Abstract
Objective: To examine whether the expression levels of endogenous H2S synthases and hedgehog (Hh) signaling pathway components correlate with the clinicopathological characteristics of papillary thyroid cancer (PTC) patients. Methods: A retrospective analysis was conducted of clinicopathological data obtained from 176 patients diagnosed with PTC, and immunohistochemical methods were used to detect the expression levels of endogenous H2S synthases cystathionine γ-lyase (CSE), cystathionine β-synthase (CBS), and 3-mercaptopyruvate sulfurtransferase (MPST), as well as three molecules in the Hh signaling pathway: sonic hedgehog (SHH), patched (PTCH), and smoothened (SMO). Specimens of PTC tissue (n=176) and normal para-cancerous thyroid tissue (n=134) were obtained from 176 patients who underwent a total thyroidectomy or thyroid glandular follicle and isthmus resection and analyzed by immunohistochemical methods for their levels of CSE, CBS, MPST, SHH, PTCH, and SMO expression. Results: We found that CSE was overexpressed in PTC tissues, while CBS and MPST were only slightly expressed in PTC tissues at levels similar to those in adjacent normal tissues. The levels of CSE expression were positively correlated with tumor size, extrathyroidal extension (ETE), and lymph node metastasis (LNM), but were not correlated with patient gender, age, or TNM stage. SHH, PTCH, and SMO Hh signaling pathway components were widely expressed in PTC tissues, and their expression correlated with larger tumor size, ETE, and LNM, but not with patient gender, age, or TNM stage, suggesting that activation of the Hh signaling pathway is involved in thyroid tumor progression. Conclusions: These data suggest that a high level of CSE expression accompanied by Hh signaling pathway activation is involved in the pathogenesis and progression of PTC.
Keywords: Papillary thyroid cancer, hydrogen sulfide synthases, Hh signaling pathway
Introduction
Thyroid cancer is the most common malignancy of the human endocrine system, and head and neck tumors are becoming the most frequent types of solid tumors seen in the clinic [1,2]. Since 2010, thyroid cancer has been the fifth most common malignancy among females in the United States, and ~56,000 new cases of thyroid cancer are diagnosed annually [3]. In Italy, thyroid cancer is the second most common cancer among females aged <45 years [4]. In 2011, thyroid cancer was the most prevalent malignant tumor in South Korea [5]. The China Cancer Registration Center 2012 annual report showed that thyroid cancer was the fourth most prevalent malignancy found in urban areas, and from 2003 to 2007, had an annual increase of 14.5%. Nearly 90,000 new cases of thyroid cancer were reported in China in 2015, and ~6,800 people died of the disease. Particularly noteworthy is the finding was that thyroid cancer is the most frequently diagnosed malignancy among women <30 years old [6].
Many studies have suggested that extrathyroidal extension (ETE) and lymph node metastasis are responsible for the significant increase in the risk of death among patients with thyroid cancer [7-9]. Although the majority of papillary thyroid cancers (PTCs) have a favorable prognosis, ~10% of PTCs have a poor prognosis due to the presence of distant metastases and a loss of cellular differentiation.
Hydrogen sulfide (H2S) is an important gas signaling molecule with numerous biologic effects [10]. Meng JL et al. confirmed that H2S helps to protect nerve cells against hypoxia-induced oxidative stress injuries [11]. In recent years, studies have shown that H2S plays an important role in the pathophysiology of tumors [12]. Increased levels of cystathionine β-synthase (CBS) [13] or cystathionine γ-lyase (CSE) expression occur in a variety of tumor cells [14]; furthermore, tumor cell survival depends on the H2S produced by CBS [15] or CSE [16]. Studies have shown that endogenous H2S can promote proliferation, invasion, and migration in tumor cells, including colon cancer [17,18] and breast cancer [19] cells. However, another report suggested that increasing H2S concentrations can also exert an anti-tumor effect [20,21]. Szabo pointed out that reduced levels of H2S production or increased H2S concentrations over a certain threshold exert an anti-tumor effect [22]. In this study, we explored how endogenous H2S affects the invasion and migration of PTC cells.
