Abstract
Non-small cell lung cancer (NSCLC) remains the leading cause of deaths around the world. Therefore, improving the diagnostic and treatments of NSCLC are urgently needed. The microRNA-34a (miR-34a) and SIRT6 are associated with NSCLC. miR-34a is downregulated in three NSCLC cells lines (A549, H460, and H1299). The functions of SIRT6 in NSCLC are controversial. Some reports have shown that SIRT6 is downregulated in NSCLC cells, while other reports have shown that SIRT6 is upregulated in NSCLC tissues as well as SIRT6 overexpression is associated with the poor prognosis of NSCLC. SIRT6 is a direct target of miR-34a in human keratinocytes (HKCs). However, the relationship between SIRT6 and miR-34a in NSCLC has not been investigated. In this study, we found that the SIRT6 was upregulated in NSCLC tissues while miR-34a was downregulated in NSCLC tissues compared with those in their normal counterparts. Overexpression of miR34a or downregulation of SIRT6 promoted A549 cells apoptosis, cell cycle arrest in vitro and further inhibited the tumor formation in vivo. SIRT6 was indeed the target gene of miR-34a, which was proved by the luciferase reporter data. Therefore, we conclude that SIRT6 was the target gene of miR-34a in NSCLC. miR-34a acted as a cancer suppressor in NSCLC via targeting the SIRT6.
Keywords: Non-small cell lung cancer (NSCLC), SIRT6, microRNA-34a (miR-34a)
Introduction
Lung cancer is a tumor arising from the respiratory epithelium, which remains the leading cause of cancer-related deaths worldwide [1]. Cigarette smoking, second or passive smoking, diet and food supplements, alcohol, and air pollution increase the risk of lung cancer [2]. Histologically, lung cancer is divided into small cell lung cancer (NSCLC), adenocarcinoma, squamous cell carcinoma (SCC) and large cell carcinoma. Among the four types of lung cancers, adenocarcinoma, SCC and large cell carcinoma are usually grouped into non-small cell lung cancer (NSCLC) [3]. NSCLC accounts for approximately 80% of all lung cancers [4]. The 5-year survival rate of lung cancer is about 15%. Currently, there are 5 main methods (surgery, chemotherapy, radiation therapy, targeted therapy and immunotherapy) to treat NSCLC [5,6]. Nevertheless, NSCLC cannot be cured with currently available therapeutic modalities and acquired resistance remains the challenges to be overcome [7]. Therefore, improvements in diagnostic and treatments of NSCLC are necessary.
MicroRNAs (miRNAs), small noncoding RNAs (17-25 nucleotide), regulate about 20%-30% of the genes in the human genome via binding to the complementary sequences of 3’-untranslated regions (3’-UTR) of their target mRNA [8]. miRNAs are double-edged sword in cancers, which can regulate various cell processes, such as cell proliferation, differentiation, migration, and apoptosis [8]. The microRNA-34a (miR-34a) is upregulated in Chronic Lymphocytic Leukemia (CLL) [8-10]. While miR-34a is downregulated in some human cancers, including breast cancer, prostate cancer, osteosarcoma, and lung cancer [11]. miR-34a is downregulated in three NSCLC cells lines (A549, H460, and H1299) [12,13]. miR-34a inhibits the holoclone formation, clonogenic expansion and tumor regeneration of the three NSCLC cells [12]. Therefore, miR-34a negatively regulates the tumorigenic properties of NSCLC cells and CD44hi lung cancer stem cells (CSCs) [12,14]. Another report has shown that miR-34a and miR15a/16 act synergistically to arrest the NSCLC cell cycle in G1-G0, while only miR-34a could induce the NSCLC apoptosis [15].
SIRT6 is a NAD+-dependent histone deacetylase belonging to sirtuin family, which can regulate transcription, genomic DNA stability and repair based on its histone deacetylation function [16-18]. More importantly, SIRT6 can regulate autophagy, which is an important process in cancer cell survival and chemoresistance [19]. Thence, SIRT6 has been studied due to its potential function in overcoming drug resistance in cancers. However, the functions of SIRT6 in NSCLC studied are inconsistent. The SIRT6 is downregulated in human NSCLC tissue and cell lines [20,21]. Overexpression of SIRT6 could inhibit the proliferation of NSCLC cells [20]. Conversely, knockdown of SIRT6 using small interfering RNA (siRNA) promotes the proliferation of NSCLC cells [20]. SIRT6 inhibits the proliferation of NSCLC cells through downregulation of Twist1 [20]. Taken together, SIRT6 plays an anticancer role by promoting apoptosis in NSCLC [22]. In NSCLC, SIRT6 is mainly present in the cytoplasm. However, high cytoplasmic versus low nuclear expression of SIRT6 in primary cancer tissues from patients with NSCLC, is associated with more aggressive cancer and with poor prognosis [23]. Additionally, downregulation of SIRT6 with siRNA in A549 cells improves the sensitivity of A549 cells to paclitaxel and promotes the γ-ray-induced apoptosis of NSCLC cells [23]. All these results suggest that SIRT6 might acts as an oncogene in the tumorigenesis and progression of NSCLC [24].
As mentioned above, the functions of SIRT6 in NSCLC are contradictory. The relationship between SIRT6 and miR-34a in cancers has also been investigated [25,26]. Previous reports have shown that SIRT6 is a direct target of miR-34a in human keratinocytes (HKCs) [25]. SIRT6 is oppositely expressed to miR-34a in normal keratinocytes and keratinocyte-derived tumors. SIRT6 is downregulated by increased amounts of miR-34a. SIRT6 has a miR-34a putative binding site in its 3’-UTR region [25]. Furthermore, downregulation of SIRT6 promotes the pro-differentiation effect of miR-34a, while overexpression of SIRT6 counteracts the miR-34a pro-differentiation effects. SIRT6 level is decrease during keratinocyte differentiation [25]. All these results suggest that SIRT6 and miR-34a are against with each other. The negative correlation between SIRT6 and miR-34a expression in HKCs have uncovered [25,26]. Both SIRT6 and miR-34a were associated with NSCLC. However, the relationship between SIRT6 and miR-34a in NSCLC has not been investigated.
