for up to 120 min. After the dye had circulated for 120 min, the chest cavity was opened, and the left ventricle was cannulated. Each rat was perfused with 0.05 M citrate buffer, pH 3.5 (37°C), for 2 min at 66 mL/min to clear the dye. After the perfusion, the eyes were enucleated immediately, and the retinas were carefully dissected under an operating microscope. Evans blue in retinas and blood samples were detected as described previously [20]. Retinal vascular permeability was calculated using the following equation, and the results were expressed in µL plasma/g retina dry weight/h: Retinal vascular permeability = Evans blue (µg)/retina dry weight (g)/time-averaged Evans blue concentration (µg)/plasma (µL) × circulation time (h).
Apoptosis
Apoptotic cells in the HRMECs cultures wasdetected using the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) system (Promega, Madison, WI) according to the manufacturer’s instructions.
Tube formation assay
The HRMECs tube formation assay was conducted on Matrigel (BD Biosciences, Bedford, MA) according to the manufacturer’s protocol. HRMECs were plated at 4×105 cells/well in 24-well plates precoated with 300 μL Matrigel (10 mg/mL) in the absence of VEGF. After 24 h of incubation with or without the fluorescent dye calcein (8 µg/mL, 300 µL/well; BD Biocoat) under a 5% CO2-humidified atmosphere at 37°C, cell organization was examined under an inverted photomicroscope at 100× magnification. A minimum of three fields were analyzed per image using ImageJ software (National Institutes of Health).
Statistical analysis
The results are expressed as the mean ± standard deviation (SD). Group means were compared by one-way ANOVA using the GraphPad Prism 4.0 software system (Graph Pad, San Diego, CA) and the statistical software program SPSS version 17.0 for Windows (SPSS, Chicago, IL). In all comparisons, a value of P<0.05 was considered to indicate a statistically significant difference.
Results
Increased expression of PFN1 and HIF-1α under HG conditions in vivo and in vitro
PFN1 is a critical regulator of actin, the major cell skeleton, and HIF-1α, a single transcriptional activator, mediate acclimation of cells, tissues, and the organism to conditions of HG-evoked low oxygen tension [21]. Initially, to determine whether PFN1 and HIF-1α expression level is upregulated in DR, we incubated HRMECs in HG for 48 or 72 h. As shown in Figure 1A-C, chronic HG resulted in significantly increased levels of PFN1 and HIF-1α protein or mRNA compared with the NG group (P<0.01).
Figure 1.
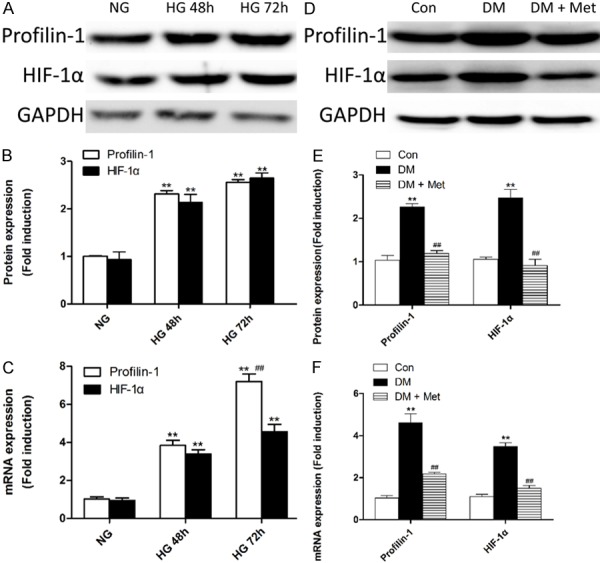
Activation of the HIF-1α/Profilin-1 pathway under HG conditions in vivo and in vitro. (A-C) Western blotting (A) and quantification (B) of HIF-1α and Profilin-1 protein or real-time RT-PCR analysis of mRNA (C) expression profiles in the following cell treatment groups: NG, HG for 48 h (HG 48 h), and HG 72 h. **P<0.01 vs. NG; ##P<0.01 vs. HG. (D-F) Western blotting (D) and quantification (E) of HIF-1α and Profilin-1 protein or real-time RT-PCR analysis of mRNA (F) expression profiles in the control rat group (Con), diabetic rat group (DM), and DM plus metformin group (DM + Met). **P<0.01 vs. Con; ##P<0.01 vs. DM. Means ± SD. n=3.
