Abstract
A novel microRNA, miR-520d-5p, can inhibit proliferation of osteosarcoma cells, but the biological role of miR-520d-5p in lung cancer is notknown. Midazolam can induce apoptosis in many kinds of cancer cells, but there are no reportson its use in lung cancer. We investigated the roles of midazolam and miR-520d-5p in apoptosis induction in a non-small cell lung cancer (NSCLC) cell line (A549). The expression of miR-520d-5p, a signal transducer and activator of transcription 3 (STAT3) and its related protein were measured by quantitative real-time PCR and Western blot. Apoptosis of the NSCLC cells in response to midazolam was determined by MTT assay, flow cytometry, and Western blot. Midazolam significantly induced A549 cell apoptosis and modulated expression of Bcl-2, Bax, and Caspase-3. Additionally, midazolam regulated STAT3 expression in A549 cells, and the siRNA inhibited STAT3 levels, highlighting their roles in the regulation of STAT3 signaling. Midazolam combined with the miR-520d-5p mimic and inhibitor, regulated STAT3 expression and its signaling pathway. Midazolam combined with the miR-520d-5p mimic significantly induced A549 cell apoptosis. Thus, midazolam can induce apoptosis of A549 cells by targeting STAT3 via miR-520d-5p. These findings suggest that midazolam might be a putative anti-cancer approach for NSCLC therapy.
Keywords: Cell apoptosis, miR-520d-5p, midazolam, NSCLC, STAT3
Introduction
Lung cancer is the most common cancer and the leading cause of cancer deaths in China [1]. More than 80% of all lung cancers are accounted for by non-small cell lung cancer (NSCLC) [2]. A number of in vitro studies have demonstrated that some anesthetic agents, including midazolam, have antitumor effects [3,4]. However, there are no reports regarding the underlying mechanism of the effect of midazolam on lung cancer.
In addition, microRNAs play an important role in the regulation of many kinds of cancers, including lung cancer, and gastric cancer [5,6]. miR-520d-5p is a novel microRNA that has been shown to inhibit proliferation and induce apoptosis in various cancer cells [7-9], but there is no direct evidence for a relationship between miR-520d-5p and lung cancer.
Signal transducer and activator of the transcription 3 (STAT3) signal pathway has been linked to many biological processes, such as cellular apoptosis and proliferation [10,11]. STAT3 signaling plays an important role in the anti-apoptotic pathway, which is activated in various cancers, including lung cancer [12,13]. miR-520d-5p has been identified as a direct STAT3 target in the proliferation of gastric cancer cell lines [14], although this association has not been verified in lung cancer cells.
We aimed to investigate the effect of miR-520d-5p/STAT3 in response to midazolam, a common anesthetic agent, on apoptosis in human NSCLC A549 cells.
Materials and methods
Cell culture, reagents and antibodies
Non-small-cell carcinoma (A549) cells were provided by the Shanghai Institute of Cell Biology, Chinese Academy of Sciences. The cells were cultured with Minimum Essential Medium (MEM) supplemented with 10% heat-inactivated fetal calf serum (FBS; HyClone, Logan, UT, USA) in a humidified atmosphere with 5% CO2 (37°C). Cells were treated with different concentrations of midazolam (0, 10 μg/mL, 20 μg/mL, 40 μg/mL, and 80 μg/mL) for 24 h and 48 h under controlled conditions.
The antibodies used were as follows: rabbit anti-human-STAT3 (1:800; Bioworld Technology, co. Ltd), rabbit anti-human Bcl-2 associated X protein (Bax, 1:500; Bioworld Technology, co. Ltd), B cell lymphoma 2 (Bcl-2, 1:500; Bioworld Technology, co. Ltd), rabbit anti-human Caspase-3 (1:500; Bioworld Technology, co. Ltd), and rabbit anti-human GAPDH (1:4000; Bioworld Technology, co. Ltd).