In this study, we verify that SIRT6 is indeed the target gene of miR34a. Both overexpression of miR-34a and downregulation of SIRT6 inhibit the tumor formation in the animal experiment. We conclude that miR-34a acts as a tumor suppressor via targeting SIRT6.
Materials and methods
mRNA and protein expression in the NSCLC tissues
NSCLC tissues and normal tissues (2 cm away from NSCLC tissue) of four NSCLC patients were obtained from Guangzhou Chest Hospital (Guangzhou, China). The total RNA was extracted from the NSCLC tissues and normal tissues in the light of the manufacture’s instruction (Invitrogen, Carlsbad, CA). The protein was extracted from the NSCLC tissues and normal tissues via using Membrane Protein Extraction Kit (Keygen Biotech).
The gene expression of miR-34a and SIRT6 in the cancer tissues and normal tissues was measured by real-time quantitative reverse transcription polymerase chain reaction (RT-PCR). cDNA was synthesized by using PrimeScript II 1st Strand cDNA Synthesis Kit (Takara, Japan). The primer sequence used in RT-PCR were showed as follows: SIRT6 forward primer: 5’ CCGGAGGAGCTGGAGCGGAAG 3’, SIRT6 reverse primer: 5’ CGTGGGCCGCGCGCTCTCAAAG 3’; 18S forward primer: 5’ CCTGGATACCGCAGCTAGGA 3’; 18S reverse primer: 5’ GCGCG-CAATACGAATGCCCC 3’; has-miR-34a-5p RT primer: 5’ CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCCCCTCTG3’; hsa-miR-34a-5p forward primer: 5’ ACACTCCAGCTGTGACTGGTTGACCAGA3’; hsa-miR-34a-5p reverse primer: 5’ CTCAACTGGTGTCGTGGA 3’; U6 forward primer: 5’ CTCGCTTCGGCAGCACA 3’; U6 reverse primer: 5’ AACGCTTCACGAATTTGCGT 3’. The gene expressions of SIRT6 and miR-34a were determined by RT-PCR using SYBR Green PC Master Mix (Toyobo, Japan). Reaction were conducted on an ABI PRISM® 7500 Sequence Detection System and performed under the following thermocycler conditions: 95°C for 5 min, followed by 40 cycles of 95°C for 15 seconds, 60°C for 15 seconds and 72°C for 30 seconds; then followed by a 60°C for 1 min and 95°C for 15 seconds. Raw Data of all samples were collected and normalized to that of the control (miR-34a was normalized to U6 small nuclear RNA and SIRT6 was normalized to 18S). The gene expression of SIRT6 and miR-34a were calculated using the relative quantification equation (RQ=2-ΔΔCt) [27].
The protein expression level of SIRT6 in NSCLC tissues and normal tissues was detected by Western Blotting (WB). Protein concentrations were determined using BCA Protein Assay Kit (Keygen Biotech). Proteins extracted from NSCLC tissues and normal tissues were separated in 10% SDS-polyacrylamide gels, electroblotted onto an Immobilon-P transfer polyvinylidene fluoride membrane 20 (Millipore, USA), detected with a rabbit anti-human SIRT6 monoclonal antibody (ab62739, 1:1000, Abcam, USA), a rabbit anti-human-GAPDH antibody (KC-5G5, 1:10,000, KangCheng bio, China) and then visualized by a commercial Immobilon Western HRP Substrate (WBKLS0500, Millipore, USA) under dark conditions.
Cell line and culture
The A549 human NSCLC cell line was obtained from the Guangzhou Runwenbio company (Guangzhou, China). A549 cells were cultured in DEME high glucose medium supplemented with 10% fetal bovine serum (FBS; Hyclone, USA) and 1% penicillin and streptomycin, on the base of standard procedures. Cell were incubated in a humidified incubator at 37°C supplied with 5% CO2. Cells were treated as follows: negative control mimic (control group); miR-34a mimics (miR-34a group); overexpression of SIRT6 (SIRT6 group); knockdown od SIRT6 expression by RNA interference (si-SIRT6 group); cotransfected with miR-34a and SIRT6 (SIRT6+miR-34a). The transfected processes were performed according to previous research [13,20]. Then mRNA expressions of SIRT6 and miR-34a in A549 cells transfected with various vectors were measured based on the above-mentioned methods.
Cell proliferation assay
Cell proliferation was measured using the Cell Counting Kit-8 assay kit (Dojindo, Kumamato, Japan). The absorbance at 450 nm (OD450) of all samples was measured. The proliferation rates of cells from day 0 to day 5 were calculated based on OD450 values.
Cell cycle assay by flow cytometry
The A549 cells were treated with NC, miR-34a, si-SIRT6, and SIRT6+miR-34a as above-mentioned and were cultured for 24 h. After 72 h, the cells were collected, fixed and then stained on the base of standard process. The cells were stained with a propidium iodide (PI) solution containing 100 μg/ml PI and 50 μg/ml RNase (Sigma, USA) in PBS at 37°C for 30 min in the dark. To remove cell clumps, the stained cells were passed through a nylon mesh sieve, and then the samples were analyzed by flow cytometry (BD, USA). Data were collected and analyzed by the CELL Quest and ModFit LT software.