Hyperglycemia has long been identified as major risk factor for DR. To further clarify the expression level of PFN1 in vivo, and to define the role of metformin, we examined the levels of PFN1 and HIF-1α in the retinas of diabetic animals. We found that hyperglycemia caused a significant increase in the protein and mRNA levels of PFN1 and HIF-1α compared with the control rats group (P<0.01; Figure 1D-F).
Metformin reverses HG-triggered overexpression of PFN1
The concept that ischemic retinopathyis driven by ischemia-induced angiogenic factors was proposed decades ago. HIF-1α has recently emerged as the master regulator of these angiogenic mediators [21]. Hereby, we examined the inhibiting effect of metformin on HIF-1α since metformin has been demonstrated to be a strong pharmaceutical inhibitor of HIF-1α activity [22,23]. As shown in Figure 1D, 1E, elevated protein levels of HIF-1α and PFN1, were blocked by metformin pretreatment for 48 h (P<0.01). Metformin also significantly inhibited HIF-1α and PFN1 mRNA expression (P<0.01; Figure 1F).
Moreover, we quantified PFN1 levels using immunofluorescence. Compared with the NG group, a clear increase in PFN1 expression was evident in the HG group, which was effectively inhibited by metformin in the HG + Met group (Figure 2).
Figure 2.
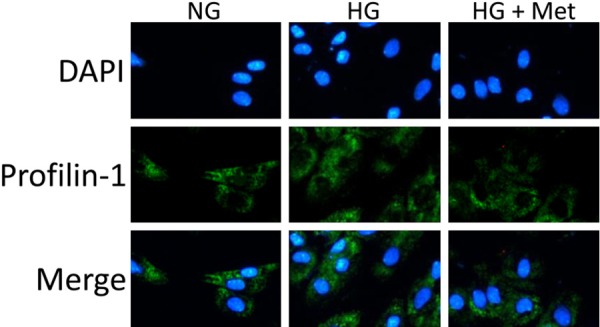
Immunofluorescent analysis indicates that high glucose (HG) induced profilin-1 activation in the HG group are effectively inhibited by treatment with metformin (HG + Met). Mean ± SD. n=3.
HG-inducedactivation of PFN1 contributes to the cell hypertonicity in vivo and in vitro
To assess alterations in the rat retina under HG conditions, we examined the permeability of HRMECs in vitro and SD rat retinas in vivo. Compared with the NG group, PFN1 knockout by shPFN1 and metformin both significantly restored HG resulted increase of BSA clearance rate (P<0.05; Figure 3A) and decrease of TEER (P<0.01; Figure 3B). Moreover, we found an ~2.5-fold increase in retinal permeability under HG conditions versus that under NG conditions in vivo, and similar results were observed in the DM + shPFN1 group and the DM + Met group (P<0.01; Figure 3C). However, the differences of permeability between the HG + shPFN1 group and the HG + Met group, and between the DM + shPFN1 group and the DM + Met group, were not significant (P>0.05; Figure 3A-C).
Figure 3.

Modulation of Profilin-1 (PFN1) contributes to hyper-permeability induced by HG in vivo and in vitro. (A, B) Utilization of the BSA clearance rate (A) and TEER of HRMECs to measure the permeability in the following cell treatment groups: NG, HG, HG + shPFN1, HG + Met. (C) PFN1 knockdown by shRNA (DM + shPFN1) and metformin (DM + Met) both significantly decrease the hyper-permeability in the DM group. **P<0.01 vs. NG; ##P<0.01 vs. HG; NS means not significant (P>0.05). Means ± SD. n=3.
PFN1 mediates cell apoptosis under HG conditions in vivo and in vitro
To evaluate the physiological effect of PFN1 in the augmented cell apoptosis induced by hyperglycemia, we examined apoptotic factors via knockdown of PFN1 in vivo. Our first observation was that the hyperglycemia induced overexpression of apoptotic molecules including p53 and BAX in the DM group, correlating with the overexpression of PFN1, were both significantly decreased in the DM + shPFN1 group via western blot in vivo (P<0.01; Figure 4A, 4B). Simultaneously, mRNA analysis showed the analogical results in vivo (Figure 4C).
Figure 4.