MTT assay
For MTT assays, 100 µl cells per well were seeded in 96 well plates and treated with midazolam in six parallel plates at designated concentrations for 24 and 48 hours, as reported [15].
LIVE/DEAD assay
A549 Cells were treated with midazolam at concentrations of 0, 10, 20, 40, and 80 μg/mL for 48 h, as described above. The LIVE/DEAD cell viability assay (Invitrogen, Carlsbad, USA) was conducted following the manufacturer’s instructions. Live cells were able to take up calcein-AM and could be identified by green fluorescent light emission at 490 nm. Dead cells were able to take up ethidium bromide homodimer and could be identified by bright red fluorescence light emission at 545 nm.
DAPI staining
Cell nuclear morphology was detected by DAPI staining under fluorescence microscopy. Cells (A549) were treated with midazolam for 48 h. The cells were washed with PBS (pH7.4), fixed with ice cold 70% ethanol, re-suspended in DAPI, and incubated at 37°C for 15 min. The cells were then washed with PBS and observed under a fluorescence microscope (Nikon Instruments Inc., NY, USA).
Flow cytometry
Flow cytometry (FCM) was performed to assess apoptosis, as previously reported [16]. A549 cells were treated with midazolam for 48 h and co-stained with annexin V-FITC and PI, according to the manufacturer’s instructions (KeyGENBioTECH. Co. Ltd., China). Apoptotic cells were separated and quantified by a FACSCalibur Flow Cytometry System (Becton Dickinson, San Jose, USA).
Western blot
A549 cells were cultured with or without midazolam for 48 h, and then all cells were harvested. The cells were washed with icecold PBS, lysed with RIPA reagent (Sigma-Aldrich, UK) for 30 min at 4°C, and centrifuged at 13,500 rpm for 15 minutes. Equal amounts of total lysed protein were combined with glyceraldehyde 3-phosphate dehydrogenase and loaded onto sodium dodecyl sulfate polyacrylamide gels and subject to electrophoresis (SDS-PAGE). The separated proteins were transferred onto a PVDF membrane (Bio-Rad, Hercules, CA, USA). After washing with PBST, the membranes were incubated with a horseradish peroxidase-conjugated secondary antibody (Amresco, USA) for 1 h at room temperature. The membranes were probed for apoptosis regulatory protein levels using specific antibodies.
MicroRNA mimics, siRNA transient transfection
The miR-520d-5p mimics, miR-520d-5p inhibitor and a STAT3 siRNA, were purchased from GenePharma (Suzhou, China). When cells reached 60% confluency they were transfected with 100 pmol of the miR-520d-5p mimic or inhibitor, or the STAT3 siRNA, using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) in Opti-MEM (Invitrogen). The sequences were as follows: STAT3: CCCGGAAATTTAACATTCT; miR-520d-5p: CUACAAAGGGAAGCCCUUUC. After 24-48 h of transfection, the A549 cells were harvested.
Real-time RT-PCR
To detect miR-520d-5p, reverse transcription-polymerase chain reaction (RT-PCR) was carried out using an all-in-oneTM miRNA quantitative RT-PCR (qRT-PCR) detection Kit (Gene Copoeia, Rockville, MD, USA). U6 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as references for miRNA and mRNA, respectively. Each sample was analyzed in triplicate. The 2-ΔΔCt method was used to calculate fold function.
Luciferase reporter assay
The possible binding sites of miR-520d-5p with STAT3 were predicted and revealed by microRNA.org-Targets and Expression (http://www.microrna.org). The 3’-untranslated region (UTR) of STAT3, harboring the seed sequence of the miR-520d-5p wild-type (or mutant) binding sites, was cloned into the pmirGLO dual-luciferase vector (Promega, USA). The vectors were transfected with miR-520d-5p mimics (Genepharma, China) into A549 cells by lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. After 48 h, A549 cells were harvested and luciferase activity was detected by the Dual-Luciferase Reporter Assay system (Promega, USA). All in vitro experiments were repeated at least three times for each condition.