The sonic hedgehog (Hh) pathway is activated in several types of malignancy and plays important roles in tumorigenesis and tumor cell proliferation [23-27]. However, the roles of the Hh signaling pathway and endogenous H2S activation and their association in PTC patients with different clinicopathological features have not yet been well documented. Thus, the present study was undertaken to examine the expression levels of three different H2S-producing enzymes (CBS, CSE, and MPST) and Hh signaling pathway molecules (SHH, PTCH, and SMO) to elucidate their clinical significance in PTC and further explore their association.
Materials and methods
Patients and tissue samples
Specimens of PTC tissue (n=176) and normal para-cancerous thyroid tissue (n=134, located 2 cm from the edge of the tumor tissue) were obtained from 176 patients (mean age 39.68±12.56 y, age range 16-77 y) who had undergone a thyroidectomy or thyroid glandular follicle and isthmus resection at the Traditional Chinese Medicine-Integrated Hospital of Southern Medical University from September 2014 to December 2016. The specimens were from 46 male patients (26%) and 130 female patients (74%). A signed written Informed Consent was obtained from each subject. The pathologic diagnosis of each sample was confirmed by at least two independent experienced pathologists.
Histopathological examination
An immunohistochemical staining analysis of endogenous H2S synthases CSE, CBS, and MPST and three molecules in the Hh signaling pathway (SHH, PTCH, and SMO) was performed on sections of 10%-neutral-formalin-fixed, paraffin-embedded (FFPE) thyroid tissue that were of 2 µm thickness. After the sections were dewaxed, their antigens were retrieved, and the slides with tissue sections were treated with 3% hydrogen peroxide for 15 min. The sections were then incubated with normal goat serum for 30 min to block nonspecific-binding sites. Next, the tissue sections were then incubated with primary antibodies against CBS (1:200) (Abnova, Taipei City, Taiwan), CSE (1:200) (Abnova), MPST (1:100) (Santa Cruz Biotechnology, Dallas TX, USA), SHH (EP1190Y; Novus Biologicals, Inc., Littleton, CO, USA), PTCH (H0267, sc-9016; Santa Cruz Biotechnology,), and SMO (H-300, sc-13943; Santa Cruz Biotechnology) overnight at 4°C. The slides were then washed with phosphate-buffered saline containing 0.1% v/v Tween-20 and incubated with horse radish peroxidase-conjugated goat anti-mouse immunoglobulin (Santa Cruz Biotechnology) for 20 min at room temperature. Finally, the slides were treated with peroxidase-conjugated streptavidin and stained with DAB. Images were photographed using a confocal microscope (Olympus, Tokyo, Japan). Staining intensity was scored as “-” (negative), “+” (moderate) or “++” (strong). The extent of staining was scored as “-” (<10% of thyroid cells stained), “+” (10%-50% stained) or “++” (>50% stained). The immunohistochemistry results were evaluated by two independent pathologists.
Statistical analysis
All data were analyzed using IBM SPSS Statistics for the Social Sciences, Version 20 (IBM Corp., Armonk, NY, USA). P-values <0.05 were considered statistically significant. Descriptive statistics were applied based on the distribution of the variables. The X2 test or Fisher’s exact test was used to determine whether there were significant differences in the levels of CSE, CBS, MPST, SHH, PTCH, and SMO expression in the PTC tissues vs. the adjacent normal thyroid tissues. Those tests were also used to determine whether there were significant differences in CSE, SHH, PTCH, and SMO expression among patients of different age and gender, and whether CSE, SHH, PTCH, and SMO expression correlated with tumor size, the presence of ETE and LNM, and TNM stage. Spearman’s correlation test was used to assess the association between CSE expression and the expression of Hh signaling pathway molecules.