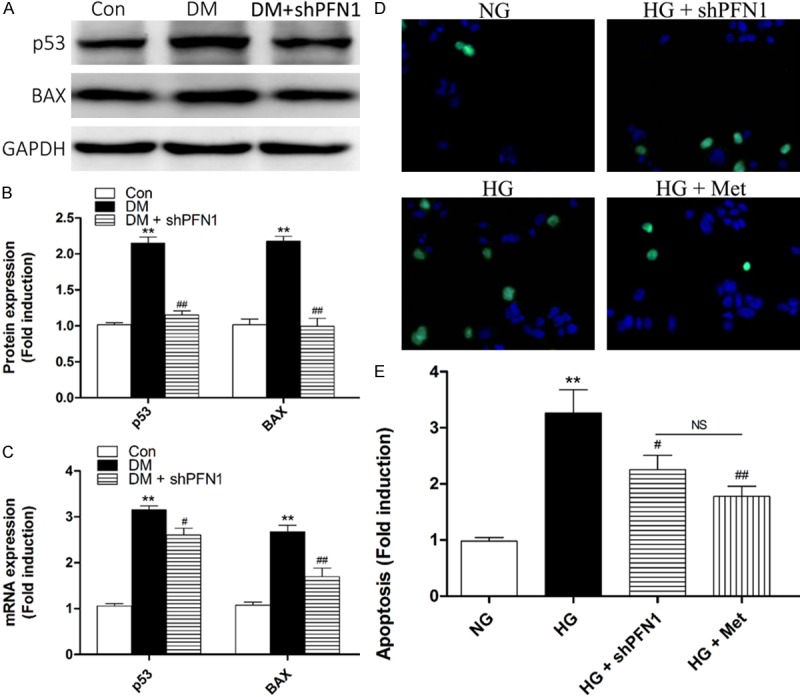
Profilin-1 (PFN1) modulates apoptosis under HG conditions in vivo and in vitro. (A-C) Western blotting (A) and quantification (B) of p53 and BAX protein or real-time RT-PCR analysis of mRNA (C) expression profiles in control rats (Con), diabetic rats (DM), and DM plus shPFN1 (DM + shPFN1). **P<0.01 vs. Con; #P<0.05 vs. DM; ##P<0.01 vs. DM. (D-F) Fluorescence microscope (D) and quantification analysis (E) of cellular apoptosis levels by TUNEL in the following cell treatment groups: NG, HG, HG plus shPFN1 (HG + shPFN1), and HG plus metformin (HG + Met). **P<0.01 vs. NG; #P<0.05vs. HG; ##P<0.01 vs. HG; NS means not significant (P>0.05). Means ± SD. n=3.
To confirm these results, we retested apoptosis in vitro via using TUNEL assays. Compared with the NG group, the number of apoptotic cells in the HG group was significantly increased (P<0.01; Figure 4D, 4E). Furthermore, the numbers of apoptotic cells in the HG + shPFN1 group (P<0.05; Figure 4D, 4E) and HG + Met group (P<0.01; Figure 4D, 4E) were both significantly decreased compared with the HG group. However, the difference between the HG + shPFN1 group and the HG + Met group was not significant (P>0.05; Figure 4D, 4E).
PFN1 mediates angiogenesis under HG conditions in vitro
Regarding the pivotal role of PFN1 in the reorganization and redistribution of actin and the indispensable part of actin in the mitosis and proliferation of endothelial cells [24], we hypothesized that PFN1 may also be involved in the pathological angiogenesis of HRMECs due to HG. Thus, we performed tube formation assays to measureangiogenesis using Matrigel in vitro.
Similar to the previous observations, HG conditions markedly stimulated promotion of tube formation in vitro (P<0.01; Figure 5A, 5B). Subsequently, this promotion was significantly decreased in the HG + shPFN1 group and the HG + Met group (P<0.01; Figure 5A, 5B). These results suggest an important role for PFN1 in this characteristic response to HG.
Figure 5.