Cell apoptosis analysis by flow cytometry
The A549 cells treated with NC, miR-34a, si-SIRT6, and SIRT6+miR-34a were harvested and washed after 48 h incubation. The cell apoptosis was analyzed by Annexin V-FITC/PI Apoptosis Detection Kit (Cataog no. KGA106; Nanjing Keygen Biotech Co., Ltd) according to manufacturer’s instruction. After staining, all samples were immediately measured on FACSort flow cytometry (BD, USA). The data were analyzed using the Cell Quest 3.0 software (BD, USA).
Luciferase reporter assay
The SIRT6-3’-UTR fragment containing putative binding sites for miR-34a was obtained and ligased (Takara, Japan) into the digested psiCheck-2 plasmid (Promega, USA) between the XhoI and NotI sites. SIRT6-3’-UTR-targeted site mutations were generated using the KOD-plus mutagenesis kit (Toyobo, Japan) in the light of the manufacturer’s protocol. A549 cells were plated in a 96-well plate and then co-transfected with 200 ng/μl of plasmid and 50 nM miR-34a or NC. After co-transfection 48 h, luciferase activity was detected by the Dual-Glo luciferase assay kit (Promega, USA). The transfections were performed in duplicate and repeated 3 times.
Tumor formation in BALB/c nude mice
All experimental procedures involving animals were according to the Guide for the Care and Use of Laboratory Animals (8th edition) [28]. A549 cells were pre-treated with the miR-34a mimics, si-SIRT6, SIRT6+miR-34a, and NC, respectively, and were incubated for 24 h. Then the cells were suspended with 100 μl PBS (4×106 cells/ml) and injected into right flank of the same BALB/C female athymic nude mouse at 5-6 weeks of age (5 mice for each group, n=5). The tumor size was monitored by measuring the length (L) and width (W) with calipers, and the volumes were calculated using the formula: (L×W2)/2. The tumor weight was also measured in the last day of the experiment.
Statistical analysis
All statistical analyses were performed with Statistical Package for the Social Sciences (SPSS) 19.0 software. The data are presented as the mean ± SD from three separate experiments. Data were analyzed by One Way ANOVA analysis of variance for multiple comparisons. The statistical analysis was determined using LSD-t test for comparisons between control groups and other groups. During heterogeneity of variance, Tamhane’s T2 and Dunnett’s T3 were conducted. Statistical significance was determined by paired or unpaired Student’s t-test in cases of standardized expression data. Differences were considered statistically significant at P<0.05.
Results
Expression of SIRT6 and miR-34a in NSCLC tissues and normal tissues
The mRNA expression of SIRT6 and miR-34a-5p in NSCLC tissues and normal tissues were determined by RT-PCR (Figure 1). The protein expression of SIRT6 in the NSCLC tissues and normal tissues were detected by WB with GAPDH as control (Figure 1). The gene and protein expression of SIRT6 in NSCLC tissues were higher than that in normal tissues. The gene expression of miR-34a-5p detected in NSCLC tissues was lower than that in normal tissues. The expression of SIRT6 and miR-34a were negatively correlated with each other.
Figure 1.
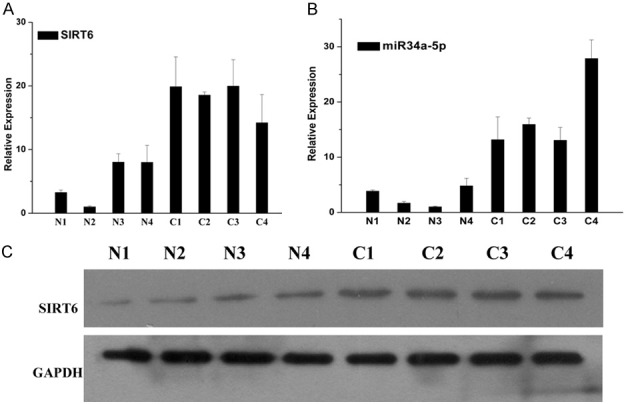
Expression of SIRT6 and miR-34a in four pairs of NSCLC tissues and normal tissues. N1, N2, N3 and N4 represent four normal tissues. C1, C2, C3, and C4 represent four NSCLC tissues. A. The mRNA expression of SIRT6 in NSCLC tissues and normal tissues, which were detected by RT-PCR. B. The mRNA expression of miR-34a in NSCLC tissues and normal tissues, which were detected by RT-PCR. C. The protein expression of SIRT6 in NSCLC tissues and normal tissues, which were detected by WB.
Regulation of SIRT6 and miR-34a expression by transfection in cell lines
The gene expression of SIRT6 and miR-34a in A549 cells transfected with negative control (NC), miR-34a, SIRT6 and SIRT6+miR-34a were measured by RT-PCR (Figure 2). The results showed that the expression of SIRT6 in the A549 cells transfected with miR-34a were decreased compared with that in A549 cells transfected with NC. The expression of SIRT6 was significantly increased in A549 cells transfected with SIRT6. While the SIRT6 expression in the group A549 cells transfected with SIRT6+miR-34a were obviously decreased compared with that in group A549 cells transfected with SIRT6. The expression of miR-34a in A549 cells transfected with SIRT6 was lower than that in A549 cells transfected with NC. The expression of miR-34a in A549 cells transfected with SIRT6+miR-34a were lower than that in A549 cells transfected with miR-34a. All the results suggested that the expressions of miR-34a and SIRT6 were negatively related with each other.
Figure 2.