Statistical analysis
All statistical data were analyzed using the SPSS 11.0 software (Chicago, IL). All data are expressed as the mean ± standard deviation (SD). Statistical analyses were performed using the student’s t-test or analysis of variance (ANOVA). A P value <0.05 is considered to be statistically significant.
Results
The viability of A549 cells treated with different concentrations of midazolam for 24 and 48 hours is shown in Figure 1A. According to the results, midazolam, at concentrations greater than 20 μg/mL, could significantly inhibit A549 cell viability after 24 h or 48 h of treatment (P<0.01). The outcomes indicated a time response relationship between midazolam and A549 cell viability. Consequently, we chose 48 h as the standard treatment time for midazolam. The DAPI staining assay showed fragmented chromatin and apoptotic bodies in the A459 cells following treatment with midazolam, indicating apoptosis (Figure 1B). Similarly, LIVE/DEAD staining assays revealed an increase in A549 cell death after midazolam treatment compared with the control group (Figure 1C). Flow cytometry analysis revealed that midazolam induced apoptosis of A549 cells, compared with control cells (P<0.01) (Figure 1D). To explore the mechanism of midazolam induced apoptosis, expression levels of the anti-apoptotic protein, Bcl-2, as well as the pro-apoptotic proteins, Bax and caspase-3, were determined by Western blot. We found that treatment of A549 cells with midazolam resulted in a decrease in Bcl-2 levels in a dose-dependent manner (Figure 1E). Conversely, Bax and caspase-3 levels increased in a dose-dependent manner (Figure 1E).
Figure 1.
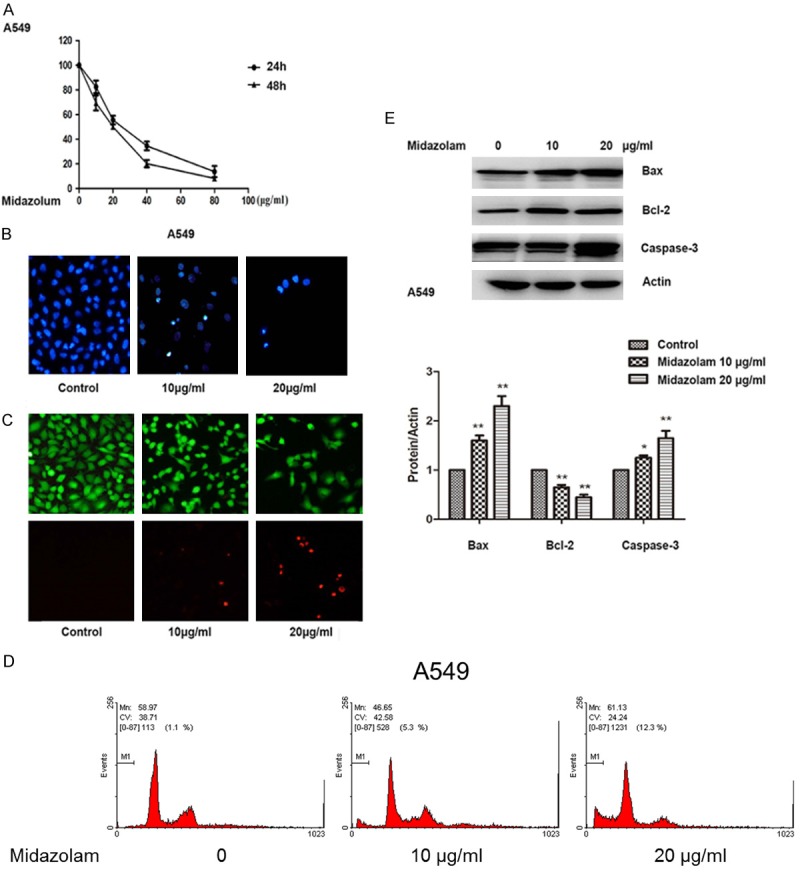
Induction of apoptosis in A549 cells with midazolam. A: Cell viability of A549 cells treated with different concentrations of midazolam for 24 h or 48 h detected by MTT assay. B: Detection of the mode of cell death by DAPI staining assay. C: LIVE/DEAD staining assay of A549 cells following treatment with midazolam at varying concentrations. D: Flow cytometry analysis of A549 cells following treatment with midazolam at varying concentrations. E: Relative expression levels of Bcl-2, Bax, and caspase 3 following treatment with midazolam at varying concentrations for 48 h. Cells were observed using a microscope (original magnification ×20). *; P<0.05, **; P<0.01 compared with control.
