Abstract
Background and Aim: Ischemia-reperfusion (I/R) injury is an unavoidable event occurring during heart transplantation and is a key factor in graft failure and long-term survival rate of recipients. Therefore, there is an urgent need for the development of new therapies to prevent I/R injury. Clusterin is a hetero-dimeric glycoprotein with an antiapoptotic function. In this study, we investigated whether clusterin was cardioprotective in heart transplantation against I/R injury using an in vivo rat model and an in vitro cell culture system and we examined the underlying mechanisms of I/R injury. Methods: Heart grafts from wild-type C57BL/6 mice were preserved in UW solution (control) or UW solution containing recombinant human apolipoprotein J (hr clusterin) for 24 hours. The preserved hearts were implanted into recipient mice of the same strain as the donors for 72 hours. The heart grafts were then taken for histopathological and gene expression analyses. An in vitro ischemia reperfusion model using H9C2 cells or H9C2/clusterin cDNA cells was constructed. The expression of clusterin, p65, Bax, Bcl-xL, IL-1β, and TNF-α protein and mRNA in heart tissue and H9C2 cells was detected by Western blot, reverse transcription-polymerase chain reaction (RT-PCR), and quantitative RT-PCR assays. IL-1β and TNF-α protein was detected by enzyme-linked immunosorbent assays. NF-kB activity was detected by an electrophoretic mobility shift assay and cell apoptosis was detected by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling and flow cytometric analyses. Results: Cold I/R caused severe morphologic myocardial injury to heart grafts from wild-type C57BL/6 mice whereas grafts from hr clusterin preservation showed less damage, as demonstrated by decreased cell apoptosis/death, decreased neutrophil infiltration, and the preservation of the normal structure of the heart. Clusterin reduced expression of p65, pre-inflammatory IL-1β, TNF-α, and the pro-apoptotic gene Bax while it enhanced expression of the anti-apoptotic gene Bcl-xL in vitro and in vivo. Clusterin inhibited cell apoptosis/death and reduced pre-inflammation. Conclusion: Clusterin is a promising target for preventing cold I/R injury in heart transplantation. This study also shows that the resultant protective effects of clusterin are mediated by NF-κB signaling and Bax/Bcl-xL expression.
Keywords: Ischemia reperfusion injury, heart transplantation, clusterin, NF-kB, Bax, Bcl-XL
Introduction
Cardiac transplantation is the last resort for patients with end-stage heart failure. Ischemia-reperfusion (I/R) injury is a major issue in cardiac transplantation. I/R injury is associated with increased primary organ dysfunction and subsequent delayed organ function after cardiac transplantation. To improve the rate of successful heart transplantations, organ preservation should be optimized. However, the functional depression of cardiac grafts in postoperative recovery is not exceptional and the vitality of the transplanted tissue depends considerably on cardioplegic and storage conditions. At present, heart preservation is limited to 4-6 hours of cold ischemic storage [1]. Reperfusion injury occurs when there has been inadequate myocardial protection during the preceding ischemic period.
Extended cold ischemic times during heart transplantation have been associated with an increased risk of developing graft vasculopathy and failure in animal models [2,3] and humans [4]. Moreover, prolonged times between donor brain death and organ retrieval have been associated with increased mortality in cardiac transplant recipients [5]. Graft coronary microvascular dysfunction after ischemia and reperfusion can culminate in primary graft failure or untreatable chronic rejection [6]. Cold ischemia stimulates the expression of inflammatory mediators acting as “danger signals” and amplifying tissue injury and graft rejection.
In recent years, maintaining organ viability has become more challenging because the shortage of donors has led to a broader criteria for donor acceptability and, consequently, to organs with greater compromise [7]. RNA interference can be used to inhibit the expression of specific genes in vitro and in vivo, thereby providing an extremely useful tool for investigating gene function. However, limited success has been reported in animal studies [8]. Thus, there is an urgent need for an increased understanding of cold I/R injury and identification of new targets.
Clusterin (apolipoprotein J) is a hetero-dimeric glycoprotein secreted by a number of cell types. It has a cytoprotective effect in response to diverse stresses. Previous studies have suggested that clusterin plays important roles in cell adhesion, spermatogenesis, tumor metastasis, and lipid transportation [9]. In addition to its chaperone activity, clusterin may have an anti-apoptotic function. With regard to the role of clusterin in apoptotic cell death, multiple lines of evidence have demonstrated its cytoprotective effects in several tumors, epithelial cells, endothelial cells, and so forth [10]. Moreover, clusterin protects cells from heat shock and the action of TNF-α, suggesting that clusterin could be a survival factor related to the apoptotic pathway [11]. The anti-apoptotic effect of clusterin was also reported in the cardiac system based on its increased expression in the injured heart [12]. Clusterin reportedly protects cardiomyocytes from ischemia-induced cell death [13] or ethanol-induced cardiac injury [14].
However, the specific impact of clusterin on cold I/R injury in heart transplantation remains unknown and its underlying mechanism has not been identified. In this study, we determined that clusterin has a protective effect on cold I/R injury in heart transplantation in vivo and in vitro. We discovered that clusterin exerts its protective effect through an interaction with NF-κB signaling.
Materials and methods
Animals
C57BL/6 WT mice were purchased from Shanghai Laboratory Animal Research Center. The animals were housed under conventional conditions at the Animal Care Facility at Yantai Yuhuangding Hospital Affiliated to Qingdao University and were cared for in accordance with the guidelines established by the China Council on Animal Care.