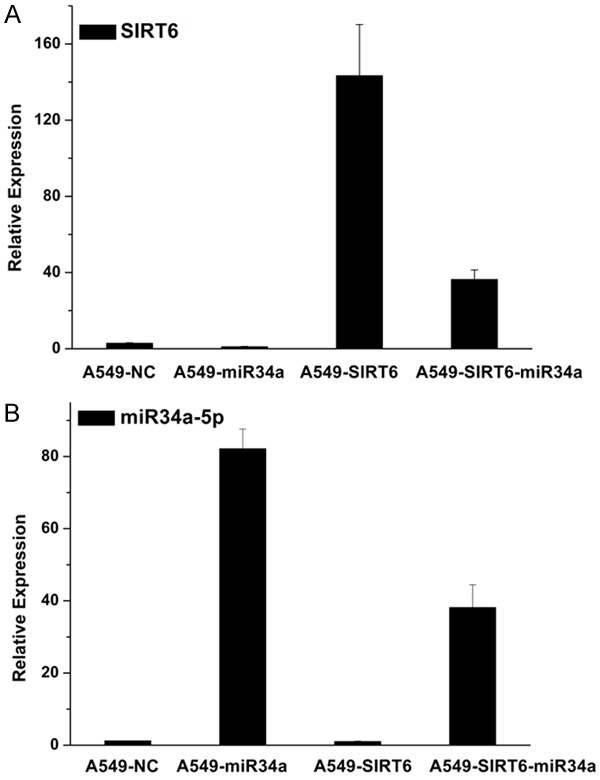
The mRNA expression of SIRT6 and miR-34a-5p in NSCLC cell line A549 transfected with negative control, miR-34a, SIRT6 and SIRT6+miR-34a. A. The expression of SIRT6 in A549 cells. B. The mRNA expression of miR-34a-5p in A549 cells.
The cells growth affected by SIRT6 and miR-34a
To determine whether miR-34a and SIRT6 mediates the growth of A549 cells, A549 cells were co-transfected with NC, miR-34a mimics, si-SIRT6 and si-SIRT6+miR-34a, then we determined the cells proliferation rate (Figure 3). Both downregulation of SIRT6 and overexpression of miR-34a inhibited the cells proliferation, which was indicated by OD450. The cells proliferation was significantly slower in A549 cells contransfected with SIRT6+miR-34a than that in control group (A549+NC).
Figure 3.
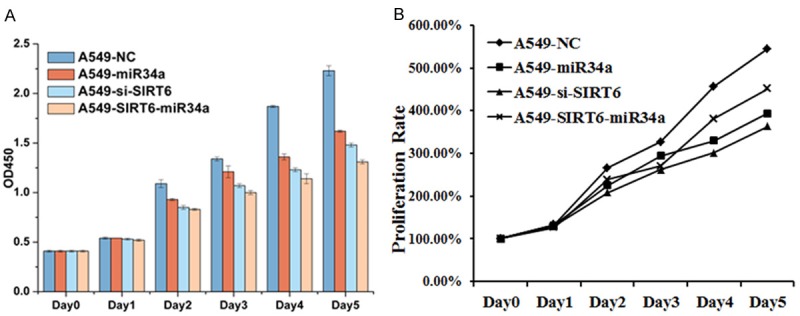
Cell growth affected by miR-34a and SIRT6. A. The effect of SIRT6 and miR-34a in cell growth. B. The proliferation rate of A549 cells transfected with different vectors. The data are presented as the mean ± SEM.
Apoptotic analysis and cell cycles of A549 cells transfected with different vectors
To determine the effect miR-34a and SIRT6 treatment on cell death and cell cycle, the apoptotic analysis and cell cycle of A549 cells transfected with different vectors were determined by flow cytometry (Figure 4). The A549 cells transfected with NC, miR-34a, si-SIRT6 and SIRT6+miR-34a were double-stained with Annexin V-FITC and PI. The shifts in cell population with the different treatments clearly indicated that apoptotic rate of the si-SIRT6-treated group is higher than that of the miR-34a, or SIRT6+miR-34a group, while the control group showed the lowest rate of apoptosis (Figure 4A and 4B). Downregulated the expression of SIRT6 promoted the cells apoptosis. We further examined the effect of miR-34a and SIRT6 on the A549 cell cycle by flow cytometry (Figure 4C). Individually, miR-34a and si-SIRT6 treatment increased the percentage of cells detected at the G1 phase. The percentage of cells at G1 phase detected in A549-SIRT6+SIRT6 were higher than that in control group, but were lower than that in A549-miR-34a or A549-si-SIRT6 group. The results showed that downregulation of the SIRT6 and overexpression of miR-34a block the cell cycle phase transition from G0/G1 to S. Both the si-SIRT6 and overexpression of miR-34a decreased the expression of SIRT6. Therefore, the SIRT6 promoted the cell progression and survival.
Figure 4.