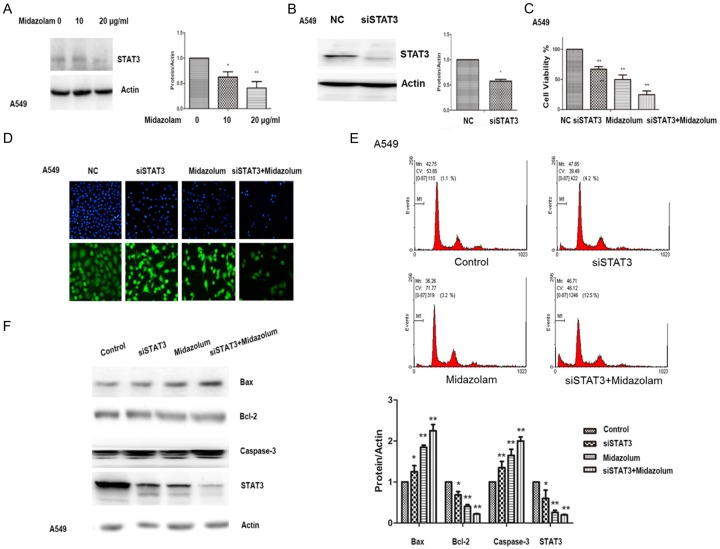
Furthermore, expression of STAT3 in A549 cells was significantly decreased in a dose related manner after midazolam treatment (P<0.01) (Figure 2A). Expression of STAT3 was also decreased by siRNA-STAT3 (siSTAT3) (Figure 2B). The cell viability of A549 cells increased with siSTAT3 treatment (Figure 2C). DAPI staining showed that midazolam treatment (10 μg/mL) combined with siSTAT3 transfection significantly induced cell death compared with other treatment groups (Figure 2D). Similarly, flow cytometry showed that midazolam treatment (10 μg/mL) combined with siSTAT3 transfection significantly induced A549 cell apoptosis, compared with other treatment groups (Figure 2E). A combination of midazolam treatment and transfection with siSTAT3 resulted in an increase in A549 cell caspase-3, compared with other treatments. Similarly, Western blots showed that Bcl-2 levels decreased, and Bax levels increased when the A549 cells were simultaneously treated with midazolam and transfected with siSTAT3. STAT3 levels were significantly decreased on Western blot after midazolam treatment (10 μg/mL) combined with siSTAT3 transfection (Figure 2F).
Figure 2.
STAT3 inhibits midazolam induced apoptosis of A549 cells. A: Expression levels of STAT3 following treatment with midazolam at varying concentrations for 48 h. B: Decreased activation of STAT3 by siRNA-STAT3 (siSTAT3). C: Cell viability of A549 cells that were treated with siSTAT3. D: DAPI staining assay of A549 cells treated with midazolam (10 µg/mL) and transfected with siSTAT3. E: Flow cytometry of A549 cells treated with midazolam (10 µg/mL) and transfected with siSTAT3. F: Relative expression levels of Bcl-2, Bax, caspase 3, and STAT3 after 10 μg/mL midazolam treatment and siSTAT3 transfection, alone or in combination, for 48 h. *; P<0.05, **; P<0.01 compared with control.