Cell line and cell culture
The rat heart cell line H9C2 was purchased from ATCC (Shanghai, China) and cultured and maintained in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Hangzhou, China), which was supplemented with 10% fetal bovine serum (FBS; Sigma, Hangzhou, China) and 100 U/mL penicillin and streptomycin.
Heterotopic heart transplantation with prolonged ischemia reperfusion
Heterotypic cardiac transplantation was performed as described previously [15]. The heart was removed immediately from the donor and placed in chilled Euro-Collins solution on ice. The heart was perfused with a recombinant human apolipoprotein-J (hr clusterin) solution through the inferior vena cava and aorta until the vessels of the heart turned clear. The tube was kept there to support the lumen of the inferior vena cava and tied with silk sutures. The donor heart was then excised and immersed in a 10 μg hr clusterin solution at 4°C for 24 hours. Before anastomosis to the recipient was performed, the donor heart was flushed with fresh hr clusterin solution through the tube to wash out potentially harmful cellular metabolites that accumulate during a period of cold ischemia. Then, the tube was removed and the inferior vena cava was ligated permanently. The preserved heart was implanted into a recipient mouse of the same strain as the donor. On day 3 post-transplantation, the transplant mice were sacrificed and heart grafts were taken for histopathological and gene expression analyses.
Adenovirus infection
H9C2 cells (80,000 cells/well) were plated in a 6-well plate, cultured overnight, and then infected with a human clusterin cDNA expression adenovirus (SignaGen Laboratories, Rockville, MD, USA) at a multiplicity of infection of 100 in 600 μL FBS-free DMEM for 6 hours. Then, 600 μL culture medium containing 20% FBS was added to the infected cells and the cells were cultured overnight.
In vitro ischemia reperfusion model
H9C2 cells or H9C2/clusterin cDNA cells were plated in a 6-well plate (80,000 cells/well) and cultured in DMEM supplemented with 10% FBS and 100 U/mL penicillin and streptomycin, overnight. The medium was replaced with deoxygenized phosphate-buffered saline (PBS) and then placed in an InvivO2 Hypoxia Workstation (Baker Ruskinn, Sanford, MA, USA) with 0% O2 at 10°C for 16 hours. After 24 hours of hypoxia treatment, PBS was removed and a new complete culture medium was added to the cells. The cells were moved to a normal culture environment with 5% CO2 and 28% O2 at 37°C for 24 hours.
Western blot assay
Proteins were isolated from total cells using 14,000×g centrifugation for 10 minutes at 4°C and the supernatant was collected; nuclear fractions were isolated using a Nuclear/Cytosol Fractionation Kit (BioVision, Inc., Mountain View, CA, USA). Proteins quantified using a Lowry assay were separated by 10% SDS-PAGE, transferred onto a nitrocellulose membrane, and blocked overnight in blocking solution (10% of 10× TBS pH 7.6, 0.1% Tween 20, and 5% w/v of nonfat dry milk). The membrane was then incubated with a 1:200 dilution of rabbit polyclonal anti-NF-κB/p65, anti-Bax, anti-Bcl-xL, or anti-clusterin antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, Shanghai, China) and 10 μL rabbit polyclonal anti-β-actin antibody (Abcam, Shanghai, China) as a loading control, in blocking solution for 1 hour at room temperature. The nitrocellulose membrane was then incubated with a 1:1000 dilution of HRP-conjugated anti-rabbit secondary antibody in blocking solution for 1 hour at room temperature. Proteins were detected by chemiluminescence and autoradiography. Band density was measured using a ChemiDoc XRES (Bio-Rad Laboratories, Hercules, CA, USA) and analyzed using Quantity One software (Bio-Rad).
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was extracted using a TRIzol® Plus RNA Purification Kit (Invitrogen, Guangzhou, China). The concentration of RNA was measured by an ND2000 spectrophotometer (Thermo Scientific, MA, USA). First-strand cDNA synthesis was performed using 2.5 μg total RNA with Invitrogen SuperScript Reverse Transcriptase (Thermo Fisher Scientific, Shanghai, China). cDNA was amplified in a 25-μL PCR mixture containing 1 μL deoxynucleotide triphosphates, 1× PCR buffer, 2.5 mM MgCl2, and 1 U DNA Taq polymerase (Promega, Madison, WI, USA) with 25 pmol of primers specific for clusterin (Integrated DNA Technologies, Shanghai, China), Bcl-xL (sense: 5’-TATTGGTGAGTCGGATTGCA-3’, antisense: 5’-GCTCTCGGGTGCTGTATTGT-3’), Bax (sense: 5’- AGGCCTCCTCTCCTACTTCG-3’, antisense: 5’-AAATGCCTTTCCCCtTTCCCC-3’), IL-1β (sense: 5’-GTCTTCCGCCTCTCGGTAAT-3’, antisense: 5’-AGAGATACGGATCGCACAGG-3’), or TNF-α (sense: 5’-CTTTTGGAGTTTGAGGTAGTATACCTA-3’, antisense: 5’-GCTGCGCAGAATGAGATGAGTTGTC-3’). PCR was performed according to the manufacturer’s protocol for Applied Biosystems Power SYBR® Green PCR Master Mix and RT-PCR as follows: enzyme activation step: 10-min hold at 95°C for AmpliTaq Gold® enzyme activation, followed by 35 cycles of PCR (15 s at 95°C and 60 s at 60°C). GAPDH was used as an internal control (sense: 5’-TCACAGCGGCCCTCCTGACACCTA-3’, antisense: 5’-GCGAGTATACTCCTTCTCGATCCT-3’). GAPDH PCR conditions consisted of 17 cycles (94°C for 30 s, 52°C for 30 s, and 72°C for 1 min). After cycling, the samples were incubated at 72°C for 10 minutes. The reproducibility of the quantitative measurements was evaluated by three independent cDNA syntheses and PCR amplification from each preparation of mRNA. The relative mRNA expression level was determined as a ratio of the signal intensity to that of GAPDH.