Results
CSE was overexpressed in PTC tissue
Immunohistochemical staining was performed to detect endogenous CSE, CBS, and MPST expression, and the results showed that all three of these H2S synthesizing enzymes were present mostly in the cytoplasm. Examples of PTC tissue with CSE staining intensities graded as negative (-), moderate (+), and positive (++) are shown in Figure 1. As shown in Table 1, there was a selective upregulation of CBS expression in PTC tissues. Overall, CSE was expressed in 86.93% of the PTC tissue specimens vs. 23.88% of the adjacent non-cancerous tissue specimens. However, expression of the other two H2S-producing enzymes (CSE and MPST) was not upregulated in the tumor tissues. The positive expression rates for CBE and MPST were 29.55% (52/176) and 26.70% (47/176), respectively, in PTC tissues, and 20.15% (27/134) and 26.87% (36/134), respectively, in adjacent normal thyroid tissues.
Figure 1.
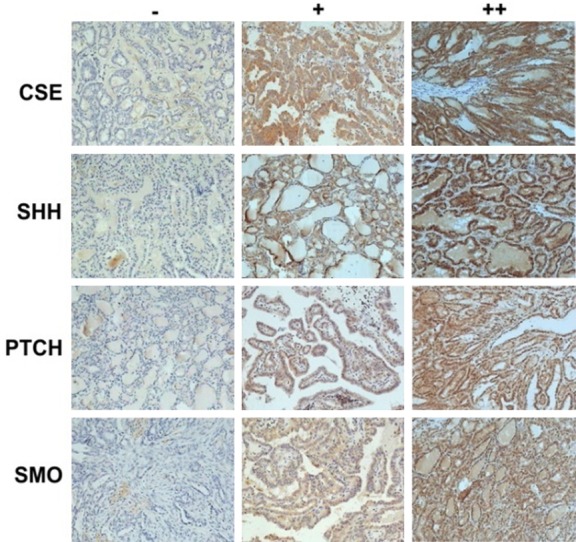
Immunohistochemical staining of CSE, SHH, PTCH, and SMO. The expression levels of CSE and three Hh signaling molecules in PTC tissues were analyzed by IHC staining performed using specific antibodies against PTC. The expression profiles of CSE and three Hh signaling molecules in PTC tissues were graded as “-” (negative), “+” (moderate), or “++” (strong) (×400).
Table 1.
Extent of CBS, CSE, and MPST staining in PTC tissues and adjacent normal tissues
| PTC (n=176) | Adjacent normal tissues (n=134) | p-value | ||
|---|---|---|---|---|
| CSE | - | 23 | 102 | 0.000 |
| + | 48 | 26 | ||
| ++ | 105 | 6 | ||
| CBS | - | 124 | 107 | 0.117 |
| + | 43 | 24 | ||
| ++ | 9 | 3 | ||
| MPST | - | 129 | 98 | 0.975 |
| + | 37 | 23 | ||
| ++ | 10 | 13 | ||
“-” (negative, <10% positive cells); “+” (moderate, 10-50% positive cells); “++” (strong, >50% positive cells).
Expression of Hh signaling pathway components was upregulated in PTC tissue
IHC staining was performed to analyze the expression of three molecules in the Hh pathway (SHH, PTCH, and SMO) in PTC tissues. SHH, PTCH, and SMO were present mostly in the cytoplasm. Examples of negative (-), moderate (+), and positive (++) staining for SHH, PTCH, and SMO expression in PTC tissues are shown in Figure 1. As shown in Table 2, the positive expression rates for SHH, PTCH, and SMO in samples of PTC tissue were 63.07%, 67.61%, and 59.66%, respectively, whereas those rates in adjacent normal thyroid tissues were 18.66%, 17.16%, and 26.87%, respectively. These results showed that expression of Hh signaling pathway components SHH, PTCH, and SMO was upregulated in PTC specimens, when compared to their expression in adjacent normal tissues, and the differences were statistically significant (all P values <0.001) (Table 2).
Table 2.
Expression of CSE and three Hh signaling pathway components in PTC tissues
| PTC (n=176) | Adjacent normal tissues (n=134) | p-value | ||
|---|---|---|---|---|
| SHH | - | 65 | 109 | 0.000 |
| + | 59 | 13 | ||
| ++ | 52 | 12 | ||
| PTCH | - | 57 | 111 | 0.000 |
| + | 71 | 13 | ||
| ++ | 48 | 10 | ||
| SMO | - | 71 | 98 | 0.000 |
| + | 74 | 27 | ||
| ++ | 31 | 9 | ||
“-” (negative, <10% positive cells); “+” (moderate, 10-50% positive cells); “++” (strong, >50% positive cells).