The online server microRNA.org-Targets and Expression (http://www.microrna.org) predicted a targeting relationship between STAT3 and miR-520d-5p (mirSVR score: -0.8232, PhastCons score: 0.5581; Figure 3A). We found that STAT3 protein expression was significantly suppressed by the miR-520d-5p mimic (P<0.01, Figure 3B), while the miR-520d-5p inhibitor significantly increased STAT3 protein expression (P<0.01, Figure 3C). Furthermore, we carried out a dual-luciferase reporter assay to verify this targeting relationship. We found that miR-520d-5p could significantly suppress the luciferase activity of the reporter with STAT3, whereas the STAT3-mut luciferase expression was not significantly regulated by miR-520d-5p (Figure 3D), confirming that the site in the STAT 3-3’UTR is the regulatory target site of miR-520d-5p.
Figure 3.
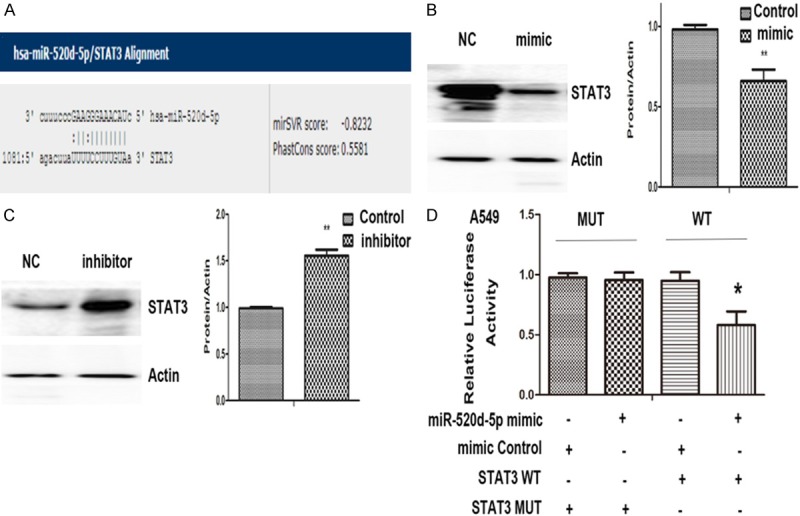
STAT3is atarget gene of miR-520d-5p. A: Possible binding sites of miR-520d-5p and STAT3 were predicted by microRNA. org-Targets and Expression (http://www.microrna.org). B: Relative expression levels of STAT3 with miR-520d-5p mimic transfection. C: Relative expression levels of STAT3 with miR-520d-5p inhibitor transfection. D: The relative luciferase activities. *; P<0.05, **; P<0.01 compared with control.
qRT-PCR showed that midazolam treatment significantly increased the level of miR-520d-5p expression in A549 cells (P<0.01, Figure 4A). To verify the mechanism of midazolam on A549 cells, cells were treated with midazolam, or transfected with miR-520d-5p inhibitor or miR-520d-5p mimic, or transfected with either miR-520d-5p mimic or miR-520d-5p inhibitor in combination with midazolam treatment. Cell viability assays revealed that midazolam treatment in combination with miR-520d-5p mimic transfection significantly inhibited A549 cell viability (Figure 4B). Flow cytometry also showed that midazolam treatment combined with miR-520d-5p mimic transfection significantly induced A549 cell apoptosis (P<0.05, Figure 4C). The effect of midazolam treatment in combination with miR-520d-5p inhibitor transfection was not significant compared with the other treatments (P>0.05, Figure 4D). We found that midazolam combined with transfection with the miR-520d-5p mimic significantly increased Bax and caspase-3 expression, compared with the control (P<0.05). Midazolam treatment alone significantly decreased the expression of Bcl-2 and STAT3 in A549 cells when compared with cells transfected with miR-520d-5p inhibitor alone, or in combination with midazolam treatment (P<0.01, Figure 4F). Midazolam may thus upregulate miR-520d-5p and suppress the activation of the STAT3 pathway to promote apoptosis of A549 cells.