Quantitative PCR (qPCR)
qPCR was conducted in a 10 μL PCR with 1× SYBR Green mixture (Bio-Rad), 100 nM primers, and 1 μL cDNA with the following thermal profiling: an initial activation step at 95°C for 2 minutes, followed by 35 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 20 s. Expression levels between I/R and non-I/R were quantitatively compared using the ΔΔCt method with GAPDH as the endogenous control for mRNA expression.
Tissue preparation for TNF-α and IL-1β measurements
The basal side of heart tissue was rinsed with ice-cold saline to remove any red blood cells or clots and then homogenized with a high-intensity ultrasonic liquid processor in 1:10 (w/v) PBS that contained 1% Triton X-100 and a protease inhibitor cocktail. The homogenate was centrifuged at 2,500×g for 20 minutes at 4°C. The supernatant was collected for determination of TNF-α and IL-1β according to the manufacturer’s instructions, using enzyme-linked immunosorbent assay (ELISA) kits (RayBiotech, Inc., Norcross, GA, USA).
Electrophoretic mobility shift assay (EMSA)
Nuclear and cytosolic proteins were prepared from cells and tissues [16]. The oligonucleotides of NF-kB: 5’AGTTGAGGGGACTTTCCCAGGCC-3’ were synthesized by Applied Biosystems and annealed oligonucleotides were labeled with [γ-32P] ATP (3000 Ci/mmol, Shanghai, China) with T4 polynucleotide kinase. Commercially available antibodies for p65 RelA (sc-372) were used for EMSA. EMSAs were carried out utilizing an infrared dye 700 labeled oligonucleotide (IR oligo) consisting the NF-kB consensus binding sequence. The binding reaction consisted of 1× binding buffer (10 mM Tris, 50 mM KCl, 1 mM DTT, pH 7.5), sterile water, 1 μg of poly[dI-dC] (Amersham Pharmacia Biotech, USA) in 10 mM Tris and 1 mM EDTA pH 7.5, 2.5 mM DTT/0.25% Tween 20, and 1 μl of NF-kB IR oligo. Five μg of nuclear protein was added to the mixture and incubated at room temperature for 30 minutes in darkness. For supershift analysis, the above mixture was incubated with 5 μg of NF-kB antibody p50 (SC-7178X Santa Cruz) or p65 (SC-109X, Santa Cruz) for an additional 30 minutes. The nuclear protein-DNA complexes were separated in native 4% polyacrylamide gels and scanned in a Li-Cor Odyssey infrared imaging system (Lincoln, Nebraska).
Blood sampling for measurement of plasma cTnI
At the end of the experiment, about 2 mL of blood was collected from the heart. The blood sample was placed in a tube containing disodium EDTA (22 mg/mL) as anti-coagulant, mixed thoroughly, and then centrifuged at 3000 rpm for 15 minutes. It was then used for determination of plasma cTnI according to the manufacturer’s instructions and guidelines using enzyme-linked immunosorbent assay (ELISA) kits (Life Diagnostics, USA).
Histological analysis
Heart grafts were collected from mice and tissue slices and harvested into 4% formalin before being processed for paraffin wax embedding. Once embedded, tissue sections were cut at 2 μm using a sledge microtome (Leitz, Germany) and sections were stained with hematoxylin and eosin stain (H&E). Hematoxylin and eosin slides were prepared and morphological assessment of cardiac injury was performed using a semi-quantitative scale of 0 to 4 (4 being most severe) [17]. Hearts were assigned a score of 5 if they failed to function immediately. In functioning cardiac grafts, the myocardium was assessed for the following: myocytolysis (dissolution of myocytes), myocardial necrosis, and inflammatory infiltrate. The degree of injury was measured by the extent of myocardium involved in the biventricular section, 1<10%, 2≥10% and ≤30%, 3≥30% and <60%, 4≥60%.
Apoptosis in I/R injured heart in vivo and cells in vitro
A fluorescein in situ cell death detection kit was used, according to the manufacturer’s instructions, for TUNEL assay (Roche Applied Science, Mannheim, Germany). Positive cells were counted in ten non-overlapping fields of outer medulla in each sample (magnification, ×200). The activity of caspase-3 in heart was measured using a Caspase-3 Colorimetric Assay Kit, following the manufacturer’s protocol (Beyotime, China) [18].
Flow cytometric analysis
Cell apoptosis was determined using a FITC Annexin V apoptosis kit (BD Pharmingen, Franklin Lakes, NJ, USA), according to the manufacturer’s instructions. In brief, cells were washed with ice-cold PBS and resuspended in binding buffer (10 mmol/L HEPES, pH 7.4, 140 mmol/L NaCl, and 2.5 mmol/L CaCl2) at a concentration of 1×106 cells/mL. Cells were stained with annexin V-FITC and propidium (PI) for 15 minutes in the dark before analysis by a flow cytometer (Beckman Coulter Inc., Miami, FL, USA).