Correlation between upregulation of CSE and clinicopathological parameters in PTC patients
The clinicopathological characteristics (gender, age, tumor size, ETE, LNM, and tumor stage) of the PTC patients who provided the 176 PTC lesions and the CSE expression levels in those lesions as determined by IHC were analyzed, and the results are shown in Table 3. We found that CSE expression was positively correlated with tumor size (P=0.011), ETE (P=0.048), and LNM (P=0.005). No significant associations were found between CSE expression and gender (P=0.616), age (P=0.355), and TNM stage (P=0.416) of the PTC patients.
Table 3.
Associations between CSE expression and the clinical/pathological characteristics of PTC patients
| Total | CSE (-/+) | 2 | p-value | ||
|---|---|---|---|---|---|
| Gender | Female | 130 | 16/114 | 0.253 | 0.616 |
| Male | 46 | 7/39 | |||
| Age | <45 | 115 | 13/102 | 0.909 | 0.355 |
| ≥45 | 61 | 10/51 | |||
| Tumor size | ≤2 cm | 111 | 20/91 | 6.482 | 0.011 |
| >2 cm | 65 | 3/62 | |||
| ETE | Yes | 25 | 0/25 | 4.380 | 0.048 |
| No | 151 | 23/128 | |||
| LNM | Yes | 106 | 7/99 | 9.804 | 0.003 |
| No | 70 | 16/54 | |||
| TNM stage | I+II | 138 | 20/118 | 1.142 | 0.416 |
| III+IV | 38 | 3/35 |
P-values are based on chi-square test; “-” (negative), “+” (positive). ETE, extrathyroidal extension; LNM, lymph node metastasis.
Relationship between activation of the Hh signaling pathway and clinicopathologic characteristics of PTC patients
As shown in Table 4, we found that expression of the Hh signaling pathway components SHH, PTCH, and SMO was positively correlated with tumor size (P=0.010, P=0.047, P=0.026, respectively), ETE (P=0.024, P=0.020, P=0.028, respectively), and LNM (P=0.008, P=0.0268, P=0.013, respectively). SHH, PTCH, and SMO expression were not significantly associated with gender (P=0.726, P=0.583, P=0.227, respectively), age (P=0.418, P=0.736, P=0.424, respectively) or TNM stage (P=0.455, P=0.436, P=0.578, respectively) of the PTC patients.
Table 4.
Associations between expression of the Hh signaling pathway components and the clinical/pathological characteristics of PTC patients
| Total | SHH (-/+) | p-value | PTCH (-/+) | p-value | SMO (-/+) | p-value | ||
|---|---|---|---|---|---|---|---|---|
| Gender | Female | 130 | 47/83 | 0.726 | 44/86 | 0.583 | 56/74 | 0.227 |
| Male | 46 | 18/28 | 13/33 | 15/31 | ||||
| Age | <45 | 115 | 40/75 | 0.418 | 36/79 | 0.736 | 49/66 | 0.424 |
| ≥45 | 61 | 25/36 | 21/40 | 22/39 | ||||
| Tumor size | ≤2 cm | 111 | 49/62 | 0.010 | 42/69 | 0.047 | 52/59 | 0.026 |
| >2 cm | 65 | 16/49 | 15/50 | 19/46 | ||||
| ETE | Yes | 25 | 4/21 | 0.024 | 3/22 | 0.020 | 5/20 | 0.028 |
| No | 151 | 61/90 | 54/97 | 66/85 | ||||
| LNM | Yes | 106 | 34/72 | 0.008 | 32/74 | 0.026 | 38/68 | 0.013 |
| No | 70 | 37/33 | 33/37 | 39/31 | ||||
| TNM stage | I+II | 138 | 49/89 | 0.455 | 47/91 | 0.436 | 54/84 | 0.578 |
| III+IV | 38 | 16/22 | 10/28 | 17/21 |
P-values based on the chi-square test; “-” (negative), “+” (positive). ETE, extrathyroidal extension; LNM, lymph node metastasis.