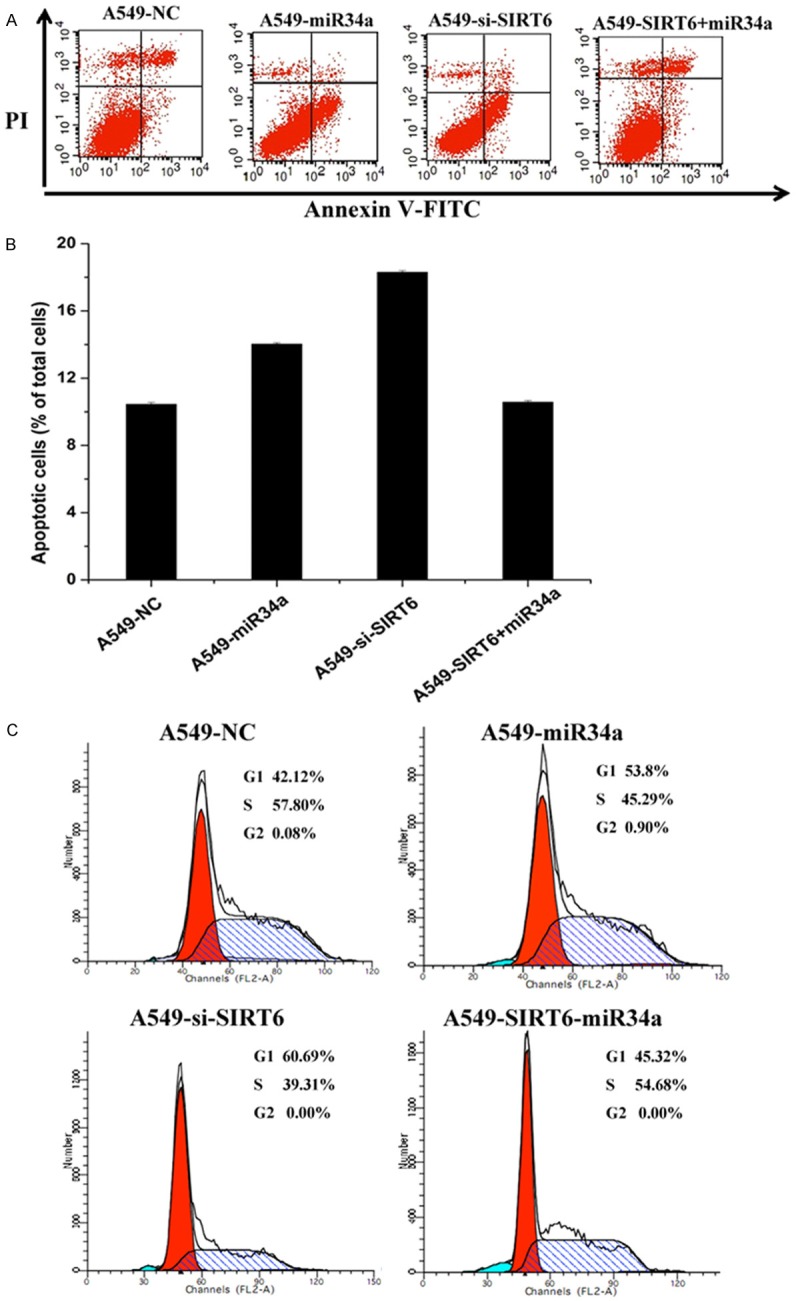
Apoptotic analysis of A549 cells transfected with different vectors. The data are presented as the mean ± SEM. A. Cells were stained with Annexin V-FITC/PI and analyzed by flow cytometry to determine the population of cells at the early a late apoptosis in the different treatment groups: A549 cells transfected with negative control mimic-treated (control), miR-34a, si-SIRT6 and SIRT6+miR-34a. B. The percentage of apoptotic cells in each group relative to the cells was used to evaluate the apoptotic rates. C. Cell cycle profile changes of A549 cells transfected with different vectors were assessed by flow cytometry using PI staining to measure the DNA content. The data are presented as the average of triplicated values.
SIRT6 is a direct target of miR-34a in A549 cells
To determine the molecular mechanism by which miR-34a induces cells growth arrest and senescence, we used 3 open-target prediction programs, such as piTar, TargetScan and miRanda, to predict the targets of miR-34a. To confirm our previous hypothesis, we compared the sequence of 3’-UTR of SIRT6 with miR-34a. The 3’-UTR of SIRT6 mRNA contained a complementary site for the seed region of miR-34a (Figure 5A), and the highly-matched bases attract our attention to investigate whether SIRT6 is a putative target of miR-34a, SIRT6 is a notably attractive candidate because it plays important roles in the development and progression of NSCLC.
Figure 5.
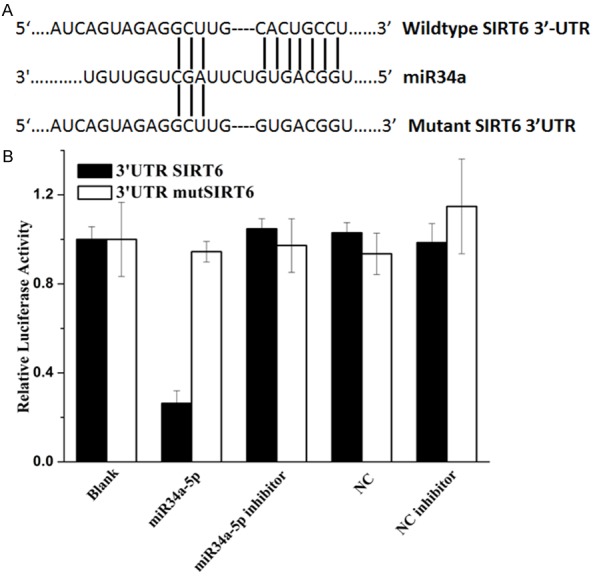
SIRT6 is a direct target of miR-34a. A. Sequence alignment between miR-34a and the 3’-UTR of the human SIRT6 mRNA seed-matching region or seed-mutated region. B. Relative luciferase activity was calculated as follows: (Rluc miRNA/Luc miRNA)/(Rluc no-target/Luc nontarget). The data are presented as the average of triplicate values.
To determine whether SIRT6 is the direct target gene for miR-34a, a dual-luciferase reporter system was employed, the results showed in Figure 5B. The luciferases reporter assay indicated that the luciferase activity of reporter containing the SIRT6 gene’s wide-type 3’-UTR decreased (70%) following treatment with miR-34a mimics. By contrast, the inhibitory effect of miR-34a mimics was abolished in the mutated construct. Moreover, the decrease of luciferase activity induced by miR-34a was inhibited when miR-34a inhibitor was introduced. The results indicated that miR-34a most likely suppresses gene expression of SIRT6 through miR-34a-binding sequence at the 3’-UTR of SIRT6. Therefore, the data indicated that miR-34a reduces SIRT6 expression by inhibiting translation and/or causing mRNA instability.