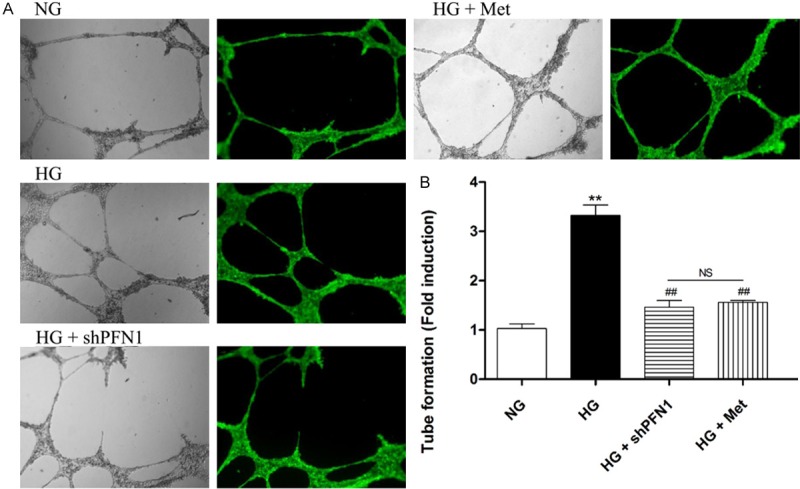
Profilin-1 (PFN1) modulates tube formation in vitro. Fluorescence microscope of tube formation (A) without (left) or with (right) dye fluorescent calcein and quantification analysis (B) in the following cell treatment groups: NG, HG, HG plus shPFN1 (HG + shPFN1), and HG plus metformin (HG + Met). **P<0.01 versus NG; ##P<0.01 versus HG; NS means not significant (P>0.05). Means ± SD. n=3.
Discussion
DR, one of the most devastating acquired vascular complications of diabetes mellitus, is responsible for affecting overall life quality worldwide [25]. DR manifests in two stages, first, the retinal microvasculature is compromised and capillary degeneration occurs; subsequently, an over-compensatory angiogenic response is initiated [26]. In DR, various growth factors, vasoactive agents and adhesion molecules result in increased blood flow, increased capillary permeability, increased apoptosis, andangiogenesis and microvascular remodeling [4]. Although landmark cross-sectional studies have confirmed the strong relationship between chronic hyperglycemia and the progression of DR, the underlying mechanism of how hyperglycemia causes MVED remains an enigma.
Essentially, PDR involves marked retinal vascular malfunction, and multiple proangiogenic factors (e.g., HIF-1α and profilin-1) portray pivotal roles in the progression of angiogenesis and represent significant targets for therapeutic intervention in PDR [4]. Notably, tube formation and sprouting progression of angiogenesis undoubtedly refer to various cell movements, and profilin-1 has been crucial in these steps according to previous research. Furthermore, there is also controversy as to PFN1 has both pro- and anti-angiogenic effects [1]. In this study, we provide proof that PFN1 is a proangiogenic factor like HIF-1α and VEGF which have been depicted before based on morphological experiments.
PFN1 is originally identified as an actin-associated protein, and has been linked to many biological functions including physiological and pathological processes, such as tumorigenesis, angiogenesis and apoptosis [12,27]. Previous research has demonstrated that PFN1, a commonly recognized intracellular actin-binding protein, plays a crucial role in inflammatory and angiogenic disease, which has linked the actin cytoskeleton and its dynamics directly to MVED. PFN1 is overexpressed in cultured endothelial cells and up-regulated profilin-1 triggers endothelial dysfunction [10]. In this study, our findings first demonstrated that PFN1 is pivotally engaged in the regulation of cell hyperpermeability, cell apoptosis, and angiogenesis under HG conditions. The role for PFN1 in MVED regulation was further supported by the efficient inhibition of PFN1 by metformin because metformin mediates as an upstream inhibitor of HIF-1α under HG conditions.
As far as clinical significance, we selectively chose p53 and BAX to explore the correlation between the increased apoptosis and PFN1 under HG conditions. For the purpose of testing the previous hypothesis, we took advantage of two negative control groups of PFN1 including shRNA and pharmacological inhibitor metformin.First, PFN1 activation triggered by HG was clearly verified. Subsequently, the potential role of PFN1 signaling early stages of endothelial dysfunction was examined. Of note, we found that PFN1 inhibition critically contributed to MVED restoration under HG conditions.
We next went on to assess the forward dysfunction of endothelium described above, such as tube formation. The results shown in the figures demonstrated up-regulation and recovery of tube formation are mediated by fluctuations of PFN1 protein level. This finding opens a new perspective for the prevention, treatment, and control of DR.
In conclusion, our results provide new insight of PFN1 in the regulation of MVED triggered by HG. We also provide evidence that metformin efficiently reverses MVED resulting from HG. Current therapies, which include pan-retinal photocoagulation and vitrectomy, have remained unaltered for several decades [26]. Thus, PFN1 bypasses canonical, multistep intracellular signaling events to initiate endothelial permeability, apoptosis, and angiogenesis, and might serve as a selective therapeutic target for anti-angiogenic therapy in DR.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 814-20108002 and 81700847) and the Natural Science Foundation of Jiangsu Province (Grants No. BK20170365).