Relationship between upregulation of CSE and an aberrant Hh signaling pathway in PTC tissue
Our results showed that high levels of CSE expression in PTC tissues were accompanied by an activated Hh signaling pathway, as significant positive correlations between CSE expression and SHH, PTCH, and SMO expression were found in PTC tissues (r=0.266, P<0.001; r=0.225, P<0.001; r=0.295, P<0.001, respectively) (Tables 3 and 4).
Discussion
Hydrogen sulfide (H2S) is a colorless and highly water soluble gas with an irritating smell. Although atmospheric H2S gas is toxic, endogenous H2S, whose formation is catalyzed by cystathionine β-synthase (CBS), cystathionine-γ-lyase (CSE) derived from L-cysteine (L-Cys), and 3-mercaptopyruvate sulphur transferase (MPST, also known as 3-MST) through one-carbon metabolism and the trans-sulphuration pathway, is now considered the third gasotransmitter, along with nitric oxide (NO) and carbon monoxide (CO) [28-31]. Increasing evidence suggests that H2S is closely associated with the occurrence and development of tumors. Studies show that different H2S-associated pathways are involved in various types of cancer, and that the pathways utilized to promote cell proliferation, survival, and death are dependent on the tumor cell type [13]. Increased H2S production, mainly from CBS, but in other cell lines also from CSE, plays an essential role in the proliferation of colon and ovarian cancer cells [32,33]; furthermore, CBS silencing in glioma cells accelerates tumor cell proliferation [34]. However, the expression of endogenous H2S synthases CSE, CBS, and MPST has never been studied in PTC cells. In this study, we used immunohistochemical staining methods to analyze CSE, CBS, and MPST expression in samples of PTC tissue and adjacent normal thyroid tissue. Our results provide histopathologic evidence that endogenous H2S, synthesized by CSE, is overexpressed in PTC tissue. The other two H2S synthases (CBS and MPST) were only slightly expressed in tumor tissues at levels similar to those in adjacent normal tissues, which was not accordance with findings in other tumor tissues [32,35]. In our study, the levels of CSE expression were positively correlated with tumor size, ETE, and LNM; however, they did not significantly correlate with patient gender, age, or tumor TNM stage.
In the canonical Hh pathway, SHH binding to patched (PTCH), a 12-passtransmembrane receptor, leads to the release of smoothened (SMO), a 7-pass transmembrane protein, and the subsequent activation of GLI transcription factors. Several different mechanisms may activate Hh signaling pathways needed for tumor development. For example, the Hh signaling pathway component PTCH in tumor cells may accumulate deletion mutations that change its function, or SMO may acquire mutations that promote its abnormal sustained activation in the absence of the ligand SHH [36,37]. Also, the self-synthesis of SHH ligands in tumor cells may permit autocrine or paracrine signaling hormones to activate the Hh signaling pathway [38].
Proper function of the Hh signaling pathway is essential for thyroid organogenesis. The thyroid primordium fails to form two lobes in SHH-/- mice [39]. The Hh signaling pathway is activated in thyroid neoplasms, and contributes to increased cell proliferation [40,41]. Hh pathway-stimulated thyroid tumor cell motility and invasiveness are largely mediated by activated AKT and c-Met, with little involvement of the epithelial-mesenchymal transition process [42]. In the present study, we found that SHH, PTCH, and SMO were widely expressed in PTC tissues, but only slightly expressed in adjacent normal thyroid tissues. These observations suggest that the Hh signaling pathway is activated during thyroid tumorigenesis. This notion is consistent with the results of several other studies that showed increased expression of several Hh signaling pathway components in malignant tumors, such as breast cancer, endometrial adenocarcinoma, and ovarian carcinoma.
We also found that SHH, PTCH, and SMO were concomitantly upregulated in PTC tissues and positively correlated with a larger tumor size, ETE, and LNM, but not with patient gender, age or tumor TNM stage. These findings suggest that activation of the Hh signaling pathway may be involved in thyroid tumor progression.