SIRT6 promotes tumor growth in vivo
To confirm the tumor suppressor role of miR-34a, we established a BALB/c nude mouse xenograft model using A549 cells. The A549 cells were pre-transfected with miR-34a, SIRT6+miR-34a and si-SIRT6, respectively. Then these cells were respectively injected into female BALB/c nude mice to form tumor. The tumor volume was measured every 2 or 3 days until day 29. The final tumors weights were measured (Figure 6A and 6B). The tumor volume and weight of A549 cells treated with miR-34a mimics were significantly reduced relative to A549-NC cell group. The results indicated that miR-34a introduction significantly inhibited the tumorigenicity of A549 cells in the nude mouse xenograft model. The tumor volume and weight of A549 cells treated with si-SIRT6 was obviously reduced compared with the group A549-NC, A549-miR-34a and A549-SIRT6+miR-34a. The results suggested that downregulation of the expression of SIRT6 could inhibited the tumorigenesis. The tumor volume and weight of the group A549-SIRT6+miR-34a were higher than that of the group A549-miR-34a, which suggested that SIRT6 expression inhibited the anti-cancer activity of miR-34a. Representative photograph of NSCLC tissues formed in the nude mice were showed in Figure 6C and 6D. All the results suggested that SIRT6 indeed promoted the formation of lung cancer, while both miR-34a and downregulating the expression of SIRT6 inhibited the formation of lung cancer.
Figure 6.
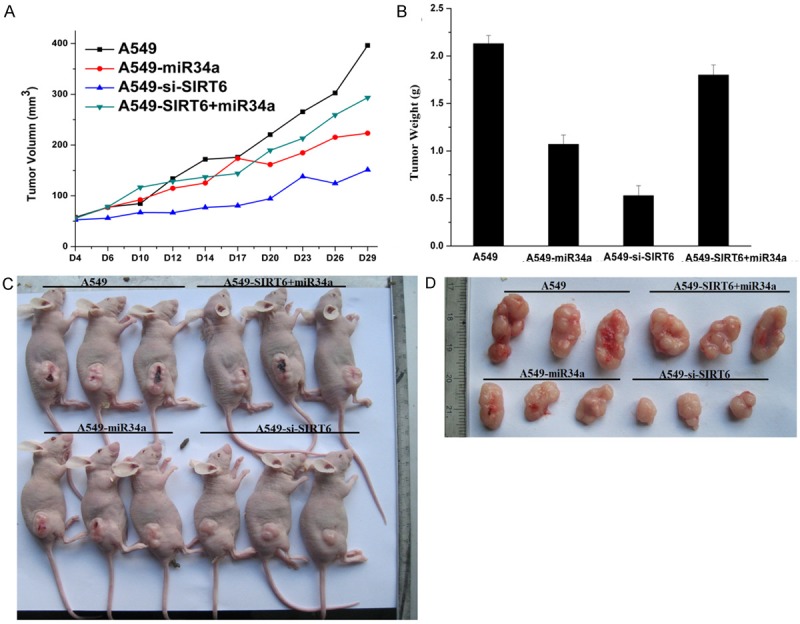
miR-34a suppresses tumor growth in vivo. A and B: Tumor volume and tumor weight in nude mice, each group contained 5 mice (n=5); the data are presented as the mean compared with A549-NC group. C and D: Representative photograph of NSCLC tissues formed when miR-34a and SIRT6-treated cells were injected into nude mice.
Discussion
Both miR-34a and SIRT6 play important roles in human cancer, and the exact mechanism in different cancers are controversial. miR-34a was upregulated in CLL, while it was downregulated in breast cancer, prostate cancer, osteosarcoma, and lung cancer [11]. SIRT6 was also downregulated in clinical samples from pancreatic cancer, colorectal cancer and hepatocellular carcinoma [29-31]. As mentioned, decreased SIRT6 might cause tumor formation and maintenance. However, other researchers showed that SIRT6 was upregulated in head and neck squamous cell carcinoma (HNSCC), squamous cell carcinomas (SCCs), and CLL, which indicated that SIRT6 acted as an oncogenic [29,32,33]. Other reports showed that high SIRT6 expression in prostate cancer and breast cancer patients were associated with their obviously poor prognoses [29]. SIRT6 improved the resistance of prostate and breast cancer cells to anticancer drugs [34,35]. SIRT6 and miR-34a were associated with the NSCLC. Currently, previous reports verified that miR-34a was downregulated in NSCLC tissues and cells and miR-34a acted as a cancer suppressor in NSCLC [12,14]. However, the functions of SIRT6 in NSCLC have no uniform conclusions. The high expression of SIRT6 in the cytoplasm in NSCLC tissues was associated with poor prognosis of NSCLC patient [23]. SIRT6 was a target gene of miR-34a in HKCs [25]. The relation of SIRT6 and miR-34a in NSCLC has not been studied, which is investigated in this paper.