Disclosure of conflict of interest
None.
References
- 1.Lu Q, Lu L, Chen W, Chen H, Xu X, Zheng Z. RhoA/mDia-1/profilin-1 signaling targets microvascular endothelial dysfunction in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2015;253:669–680. doi: 10.1007/s00417-015-2985-3. [DOI] [PubMed] [Google Scholar]
- 2.Lu Q, Zou W, Chen B, Zou C, Zhao M, Zheng Z. ANGPTL-4 correlates with vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254:1281–1288. doi: 10.1007/s00417-015-3187-8. [DOI] [PubMed] [Google Scholar]
- 3.Lu Q, Zou C, Cao H, Zhao M, Yu S, Qiu Q, Xu X, Zheng Z. Preoperative intravitreal injection of ranibizumab for patients with severe proliferative diabetic retinopathy contributes to a decreased risk of postoperative neovascular glaucoma. Acta Ophthalmol. 2016;94:414–415. doi: 10.1111/aos.13019. [DOI] [PubMed] [Google Scholar]
- 4.Lu Q, Lu P, Chen W, Lu L, Zheng Z. ANGPTL-4 induces diabetic retinal inflammation by activating Profilin-1. Exp Eye Res. 2017;166:140–150. doi: 10.1016/j.exer.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Lu Q, Lu L, Chen W, Lu P. Expression of angiopoietin-like protein 8 correlates with VEGF in patients with proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2017;255:1515–1523. doi: 10.1007/s00417-017-3676-z. [DOI] [PubMed] [Google Scholar]
- 6.Wang R, Cleary RA, Wang T, Li J, Tang DD. The association of cortactin with profilin-1 is critical for smooth muscle contraction. J Biol Chem. 2014;289:14157–14169. doi: 10.1074/jbc.M114.548099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding Z, Roy P. Profilin-1 versus profilin-2: two faces of the same coin? Breast Cancer Res. 2013;15:311. doi: 10.1186/bcr3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horrevoets AJ. Profilin-1: an unexpected molecule linking vascular inflammation to the actin cytoskeleton. Circ Res. 2007;101:328–330. doi: 10.1161/CIRCRESAHA.107.158881. [DOI] [PubMed] [Google Scholar]
- 9.Chen S, Apostolova MD, Cherian MG, Chakrabarti S. Interaction of endothelin-1 with vasoactive factors in mediating glucose-induced increased permeability in endothelial cells. Lab Invest. 2000;80:1311–1321. doi: 10.1038/labinvest.3780139. [DOI] [PubMed] [Google Scholar]
- 10.Romeo G, Frangioni JV, Kazlauskas A. Profilin acts downstream of LDL to mediate diabetic endothelial cell dysfunction. FASEB J. 2004;18:725–727. doi: 10.1096/fj.03-0841fje. [DOI] [PubMed] [Google Scholar]
- 11.Yao W, Yu X, Fang Z, Yin P, Zhao C, Li N, Wang L, Li Z, Zha X. Profilin1 facilitates staurosporine-triggered apoptosis by stabilizing the integrin beta1-actin complex in breast cancer cells. J Cell Mol Med. 2012;16:824–835. doi: 10.1111/j.1582-4934.2011.01369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan Y, Arif A, Gong Y, Jia J, Eswarappa SM, Willard B, Horowitz A, Graham LM, Penn MS, Fox PL. Stimulus-dependent phosphorylation of profilin-1 in angiogenesis. Nat Cell Biol. 2012;14:1046–1056. doi: 10.1038/ncb2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen W, Zhao M, Zhao S, Lu Q, Ni L, Zou C, Lu L, Xu X, Guan H, Zheng Z, Qiu Q. Activation of the TXNIP/NLRP3 inflammasome pathway contributes to inflammation in diabetic retinopathy: a novel inhibitory effect of minocycline. Inflamm Res. 2017;66:157–166. doi: 10.1007/s00011-016-1002-6. [DOI] [PubMed] [Google Scholar]
- 14.Lu QY, Chen W, Lu L, Zheng Z, Xu X. Involvement of RhoA/ROCK1 signaling pathway in hyperglycemia-induced microvascular endothelial dysfunction in diabetic retinopathy. Int J Clin Exp Pathol. 2014;7:7268–7277. [PMC free article] [PubMed] [Google Scholar]