Statistical analysis
Results are presented as the means ± SE of the mean. Statistical analysis was performed by two-tailed t-test or Mann-Whitney test using GraphPad Prism 4 software. Differences associated with probability values of P<0.05 were considered statistically significant.
Results
Clusterin protects the heart from I/R injury in heart transplantation
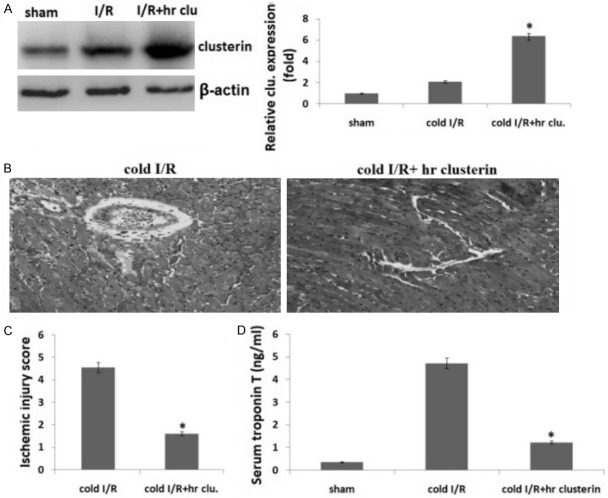
We excised donor hearts and preserved donor organs with chilled Eurocollins solution or/and recombinant human apoliprotein-J (hr clusterin) solution for 24 hours, followed by a syngeneic heterotopic heart transplantation. Twenty-four hours later, implanted hearts were harvested for protein expression by Western blot. The results showed that 24 hours cold I/R increased the expression of clusterin 1.44±0.18 folds as compared to the control grafts without I/R injury but the cold hr clusterin/I/R increased the expression of clusterin 5.7±0.48 folds as compared to the control grafts without I/R injury (Figure 1A). Perfusion of heart with hr clusterin resulted in a significant increase in clusterin levels.
Figure 1.
Clusterin protects hearts from I/R injury in heart transplantation. Preserved donor organs with chilled Eurocollins solution or/and hr clusterin solution for 24 hours, followed by a syngeneic heterotopic heart transplantation. A. Clusterin protein expression in heart tissue was detected by Western blot assay; B. H&E staining. 3 days after transplantation, heart grafts were harvested for H&E staining; C. The ischemic scores in both of the groups; D. Serum TnT was measured in sham, IR, IR+ hr clusterin treated recipients 72 hours post-transplantation. Vs. control, *P<0.01.
We next examined whether hr clusterin induced cardioprotection during cold I/R injury. We observed that the transplanted heart from wild type mice did not start to beat after implantation while the hearts from hr clusterin mice immediately beated once they were revascularized and beated strongly with steady speeds. Three days after transplantation, we harvested heart grafts to assess I/R injury by examining histopathological changes with H&E staining. Figure 1B shows the histopathological changes in heart grafts including cold I/R and cold I/R+ hr clusterin. The ischemia score is significantly higher in cold I/R groups than that of cold I/R+hr clusterin groups, indicating that hr clusterin protected heart I/R injury (Figure 1C, P<0.01). We detected cardiac TnT production and found that on 72 hours post-transplantation, the production of TnT was reduced by hr clusterin + cold I/R in comparison with cold I/R alone group (Figure 1D, P<0.01).
Clusterin inhibits cell apoptosis and reduces expression of pro-apoptotic protein in vivo
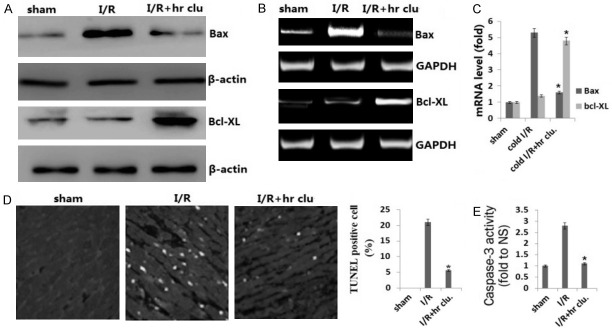
I/R induced cell apoptosis is one of the mechanisms by which I/R causes organ damage. We found pro-apoptotic gene Bax was overexpressed while the anti-apoptotic gene Bcl-XL was decreased in I/R injury heart grafts compared with non-I/R injured tissues. This gene expression change was attenuated by hr clusterin treatment by Western blot (Figure 2A), RT-PCR (Figure 2B), and qRT-PCR assay (Figure 2C).
Figure 2.
Clusterin decreased the expression of pro- and anti-apoptotic genes and reduced apoptotic cells in heart grafts. A. Expression of Bax and Bcl-XL was detected by Western blot assay; B. The expression of Bax and Bcl-XL was determined using RT-PCR. C. The expression of Bax and Bcl-XL was determined using qRT-PCR. D. Cell apoptosis was detected by TUNEL assay; E. Caspase-3 activity in heart tissue was detected with Caspase-3 colorimetric assay kit, quantitatively estimated as the fold-change relative to the I/R group. Vs. I/R, *P<0.01.