We discover that the gene expression of miR-34a is decreased in NSCLC tissues compared that in normal tissues, which is consistent with the previous results. Conversely, the gene expression and protein expression of SIRT6 in NSCLC tissues are increased compared those in normal tissues, which is inconsistent with previous reported by Han et al., but agrees with the result of Azuma et al. [20,23]. The negative correlation between SIRT6 and miR-34a in NSCLC tissues and normal tissues is consistent with the previous findings in HKCs [25,26]. Overexpression of SIRT6 in A549 cells induces the decrease of miR-34a level in A549 cells. SIRT6 is downregulated in A549 cells transfected with miR-34a mimics. Both downregulation of SIRT6 or overexpression of miR-34a inhibited the proliferation of A549 cells. Downregulated the expression of SIRT6 with siRNA or introduced the miR-34a in A549 cells promoted the A549 cells apoptosis. Both the si-SIRT6 and overexpression of miR-34a decreased the expression of SIRT6. Therefore, the SIRT6 promotes the A549 cell progression and survival, which is consistent with the results SIRT6 improve the drug resistance of cancer cells [34]. Additionally, downregulation of the SIRT6 and overexpression of miR-34a block the cell cycle transition from G0/G1 to S phase. The percentage of cells stay in G1 phase in A549 cells treated with miR-34a and si-SIRT6 are higher than the control group. Our result further verifies the previous results that the miR-34a and miR15a/16 act synergistically to arrest the NSCLC cell cycle in G0/G1 as well as knockdown of SIRT6 induces the arrest of G1 phase in human prostate cancer cells [15,35]. The cell cycle results further demonstrate that miR-34a or downregulation of SIRT6 promotes the cell apoptosis. The luciferase activity of reporter data shows that miR-34a indeed regulates the expression and function of SIRT6 in NSCLC via binding to the 3’-UTR of SIRT6. Therefore, the SIRT6 is a target gene of miR-34a in NSCLC, which has also been proved in HKCs [26]. Animal experiments show that introducing miR-34a or down-regulating the SIRT6 (si-SIRT6) suppresses the formation of NSCLC. All the results suggested that SIRT6 exhibits potentially oncogenic roles in the tumorigenesis and progression of NSCLC. SIRT6 is the target gene of miR34a. Therefore, miR-34a acts as a tumor suppressor in NSCLC via targeting SIRT6.
Acknowledgements
The study was supported by Applied Basic Research Project of Joint Special Fund of Kunming Medical University from Yunnan Province (No. 2017FE467(-117) and 2017FE468(-109)) and Scientific Research Fund Project from Yunnan Provincial Department of Education (2016ZDX009).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Subramanian H, Viswanathan P, Cherkezyan L, Iyengar R, Rozhok S, Verleye M, Derbas J, Czarnecki J, Roy HK, Backman V. Procedures for risk-stratification of lung cancer using buccal nanocytology. Biomedical Optics Express. 2016;7:3795–3810. doi: 10.1364/BOE.7.003795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virtanen C, Ishikawa Y, Honjoh D, Kimura M, Shimane M, Miyoshi T, Nomura H, Jones MH. Integrated classification of lung tumors and cell lines by expression profiling. Proc Natl Acad Sci U S A. 2002;99:12357–62. doi: 10.1073/pnas.192240599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117:294–9. doi: 10.1002/ijc.21183. [DOI] [PubMed] [Google Scholar]
- 5.Chan BA, Hughes BG. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res. 2015;4:36–54. doi: 10.3978/j.issn.2218-6751.2014.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non-small-cell lung cancer: recent developments. Lancet. 2013;382:709–719. doi: 10.1016/S0140-6736(13)61502-0. [DOI] [PubMed] [Google Scholar]
- 7.Zagryazhskaya A, Gyuraszova K, Zhivotovsky B. Cell death in cancer therapy of lung adenocarcinoma. Int J Dev Biol. 2015;59:119–29. doi: 10.1387/ijdb.150044bz. [DOI] [PubMed] [Google Scholar]
- 8.Baer C, Claus R, Plass C. Genome-wide epigenetic regulation of miRNAs in cancer. Cancer Res. 2013;73:473–7. doi: 10.1158/0008-5472.CAN-12-3731. [DOI] [PubMed] [Google Scholar]
- 9.Christoffersen NR, Shalgi R, Frankel LB, Leucci E, Lees M, Klausen M, Pilpel Y, Nielsen FC, Oren M, Lund AH. p53-independent upregulation of miR-34a during oncogene-induced senescence represses MYC. Cell Death Differ. 2010;17:236–45. doi: 10.1038/cdd.2009.109. [DOI] [PubMed] [Google Scholar]
- 10.Asslaber D, Piñón JD, Seyfried I, Desch P, Stöcher M, Tinhofer I, Egle A, Merkel O, Greil R. microRNA-34a expression correlates with MDM2 SNP309 polymorphism and treatment-free survival in chronic lymphocytic leukemia. Blood. 2010;115:4191–7. doi: 10.1182/blood-2009-07-234823. [DOI] [PubMed] [Google Scholar]
- 11.Li XJ, Ren ZJ, Tang JH. MicroRNA-34a: a potential therapeutic target in human cancer. Cell Death Dis. 2014;5:e1327. doi: 10.1038/cddis.2014.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi Y, Liu C, Liu X, Tang DG, Wang J. The microRNA miR-34a inhibits non-small cell lung cancer (NSCLC) growth and the CD44hi stem-like NSCLC cells. PLoS One. 2014;9:e90022. doi: 10.1371/journal.pone.0090022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji X, Wang Z, Geamanu A, Goja A, Sarkar FH, Gupta SV. Delta-tocotrienol suppresses Notch-1 pathway by upregulating miR-34a in nonsmall cell lung cancer cells. Int J Cancer. 2012;131:2668–77. doi: 10.1002/ijc.27549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen F, Hu SJ. Effect of microRNA-34a in cell cycle, differentiation, and apoptosis a review. J Biochem Mol Toxicol. 2012;26:79–86. doi: 10.1002/jbt.20412. [DOI] [PubMed] [Google Scholar]