Conclusion
In conclusion, our results suggest that high levels of CSE expression accompanied by an activated Hh signaling pathway can promote the development and progression of PTC.
Acknowledgements
This study was supported by the Guangdong Provincial Science and Technology Project (No. 2016A020215161) and the Medical Science and Technology Research Fund of Guangdong Province (No. A2016339).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Brito JP, Hay ID, Morris JC. Low risk papillary thyroid cancer. BMJ. 2014;348:g3045. doi: 10.1136/bmj.g3045. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4.Dal Maso L, Lise M, Zambon P, Falcini F, Crocetti E, Serraino D, Cirilli C, Zanetti R, Vercelli M, Ferretti S, Stracci F, De Lisi V, Busco S, Tagliabue G, Budroni M, Tumino R, Giacomin A, Franceschi S AIRTUM Working Group. Incidence of thyroid cancer in Italy, 1991-2005: time trends and age-period-cohort effects. Ann Oncol. 2011;22:957–963. doi: 10.1093/annonc/mdq467. [DOI] [PubMed] [Google Scholar]
- 5.Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2012;46:1–14. doi: 10.4143/crt.2013.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 7.Wu HS, Young MT, Ituarte PH, D’Avanzo A, Duh QY, Greenspan FS, Loh KC, Clark OH. Death from thyroid cancer of follicular cell origin. J Am Coll Surg. 2000;191:600–606. doi: 10.1016/s1072-7515(00)00731-6. [DOI] [PubMed] [Google Scholar]
- 8.Stroup AM, Harrell CJ, Herget KA. Longterm survival in young women: hazards and competing risks after thyroid cancer. J Cancer Epidemiol. 2012;2012:641372. doi: 10.1155/2012/641372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollenbeak CS, Boltz MM, Schaefer EW, Saunders BD, Goldenberg D. Recurrence of differentiated thyroid cancer in the elderly. Eur J Endocrinol. 2013;168:549–556. doi: 10.1530/EJE-12-0848. [DOI] [PubMed] [Google Scholar]
- 10.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184–99. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng JL, Mei WY, Dong YF, Wang JH, Zhao CM, Lan AP, Yang CT, Chen PX, Feng JQ, Hu CH. Heat shock protein 90 mediates cytoprotection by H2S against chemical hypoxia-induced injury in PC12 cells. Clin Exp Pharmacol Physiol. 2010;38:42–49. doi: 10.1111/j.1440-1681.2010.05462.x. [DOI] [PubMed] [Google Scholar]
- 12.Hellmich MR, Coletta C, Chao C, Szabo C. The therapeutic potential of cystathionine β-synthetase/hydrogen sulfide inhibition in cancer. Antioxid Redox Signal. 2011;22:424–48. doi: 10.1089/ars.2014.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hellmich MR, Szabo C. Hydrogen sulfide and cancer. Handb Exp Pharmacol. 2015;230:233–241. doi: 10.1007/978-3-319-18144-8_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panza E, De CP, Armogida C, Scognamiglio G, Gigantino V, Botti G, Germano D, Napolitano M, Papapetropoulos A, Bucci M, Cirino G, Ianaro A. Role of the cystathionine γ lyase/hydrogen sulfide pathway in human melanoma progression. Pigment Cell Melanoma Res. 2015;28:61–72. doi: 10.1111/pcmr.12312. [DOI] [PubMed] [Google Scholar]
- 15.Módis K, Coletta C, Asimakopoulou A, Szczesny B, Chao C, Papapetropoulos A, Hellmich MR, Szabo C. Effect of S-adenosyl-L-methionine (SAM), an allosteric activator of cystathionine-β-synthase (CBS) on colorectal cancer cell proliferation and bioenergetics in vitro. Nitric Oxide. 2011;41:146–156. doi: 10.1016/j.niox.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan K, Li N, Qi J, Yin P, Zhao C, Wang L, Li Z, Zha X. Wnt/β-catenin signaling induces the transcription of cystathionine-γ-lyase, a stimulator of tumor in colon cancer. Cell Signal. 