The representative photograph shows that TUNEL-positive cardiomyocytes were more frequently observed in the wild-type heart grafts group compared to the hr clusterin group (Figure 2D). As an executioner caspase, caspase-3 is activated in apoptotic cells both by extrinsic (death ligand) and intrinsic (mitochondrial) pathways. The caspase-3 zymogen exhibits virtually no activity until it is cleaved by an initiator caspase following apoptotic signaling events. As shown in Figure 2E, the caspase-3 activity was significantly reduced in the hr clusterin group compared to the wild-type heart grafts group.
Clusterin reduces expression of pro-inflammatory cytokine production in heart graft in vivo
It has been reported that myocardial cells, in response to I/R injury, upregulated the expression of pro-inflammatory cytokines such as TNF-a and IL-1β in I/R injured hearts. To investigate whether inflammatory cytokines are involved in TNF-a and IL-1β in I/R injured hearts, we first assessed the messenger RNA levels of TNF-a and IL-1β in I/R injured hearts by RT-PCR and qRT-PCR. In the hr clusterin groups, the levels of TNF-a and IL-1β were significantly decreased compared with I/R groups by RT-PCR (Figure 3A) and qRT-PCR assay (Figure 3B). The protein levels of TNF-a and IL-1β were also significantly decreased in the hr clusterin groups compared with I/R groups by ELISA assay (Figure 3C).
Figure 3.
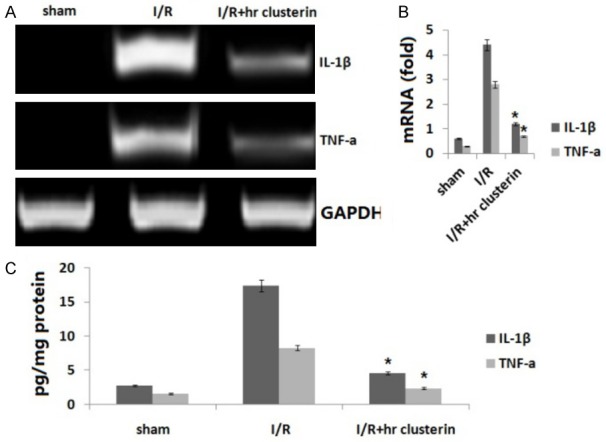
Inflammatory cytokine expression. A. Expression of TNF-a and IL-1β in I/R injured hearts was detected by RT-PCR. B. Expression of TNF-a and IL-1β was determined using qRT-PCR; C. TNF-a and IL-1β in I/R injured hearts was detected by ELISA assay. Vs. I/R, *P<0.01.
Clusterin protects cardiomyocytes from cell apoptosis induced by I/R in vitro
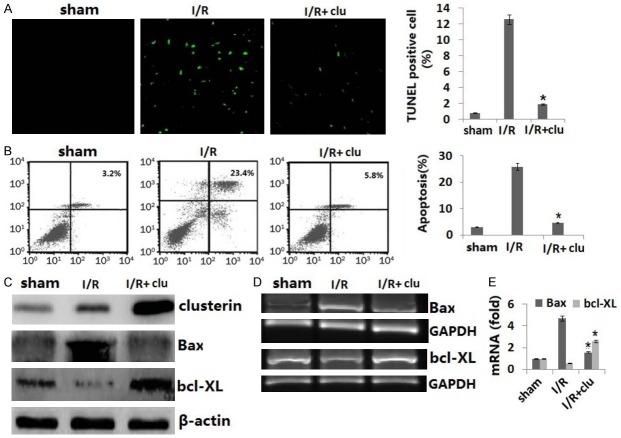
To further verify that the effect of clusterin on cell death induced by cold I/R is protective, not causative, we cultured rat H9C2 cells, the most commonly used heart cell line for in vitro studies of I/R [19]. H9C2 cells were transfected with clusterin expression adenovirus prior to exposure to 16 hours cold hypoxia at 10°C followed by 24 hours reperfusion at 37°C in vitro. Apoptosis in H9C2 cells was assessed by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining (Figure 4A) and annexin V-PI double staining (Figure 4B). We confirmed that I/R resulted in cell apoptosis and was significantly inhibited by the clusterin transfection. We also confirmed that clusterin and bax expression was upregulated both at the mRNA and protein levels in clusterin adenovirus infected cells and bcl-XL was downregulated both at the mRNA and protein levels in clusterin adenovirus infected cells (Figure 4C-E).
Figure 4.
Clusterin prevents cell apoptosis and death induced by I/R in vitro. H9C2 cells (80,000 cells/ well) were plated in six well plates and allowed to culture at 37°C 5% CO2 overnight. Cells were infected with human clusterin expression adenovirus or control null virus for 24 hours. Cells were then subjected to a hypoxia chamber with 0% O2 and 15% CO2 at 10°C for 16 h, followed by 24 hours reperfusion at 5% CO2, 28% O2 at 37°C. A. Cell apoptosis was detected by TUNEL staining; B. Cell apoptosis was detected by double staining with FITC labeled Annexin-V and PI and flow cytometry; C. Protein expression of clusterin, bax, and bcl-XL was detected by Western blot assay; D. Gene expression of clusterin, bax, and bcl-XL was detected by RT-PCR; E. Gene expression of clusterin, bax, and bcl-XL was detected by qRT-PCR. Data are the summary of three independent experiments. Vs. I/R, *P<0.01
Clusterin protects heart cells from I/R injury through the NF-κB signaling pathway
As shown in Figure 5, I/R increased NF-κBp65 (p65) translocation by Western blot assay (Figure 5A) and increased NF-κB activity by EMSA assay (Figure 5B) whereas expression of p65 in the hr clusterin I/R injured grafts was significantly decreased as compared with the wild-type I/R injured grafts and was almost recovered to the levels seen in hearts without I/R injury. These data indicate that clusterin inhibits p65 nucleus translocation.