Figure 4.
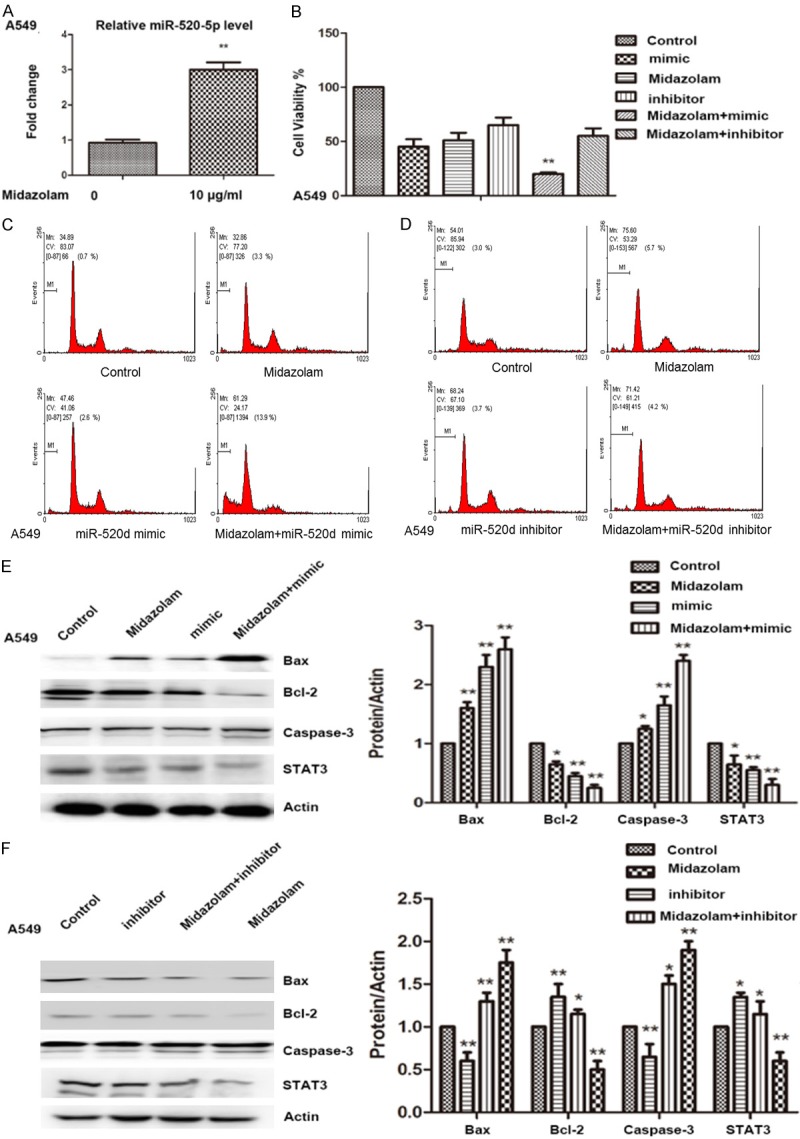
miR-520d-5p promotes midazolam induced apoptosis of A549 lung cancer cells in vitro. A: Expression levels of miR-520d-5p in A549 cells after midazolam (10 µg/mL) treatment for 48 h. B: Cell viability of A549 cells with midazolam treatment and miR-520d-5p mimic and inhibitor transfection, alone or in combination. C: Flow cytometer assay for A549 cells with midazolam (10 µg/mL) treatment and miR-520d-5p mimic transfection, alone or in combination. D: Flow cytometry of A549 cells following midazolam (10 µg/mL) treatment and miR-520d-5p inhibitor transfection, alone or in combination. E: Western blot assay for expression levels of STAT3, Bax, Bcl-2, and caspase-3 with miR-520d-5p mimic transfection and midazolam (10 µg/mL) treatment, alone or in combination. F: Western blot assay for expression levels of STAT3, Bax, Bcl-2, and caspase-3 with miR-520d-5p inhibitor transfection and midazolam (10 µg/mL) treatment, alone or in combination. *; P<0.05, **; P<0.01 compared with control.