2014;26:2801–2808. doi: 10.1016/j.cellsig.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 17.Szabo C, Coletta C, Chao C, Módis K, Szczesny B, Papapetropoulos A, Hellmich MR. Tumorderived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc Natl Acad Sci U S A. 2013;110:12474–9. doi: 10.1073/pnas.1306241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong M, Tang X, He K. [Effect of hydrogen sulfide on human colon cancer SW480 cell proliferation and migration in vitro] . Nan Fang Yi Ke Da Xue Xue Bao. 2014;34:699–703. [PubMed] [Google Scholar]
- 19.Sen S, Kawahara B, Gupta D, Tsai R, Khachatryan M, Roy-Chowdhuri S, Bose S, Yoon A, Faull K, Farias-Eisner R, Chaudhuri G. Role of cystathionine β-synthase in human breast cancer. Free Radic Biol Med. 2015;86:228–238. doi: 10.1016/j.freeradbiomed.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Lee ZW, Deng LW. Role of H2S donors in cancer biology. Handb Exp Pharmacol. 2015;230:243–65. doi: 10.1007/978-3-319-18144-8_13. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Qi Q, Yang J, Sun D, Li C, Xue Y, Jiang Q, Ye T, Xu C, Wang R. An anticancer role of hydrogen sulfide in human gastric cancer cells. Oxid Med Cell Longev. 2015;2015:636410. doi: 10.1155/2015/636410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szabo C. Gasotransmitters in cancer: from pathophysiology to experimental therapy. Nat Rev Drug Discov. 2015;15:185–203. doi: 10.1038/nrd.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, Rosenthal A, Epstein EH Jr, de Sauvage FJ. Activating smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–2. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 24.Kasperczyk H, Baumann B, Debatin KM, Fulda S. Characterization of sonic hedgehog as a novel NF-kappaB target gene that promotes NF-kappaB-mediated apoptosis resistance and tumor growth in vivo. FASEB J. 2009;23:21–33. doi: 10.1096/fj.08-111096. [DOI] [PubMed] [Google Scholar]
- 25.Wang LH, Choi YL, Hua XY, Shin YK, Song YJ, Youn SJ, Yun HY, Park SM, Kim WJ, Kim HJ, Choi JS, Kim SH. Increased expression of sonic hedgehog and altered methylation of its promoter region in gastric cancer and its related lesions. Mod Pathol. 2006;19:675–683. doi: 10.1038/modpathol.3800573. [DOI] [PubMed] [Google Scholar]
- 26.Ji Z, Mei FC, Xie J, Cheng X. Oncogenic KRAS activates hedgehog signaling pathway in pancreatic cancer cells. J Biol Chem. 2007;282:14048–55. doi: 10.1074/jbc.M611089200. [DOI] [PubMed] [Google Scholar]
- 27.Caserta TM, Kommagani R, Yuan Z, Robbins DJ, Mercer CA, Kadakia MP. p63 overexpression induces the expression of sonic hedgehog. Mol Cancer Res. 2006;4:759–768. doi: 10.1158/1541-7786.MCR-05-0149. [DOI] [PubMed] [Google Scholar]
- 28.Sekiguchi F, Sekimoto T, Ogura A, Kawabata A. Endogenous hydrogen sulfide enhances cell proliferation of human gastric cancer AGS cells. Biol Pharm Bull. 2016;39:887–90. doi: 10.1248/bpb.b15-01015. [DOI] [PubMed] [Google Scholar]
- 29.Collins N, Brewer M. Development of a clinically applicable protocol for assessment of hypoxic response through measurement of the endogenous gasotransmitter hydrogen sulfide in human plasma. J Neurosurg Anesthesiol. 2015;27:257–261. doi: 10.1097/ANA.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 30.Pouokam E, Althaus M. Epithelial electrolyte transport physiology and the gasotransmitter hydrogen sulfide. Oxid Med Cell Longev. 