Figure 5.
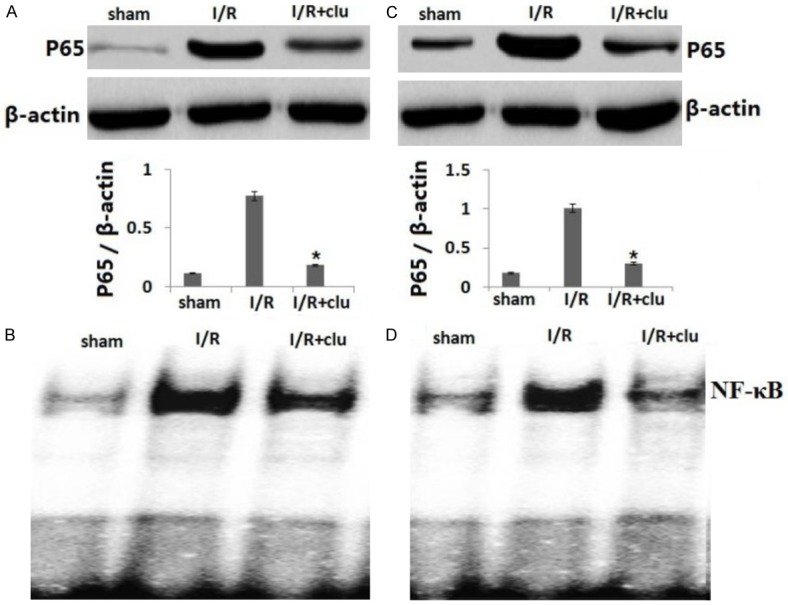
Expression of p-p65. Cells were treated and protein was extracted from the cells as Figure 4. A. The expression of p-p65 was detected by Western blotting in heart graft in vivo; B. The expression of p-p65 was detected by Western blotting in H9C2 cells induced by I/R in vitro; C. The NF-κB activity was detected by EMSA in heart graft in vivo; D. The NF-κB activity was detected by EMSA in H9C2 cells induced by I/R in vitro. Vs. I/R, *P<0.01.
As shown in Figure 5C, 5D, we found that in vitro cold hypoxia/reperfusion also increased NF-κBp65 (p65) translocation and NF-κB activity as compared with the control cells cultured in normal cell culture conditions and that transfection with clusterin expression adenovirus (clusterin cDNA) decreased p65 translocation and NF-κB activity.
Discussion
Cardiac transplantation is the last resort for patients with end-stage heart failure. However, I/R injury is a major issue in cardiac transplantation. I/R injury is associated with increased primary organ dysfunction and subsequent delayed organ function after cardiac transplantation. In the long term, this correlates with increased episodes of acute and chronic rejection. Thus, development of more effective drugs or interventions to protect the myocardium from reperfusion injury is required to provide greater clinical benefits for patients with ischemic heart disease.
Clusterin, also known as testosterone-repressed message-2, is overexpressed in the rat prostate during castration-induced programmed cell death [20]. As clusterin is usually present in resistant cells [21,22], it has been described as an anti-apoptotic factor. In cultures of renal tubular epithelial cells, downregulation of clusterin expression results in an increase of pro-inflammatory cytokine-induced cell apoptosis. In a mouse model of renal I/R injury, overexpression of clusterin is associated with a reduction of renal I/R injury and dysfunction of the kidneys and also contributes to the promotion of renal repair [23]. These results suggest that the local expression of clusterin may protect renal cells from cell death during kidney transplant rejection.
Although clusterin has long been proposed to participate in cell survival and it has been studied extensively to inhibit the pro-inflammatory cytokine TNF-α, there have been no studies carried out to investigate the link between clusterin and survival signaling pathways. Clusterin expression renders donor hearts resistant to cold I/R injury in transplantation, suggesting that upregulation of clusterin expression in donor hearts may have the potential to protect heart grafts from cold I/R injury [24,25]. In this study, we found that expression of pro-inflammatory cytokines (IL-1β and TNF-α) was impeded by hr clusterin treatment in a cold I/R model. Furthermore, we observed that hr clusterin inhibited p65, a member of the NF-κB family, and NF-κB activity. Our data suggests that the attenuation of inflammation by clusterin is mediated by inhibition of the NF-κB signaling pathway. Our study shows a new circumstance in which clusterin protects against inflammation and further supports a role for clusterin as a cardioprotective agent against inflammation under ischemic conditions.