Discussion
As lung cancer is the leading cause of cancer deaths worldwide, accounting for nearly one of every four, it is important to explore new and effective drugs to treat this disease. To the best of our knowledge, this is the first study to investigate the potential effectiveness and mechanism of midazolam on lung cancer. Our findings show that midazolam acts via miR-520d-5p, regulating STAT3 and its related protein expression. It may represent a significant potential targeting agent for the effective treatment of lung cancer.
Recent evidence indicated that midazolam may significantly contribute to the roles of Leydig tumor cells [17], human oral tumor cells [18], human head and neck squamous carcinoma cells [19], and lymphoma JeKo-1 cells [20]. To date, no reports have investigated the role of midazolam in lung cancer pathogenesis. In this current study, we found that midazolam could significantly induce apoptosis in A549 cells.
In the study of Makino et al. STAT3 was shown to have an important role in the growth of lung cancer [21]. To investigate the potential anti-cancer mechanisms of midazolam, we assessed STAT3 expression and found it to be significant, with or without midazolam intervention. Furthermore, we found that midazolam treatment suppressed STAT3 expression as well as upregulated Bax and caspase-3 expression, and downregulated Bcl-2 expression.
miRNAs are small non-coding RNAs (ncRNAs) that can affect cancer development and progression. Recently, miR-520d-5p was reported to have a substantial role as a novel cancer suppressor [22,23]. We used the online server microRNA.org-Targets and Expression (http://www.microrna.org) to predict atargeting relationship between STAT3 and miR-520d-5p. The output revealed that the STAT3 mRNA was perfectly matched by miR-520d-5p. Our study demonstrated there was an inverse correlation between miR-520d-5p and STAT3 expression in A549 cells. Interestingly, we found that the effects of midazolam on inducing A549 cell apoptosis and STAT3 expression were targeted by miR-520d-5p silencing (using siRNA) in vitro.
Our results suggest that midazolam induces A549 cell apoptosis by the miR-520d-5p/STAT3 feedback loop. Previously, Tsukerman et al. demonstrated that transcription of TWIST1, a miR-520d-5p functional target [24], was significantly increased by the activation of STAT3 and it could bind to the TWIST1 promoter [25], suggesting a detailed feedback loop (TWIST1/STAT3/miR-520) that may regulate metastasis in gastric cancer. In addition, the current study showed that there was a CypB/STAT3/miR-520d-5p feedback loop that might contribute to modulation of inflammatory signaling in gastric carcinogenesis [14]. Further studies are still needed to focus on this detailed feedback loop and its role in lung cancer, in addition to its association with midazolam.
In summary, we investigated the anti-lung cancer effects of midazolam in vitro. We show that midazolam can upregulate miR-520d-5p in A549 cells and suppress cancer cell growth by suppressing STAT3 activity and inducing apoptosis. Although midazolam did not seem to completely suppress tumor growth, it providesnovel insight for future therapeutic strategies against lung cancer.
Acknowledgements
Thanks to Dr. Sharon Forsyth in Biomedical Editing International for English editing and proofreading services. This work was financed by grants from Science and Technology Fund Project of Shenyang Medical College (No. 20171006).