2016;2016:4723416. doi: 10.1155/2016/4723416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ivanciuc T, Sbrana E, Ansar M, Bazhanov N, Szabo C, Casola A, Garofalo RP. Hydrogen sulfide is an antiviral and antiinflammatory endogenous gasotransmitter in the airways. Role in respiratory syncytial virus infection. Am J Respir Cell Mol Biol. 2016;55:684–96. doi: 10.1165/rcmb.2015-0385OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhattacharyya S, Saha S, Giri K, Lanza IR, Nair KS, Jennings NB, Rodriguezaguayo C, Lopez-Berestein G, Basal E, Weaver AL, Visscher DW, Cliby W, Sood AK, Bhattacharya R, Mukherjee P. Cystathionine beta-synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLoS One. 2013;8:e79167. doi: 10.1371/journal.pone.0079167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szabo C, Hellmich MR. Endogenously produced hydrogen sulfide supports tumor cell growth and proliferation. Cell Cycle. 2013;12:2915–2916. doi: 10.4161/cc.26064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takano N, Sarfraz Y, Gilkes DM, Chaturvedi P, Xiang L, Suematsu M, Zagzag D, Semenza GL. Decreased expression of cystathionine β-synthase promotes glioma tumorigenesis. Mol Cancer Res. 2014;12:1398–406. doi: 10.1158/1541-7786.MCR-14-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szabo C, Coletta C, Chao C, Modis K, Szczesny B, Papapetropoulos A, Hellmich MR. Tumor-derived hydrogen sulfide, produced by cystathionine-beta-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc Natl Acad Sci U S A. 2013;110:12474–12479. doi: 10.1073/pnas.1306241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang L, Su X, Xie J. Activation of hedgehog pathway in gastrointestinal cancers. Vitam Horm. 2012;88:461–472. doi: 10.1016/B978-0-12-394622-5.00020-1. [DOI] [PubMed] [Google Scholar]
- 37.Clark VE, Erson-Omay EZ, Serin A, Yin J, Cotney J, Ozduman K, Avsar T, Li J, Murray PB, Henegariu O, Yilmaz S, Gunel JM, Carrion-Grant G, Yilmaz B, Grady C, Tanrikulu B, Bakircioglu M, Kaymakcalan H, Caglayan AO, Sencar L, Ceyhun E, Atik AF, Bayri Y, Bai H, Kolb LE, Hebert RM, Omay SB, Mishra-Gorur K, Choi M, Overton JD, Holland EC, Mane S, State MW, Bilguvar K, Baehring JM, Gutin PH, Piepmeier JM, Vortmeyer A, Brennan CW, Pamir MN, Kilic T, Lifton RP, Noonan JP, Yasuno K, Gunel M. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science. 2013;339:1077–1080. doi: 10.1126/science.1233009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tickle C, Towers M. Sonic hedgehog signaling in limb development. Front Cell Dev Biol. 2017;5:14. doi: 10.3389/fcell.2017.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fagman H, Grände M, Gritlilinde A, Nilsson M. Genetic deletion of sonic hedgehog causes hemiagenesis and ectopic development of the thyroid in mouse. Am J Pathol. 2004;164:1865–72. doi: 10.1016/S0002-9440(10)63745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong W, Cui J, Tian X, He L, Wang Z, Zhang P, Zhang H. Aberrant sonic hedgehog signaling pathway and STAT3 activation in papillary thyroid cancer. Int J Clin Exp Med. 2014;7:1786–1793. [PMC free article] [PubMed] [Google Scholar]
- 41.Xu X, Ding H, Rao G, Arora S, Saclarides CP, Esparaz J, Gattuso P, Solorzano CC, Prinz RA. Activation of the sonic hedgehog pathway in thyroid neoplasms and its potential role in tumor cell proliferation. Endocr Relat Cancer. 2012;19:167–79. doi: 10.1530/ERC-11-0305. [DOI] [PubMed] [Google Scholar]
- 42.Williamson AJ, Doscas ME, Ye J, Heiden KB, Xing M, Li Y, Prinz RA, Xu X. The sonic hedgehog signaling pathway stimulates anaplastic thyroid cancer cell motility and invasiveness by activating Akt and c-Met. Oncotarget. 2016;7:10472–10485. doi: 10.18632/oncotarget.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]