Apoptosis is a mechanism of programmed cell death. This process usually involves an increased ratio of pro-apoptotic (i.e., Bax and Bim) versus anti-apoptotic (i.e., Bcl-2 and Bcl-xL) molecules. NF-κB antagonizes apoptosis and promotes cell survival by inducing the expression of pro-survival genes (Bcl-2, Bcl-xL, and IEX1) and by repressing the proapoptotic genes Bax and Bim [26,27]. Zhang et al. [28] reported the I/R-induced upregulation of the proapoptotic protein and mRNA levels of Bax, Cyto-c, Apaf-1, and caspase-9/3 while it increased the ischemia/reperfusion-induced decrease of the anti-apoptotic factor Bcl-2. Clusterin is a stress-associated cytoprotective chaperone expressed in many cancers that is upregulated in an adaptive cell survival manner by various apoptotic triggers such as Bax and Bcl-2/Bcl-xL and confers treatment resistance [29,30].
In this study, we found that the number of apoptotic cells was decreased in hr clusterin-treated grafts, as detected by TUNEL assays. Expression of the apoptotic gene Bax was reduced and the antiapoptotic gene Bcl-xL was increased in heart grafts with overexpression of clusterin compared with those in WT grafts. Additionally, our in vitro results with H9C2 cells showed that hr clusterin treatment reduced H9C2 cell apoptosis under cold hypoxia/reperfusion stress by downregulating Bax and upregulating Bcl-xL. Therefore, clusterin is an antiapoptotic protein that protects heart cells against apoptosis induced by cold I/R injury in a heart transplant model. The ability of clusterin to prevent cell apoptosis suggests that it has a potential therapeutic value in preventing I/R injury in heart transplantation.
In conclusion, this study is the first to demonstrate that clusterin is a promising target to prevent cold I/R injury in heart transplantation and to show association of clusterin with Bax. We also demonstrate that the protective effect of clusterin is mediated by Bax and NF-κB signaling.
Acknowledgements
This work was financially supported by grants from Yantai Youth Elite Scientific Research Fund (No: 2016YE026).
Disclosure of conflict of interest
None.
References
- 1.Stringham JC, Southard JH, Hegge J, Triemstra L, Fields BL, Belzer FO. Limitations of heart preservation by cold storage. Transplantation. 1992;53:287–294. doi: 10.1097/00007890-199202010-00007. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka M, Mokhtari GK, Terry RD. Prolonged cold ischemia in rat cardiac allografts promotes ischemia-reperfusion injury and the development of graft coronary artery disease in a linear fashion. J Heart Lung Transplant. 2005;24:1906–1914. doi: 10.1016/j.healun.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Schneeberger S, Amberger A, Mandl J. Cold ischemia contributes to the development of chronic rejection and mitochondrial injury after cardiac transplantation. Transpl Int. 2010;23:1282–1292. doi: 10.1111/j.1432-2277.2010.01126.x. [DOI] [PubMed] [Google Scholar]
- 4.Gaudin PB, Rayburn BK, Hutchins GM. Peritransplant injury to the myocardium associated with the development of accelerated arteriosclerosis in heart transplant recipients. Am J Surg Pathol. 1994;18:338–346. doi: 10.1097/00000478-199404000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Ramjug S, Hussain N, Yonan N. Prolonged time between donor brain death and organ retrieval results in an increased risk of mortality in cardiac transplant recipients. Interact Cardiovasc Thorac Surg. 2011;12:938–942. doi: 10.1510/icvts.2010.252809. [DOI] [PubMed] [Google Scholar]
- 6.Tuuminen R, Syrjälä S, Krebs R. Donor simvastatin treatment abolishes rat cardiac allograft ischemia/reperfusion injury and chronic rejection through microvascular protection. Circulation. 2011;124:1138–1150. doi: 10.1161/CIRCULATIONAHA.110.005249. [DOI] [PubMed] [Google Scholar]
- 7.Cobo JL, Del Río Gallegos F. Organ preservation. Med Intensiva. 2009;33:282–292. doi: 10.1016/S0210-5691(09)72196-5. [DOI] [PubMed] [Google Scholar]
- 8.Zheng X, Vladau C, Zhang X, Suzuki M, Ichim TE, Zhang ZX, Li M, Carrier E, Garcia B, Jevnikar AM, Min WP. A novel in vivo siRNA delivery system specifically targeting dendritic cells and silencing CD40 genes for immunomodulation. Blood. 2009;113:2646–2654. doi: 10.1182/blood-2008-04-151191. [DOI] [PubMed] [Google Scholar]
- 9.Jones SE, Jomary C. Clusterin. Int J Biochem Cell Biol. 2002;34:427–431. doi: 10.1016/s1357-2725(01)00155-8. [DOI] [PubMed] [Google Scholar]
- 10.Kim JH, Yu YS, Kim JH, Kim KW, Min BH. The role of clusterin in in vitro ischemia of human retinal endothelial cells. Curr Eye Res. 2007;32:693–698. doi: 10.1080/02713680701487871. [DOI] [PubMed] [Google Scholar]
- 11.Trougakos IP, Gonos ES. Regulation of clusterin/apolipoprotein J, a functional homologue to the small heat shock proteins, by oxidative stress in ageing and age-related diseases. Free Radic Res. 2006;40:1324–1334. doi: 10.1080/10715760600902310. [DOI] [PubMed] [Google Scholar]