- 15.Bandi N, Vassella E. miR-34a and miR-15a16 are co-regulated in non-small cell lung cancer and control cell cycle progression in a synergistic and Rb-dependent manner. Mol Cancer. 2011;10:1–11. doi: 10.1186/1476-4598-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao Z, Hine C, Tian X, Van Meter M, Au M, Vaidya A, Seluanov A, Gorbunova V. SIRT6 promotes DNA repair under stress by activating PARP1. Science. 2011;332:1443–1446. doi: 10.1126/science.1202723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michishita E, McCord RA, Berber E, Kioi M, Padilla-Nash H, Damian M, Cheung P, Kusumoto R, Kawahara TL, Barrett JC, Chang HY, Bohr VA, Ried T, Gozani O, Chua KF. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452:492–6. doi: 10.1038/nature06736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang WW, Zeng Y, Wu B, Deiters A, Liu WR. A chemical biology approach to reveal Sirt6-targeted histone H3 sites in nucleosomes. ACS Chem Biol. 2016;11:1973–81. doi: 10.1021/acschembio.6b00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao J, Yang X, Liu T, Zhang T, Xie QR, Xia W. Autophagy induction by SIRT6 is involved in oxidative stress-induced neuronal damage. Protein Cell. 2016;7:281–90. doi: 10.1007/s13238-016-0257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han Z, Liu L, Liu Y, Li S. Sirtuin SIRT6 suppresses cell proliferation through inhibition of Twist1 expression in non-small cell lung cancer. Int J Clin Exp Pathol. 2014;7:4774–4781. [PMC free article] [PubMed] [Google Scholar]
- 21.Cai Y, Sheng ZY, Liang SX. Radiosensitization effect of overexpression of adenovirus-mediated SIRT6 on A549 non-small cell lung cancer cells. Asian Pac J Cancer Prev. 2014;15:7297–7301. doi: 10.7314/apjcp.2014.15.17.7297. [DOI] [PubMed] [Google Scholar]
- 22.Bosch-Presegue L, Vaquero A. The dual role of sirtuins in cancer. Genes Cancer. 2011;2:648–62. doi: 10.1177/1947601911417862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azuma Y, Yokobori T, Mogi A, Altan B, Yajima T, Kosaka T, Onozato R, Yamaki E, Asao T, Nishiyama M, Kuwano H. SIRT6 expression is associated with poor prognosis and chemosensitivity in patients with non-small cell lung cancer. J Surg Oncol. 2015;112:231–7. doi: 10.1002/jso.23975. [DOI] [PubMed] [Google Scholar]
- 24.Kim EJ, Juhnn YS. Cyclic AMP signaling reduces sirtuin 6 expression in non-small cell lung cancer cells by promoting ubiquitin-proteasomal degradation via inhibition of the Raf-MEK-ERK (Raf/mitogen-activated extracellular signal-regulated kinase/extracellular signal-regulated kinase) pathway. J Biol Chem. 2015;290:9604–13. doi: 10.1074/jbc.M114.633198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lefort K, Brooks Y, Ostano P, Cario-André M, Calpini V, Guinea-Viniegra J, Albinger-Hegyi A, Hoetzenecker W, Kolfschoten I, Wagner EF, Werner S, Dotto GP. A miR-34a-SIRT6 axis in the squamous cell differentiation network. EMBO J. 2013;32:2248–63. doi: 10.1038/emboj.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dotto GP, Karine L. miR 34a SIRT6 in squamous differentiation and cancer. Cell Cycle. 2014;13:1055–1056. doi: 10.4161/cc.28378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals[J] Guide for The Care & Use of Laboratory Animals. 2011;103:1072–1073. [Google Scholar]
- 29.Lerrer B, Gertler AA, Cohen HY. The complex role of SIRT6 in carcinogenesis. Carcinogenesis. 2016;37:108–18. doi: 10.1093/carcin/bgv167. [DOI] [PubMed] [Google Scholar]
- 30.Sebastian C, Zwaans BM, Silberman DM, Gymrek M, Goren A, Zhong L, Ram O, Truelove J, Guimaraes AR, Toiber D, Cosentino C, Greenson JK, MacDonald AI, McGlynn L, Maxwell F, Edwards J, Giacosa S, Guccione E, Weissleder R, Bernstein BE, Regev A, Shiels PG, Lombard DB, Mostoslavsky R. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell. 2012;151:1185–99. doi: 10.1016/j.cell.2012.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marquardt JU, Fischer K, Baus K, Kashyap A, Ma S, Krupp M, Linke M, Teufel A, Zechner U, Strand D, Thorgeirsson SS, Galle PR, Strand S. Sirtuin-6-dependent genetic and epigenetic alterations are associated with poor clinical outcome in hepatocellular carcinoma patients. Hepatology. 2013;58:1054–64. doi: 10.1002/hep.26413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang JC, Kafeel MI, Avezbakiyev B, Chen C, Sun Y, Rathnasabapathy C, Kalavar M, He Z, Burton J, Lichter S. Histone deacetylase in chronic lymphocytic leukemia. Oncology. 2011;81:325–329. doi: 10.1159/000334577. [DOI] [PubMed] [Google Scholar]
- 33.Lu CT, Hsu CM, Lin PM, Lai CC, Lin HC, Yang CH, Hsiao HH, Liu YC, Lin HY, Lin SF, Yang MY. The potential of SIRT6 and SIRT7 as circulating markers for head and neck squamous cell carcinoma. Anticancer Res. 2014;34:7137–7143. [PubMed] [Google Scholar]
- 34.Khongkow M, Olmos Y, Gong C, Gomes AR, Monteiro LJ, Yagüe E, Cavaco TB, Khongkow P, Man EP, Laohasinnarong S, Koo CY, Harada-Shoji N, Tsang JW, Coombes RC, Schwer B, Khoo US, Lam EW. SIRT6 modulates paclitaxel and epirubicin resistance and survival in breast cancer. Carcinogenesis. 2013;34:1476–86. doi: 10.1093/carcin/bgt098. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Xie QR, Wang B, Shao J, Zhang T, Liu T, Huang G, Xia W. Inhibition of SIRT6 in prostate cancer reduces cell viability and increases sensitivity to chemotherapeutics. Protein Cell. 2013;4:702–10. doi: 10.1007/s13238-013-3054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