- 15.Falcon Technical Bulletin. Franklin Lakes, NJ: Becton Dickinson; 1998. X. QHP. G. In vitro study of cytokine-mediated activation of endothelial cell permeability using falcon cell culture inserts. [Google Scholar]
- 16.Bonner SM, O’Sullivan MA. Endothelial cell monolayers as a model system to investigate dengue shock syndrome. J Virol Methods. 1998;71:159–167. doi: 10.1016/s0166-0934(97)00211-5. [DOI] [PubMed] [Google Scholar]
- 17.Dalpiaz A, Paganetto G, Pavan B, Fogagnolo M, Medici A, Beggiato S, Perrone D. Zidovudine and ursodeoxycholic acid conjugation: design of a new prodrug potentially able to bypass the active efflux transport systems of the central nervous system. Mol Pharm. 2012;9:957–968. doi: 10.1021/mp200565g. [DOI] [PubMed] [Google Scholar]
- 18.Pavan B, Capuzzo A, Forlani G. High glucose-induced barrier impairment of human retinal pigment epithelium is ameliorated by treatment with goji berry extracts through modulation of cAMP levels. Exp Eye Res. 2014;120:50–54. doi: 10.1016/j.exer.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Peiris D, Pacheco I, Spencer C, MacLeod RJ. The extracellular calcium-sensing receptor reciprocally regulates the secretion of BMP-2 and the BMP antagonist noggin in colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2007;292:G753–766. doi: 10.1152/ajpgi.00225.2006. [DOI] [PubMed] [Google Scholar]
- 20.Qaum T, Xu Q, Joussen AM, Clemens MW, Qin W, Miyamoto K, Hassessian H, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001;42:2408–2413. [PubMed] [Google Scholar]
- 21.Xin X, Rodrigues M, Umapathi M, Kashiwabuchi F, Ma T, Babapoor-Farrokhran S, Wang S, Hu J, Bhutto I, Welsbie DS, Duh EJ, Handa JT, Eberhart CG, Lutty G, Semenza GL, Montaner S, Sodhi A. Hypoxic retinal muller cells promote vascular permeability by HIF-1-dependent up-regulation of angiopoietin-like 4. Proc Natl Acad Sci U S A. 2013;110:E3425–3434. doi: 10.1073/pnas.1217091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guimaraes TA, Farias LC, Santos ES, de Carvalho Fraga CA, Orsini LA, de Freitas Teles L, Feltenberger JD, de Jesus SF, de Souza MG, Santos SH, de Paula AM, Gomez RS, Guimarães AL. Metformin increases PDH and suppresses HIF-1alpha under hypoxic conditions and induces cell death in oral squamous cell carcinoma. Oncotarget. 2016;7:55057–55068. doi: 10.18632/oncotarget.10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takiyama Y, Harumi T, Watanabe J, Fujita Y, Honjo J, Shimizu N, Makino Y, Haneda M. Tubular injury in a rat model of type 2 diabetes is prevented by metformin: a possible role of HIF-1alpha expression and oxygen metabolism. Diabetes. 2011;60:981–992. doi: 10.2337/db10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jockusch BM, Murk K, Rothkegel M. The profile of profilins. Rev Physiol Biochem Pharmacol. 2007;159:131–149. doi: 10.1007/112_2007_704. [DOI] [PubMed] [Google Scholar]
- 25.Elahy M, Baindur-Hudson S, Cruzat VF, Newsholme P, Dass CR. Mechanisms of PEDF-mediated protection against reactive oxygen species damage in diabetic retinopathy and neuropathy. J Endocrinol. 2014;222:R129–139. doi: 10.1530/JOE-14-0065. [DOI] [PubMed] [Google Scholar]
- 26.Durham JT, Herman IM. Microvascular modifications in diabetic retinopathy. Curr Diab Rep. 2011;11:253–264. doi: 10.1007/s11892-011-0204-0. [DOI] [PubMed] [Google Scholar]
- 27.Yao W, Cai X, Liu C, Qin Y, Cheng H, Ji S, Xu W, Wu C, Chen T, Xu J, Long J, Fang Z, Qu B, Hoth M, Ni Q, Zha X, Yu X. Profilin 1 potentiates apoptosis induced by staurosporine in cancer cells. Curr Mol Med. 2013;13:417–428. [PubMed] [Google Scholar]