- 12.Miyata M, Biro S, Kaieda H, Eto H, Orihara K, Kihara T, Obata H, Matsushita N, Matsuyama T, Tei C. Apolipoprotein J/clusterin is induced in vascular smooth muscle cells after vascular injury. Circulation. 2001;104:1407–1412. doi: 10.1161/hc3701.095583. [DOI] [PubMed] [Google Scholar]
- 13.Krijnen PA, Cillessen SA, Manoe R, Muller A, Visser CA, Meijer CJ, Musters RJ, Hack CE, Aarden LA, Niessen HW. Clusterin: a protective mediator for ischemic cardiomyocytes? Am J Physiol Heart Circ Physiol. 2005;289:H2193–2202. doi: 10.1152/ajpheart.00355.2005. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Sagar MB, Wassler M, Shelat H, Geng YJ. Apolipoprotein-J prevention of fetal cardiac myoblast apoptosis induced by ethanol. Biochem Biophys Res Commun. 2007;357:157–161. doi: 10.1016/j.bbrc.2007.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirozane T, Matsumori A, Furukawa Y, Sasayama S. Experimental graft coronary artery disease in a murine heterotopic cardiac transplant model. Circulation. 1995;91:386–392. doi: 10.1161/01.cir.91.2.386. [DOI] [PubMed] [Google Scholar]
- 16.Monici MC, Aguennouz M, Mazzeo A, Messina C, Vita G. Activation of nuclear factor-kappaB in inflammatory myopathies and Duchenne muscular dystrophy. Neurology. 2003;60:993–997. doi: 10.1212/01.wnl.0000049913.27181.51. [DOI] [PubMed] [Google Scholar]
- 17.Makowka L, Zerbe TR, Chapman F, Qian SG, Sun H, Murase N, Kormos R, Snyder J, Starzl TE. Prolonged rat cardiac preservation with UW lactobionate solution. Transplant Proc. 1989;21:1350. [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, Liu Y, Han Y. Protective effects of aliskiren on ischemia-reperfusion-induced renal injury in rats. Eur J Pharmacol. 2013;718:160–166. doi: 10.1016/j.ejphar.2013.08.038. [DOI] [PubMed] [Google Scholar]
- 19.Waza AA, Andrabi K, Hussain MU. Protein kinase C (PKC) mediated interaction between conexin43 (Cx43) and K(+)(ATP) channel subunit (Kir6.1) in cardiomyocyte mitochondria: implications in cytoprotection against hypoxia induced cell apoptosis. Cell Signal. 2014;26:1909–17. doi: 10.1016/j.cellsig.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Takahashi K, Piao H, Qu P, Naruse K. 9-Phenanthrol, a TRPM4 inhibitor, protects isolated rat hearts from ischemia-reperfusion injury. PLoS One. 2013;8:e70587. doi: 10.1371/journal.pone.0070587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redondo M, Tellez T, Roldan MJ. The role of clusterin (CLU) in malignant transformation and drug resistance in breast carcinomas. Adv Cancer Res. 2009;105:21–43. doi: 10.1016/S0065-230X(09)05002-7. [DOI] [PubMed] [Google Scholar]
- 22.Muhammad LA, Saad F. The role of clusterin in prostate cancer: treatment resistance and potential as a therapeutic target. Expert Rev Anticancer Ther. 2015;15:1049–1061. doi: 10.1586/14737140.2015.1064769. [DOI] [PubMed] [Google Scholar]
- 23.Zhou W, Guan Q, Kwan CC, Chen H, Gleave ME, Nguan CY, Du C. Loss of clusterin expression Worsens renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2010;298:F568–78. doi: 10.1152/ajprenal.00399.2009. [DOI] [PubMed] [Google Scholar]
- 24.Li S, Guan Q, Chen Z, Gleave ME, Nguan CY, Du C. Reduction of cold ischemia-reperfusion injury by graft-expressing clusterin in heart transplantation. J Heart Lung Transplant. 2011;30:819–826. doi: 10.1016/j.healun.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Guan Q, Li S, Yip G, Gleave ME, Nguan CY, Du C. Decrease in donor heart injury by recombinant clusterin protein in cold preservation with University of Wisconsin solution. Surgery. 2012;151:364–371. doi: 10.1016/j.surg.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 26.Catz SD, Johnson JL. Transcriptional regulation of bcl-2 by nuclear factor kappa B and its significance in prostate cancer. Oncogene. 2001;20:7342–7351. doi: 10.1038/sj.onc.1204926. [DOI] [PubMed] [Google Scholar]
- 27.Grimm T, Schneider S, Naschberger E. EBV latent membrane protein-1 protects B cells from apoptosis by inhibition of BAX. Blood. 2005;105:3263–3269. doi: 10.1182/blood-2004-07-2752. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Zhang J, Yu P, Chen M, Peng Q, Wang Z, Dong N. Remote ischaemic preconditioning and sevoflurane postconditioning synergistically protect rats from myocardial injury induced by ischemia and reperfusion partly via inhibition TLR4/MyD88/NF-κB signaling pathway. Cell Physiol Biochem. 2017;41:22–32. doi: 10.1159/000455815. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Wang X, Zhao H, Liang B, Du Q. Clusterin confers resistance to TNF-alpha-induced apoptosis in breast cancer cells through NF-kappaB activation and Bcl-2 overexpression. J Chemother. 2012;24:348–357. doi: 10.1179/1973947812Y.0000000049. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, Kim JK, Edwards CA, Xu Z, Taichman R, Wang CY. Clusterin inhibits apoptosis by interacting with activated Bax. Nat Cell Biol. 2005;7:909–915. doi: 10.1038/ncb1291. [DOI] [PubMed] [Google Scholar]