Disclosure of conflict of interest
None.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Ye L, Wang H, Liu B. miR-211 promotes non-small cell lung cancer proliferation by targeting SRCIN1. Tumour Biol. 2016;37:1151–7. doi: 10.1007/s13277-015-3835-y. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Dou Y, Zheng X, Leng T, Lu X, Ouyang Y, Sun H, Xing F, Mai J, Gu J, Lu B, Yan G, Lin J, Zhu W. TRPM7 channel inhibition mediates midazolam-induced proliferation loss in human malignant glioma. Tumour Biol. 2016;37:14721–31. doi: 10.1007/s13277-016-5317-2. [DOI] [PubMed] [Google Scholar]
- 4.So EC, Chen YC, Wang SC, Wu CC, Huang MC, Lai MS, Pan BS, Kang FC, Huang BM. Midazolam regulated caspase pathway, endoplasmic reticulum stress, autophagy, and cell cycle to induce apoptosis in MA-10 mouse Leydig tumor cells. OncoTargetsTher. 2016;9:2519–33. doi: 10.2147/OTT.S101671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng Z, Pan L, Niu Z, Li W, Dang X, Wan L, Zhang R, Yang S. Identification of microRNAs as potential biomarkers for lung adenocarcinoma using integrating genomics analysis. Oncotarget. 2017;8:64143–56. doi: 10.18632/oncotarget.19358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suarez-Arriaga MC, Torres J, Camorlinga-Ponce M, Gomez-Delgado A, Pina-Sanchez P, Valdez-Salazar HA, Ribas-Aparicio RM, Fuentes-Pananá EM, Ruiz-Tachiquín ME. A proposed method for the relative quantification of levels of circulating microRNAs in the plasma of gastric cancer patients. Oncol Lett. 2017;13:3109–17. doi: 10.3892/ol.2017.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu N, Song Y, Pang L, Chen Z. CRCT1 regulated by microRNA-520 g inhibits proliferation and induces apoptosis in esophageal squamous cell cancer. Tumour Biol. 2016;37:8271–9. doi: 10.1007/s13277-015-4730-2. [DOI] [PubMed] [Google Scholar]
- 8.Lu YC, Cheng AJ, Lee LY, You GR, Li YL, Chen HY, Chang JT. MiR-520b as a novel molecular target for suppressing stemness phenotype of head-neck cancer by inhibiting CD44. SciRep. 2017;7:2042. doi: 10.1038/s41598-017-02058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Xu Y, Chen X, Xiao J. Dexmedetomidine inhibits osteosarcoma cell proliferation and migration, and promotes apoptosis by regulating MiR-520a-3p. Oncol Res. 2017 doi: 10.3727/096504017X14982578608217. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu YH, Wei XL, Hu GQ, Wang TX. Quinolone-indolone conjugate induces apoptosis by inhibiting the EGFR-STAT3-HK2 pathway in human cancer cells. Mol Med Rep. 2015;12:2749–56. doi: 10.3892/mmr.2015.3716. [DOI] [PubMed] [Google Scholar]
- 11.Zhang M, Bian ZG, Zhang Y, Wang JH, Kan L, Wang X, Niu HY, He P. Cucurbitacin B inhibits proliferation and induces apoptosis via STAT3 pathway inhibition in A549 lung cancer cells. Mol Med Rep. 2014;10:2905–11. doi: 10.3892/mmr.2014.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M, Lin T, Wang Y, Gao S, Yang Z, Hong X, Chen G. CXCL12 suppresses cisplatin-induced apoptosis through activation of JAK2/STAT3 signaling in human non-small-cell lung cancer cells. Onco Targets Ther. 2017;10:3215–24. doi: 10.2147/OTT.S133055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jorvig JE, Chakraborty A. Zerumbone inhibits growth of hormone refractory prostate cancer cells by inhibiting JAK2/STAT3 pathway and increases paclitaxel sensitivity. Anticancer Drugs. 2015;26:160–6. doi: 10.1097/CAD.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 14.Li T, Guo H, Zhao X, Jin J, Zhang L, Li H, Lu Y, Nie Y, Wu K, Shi Y, Fan D. Gastric cancer cell proliferation and survival is enabled by a cyclophilin B/STAT3/miR-520d-5p signaling feedback loop. Cancer Res. 2017;77:1227–40. doi: 10.1158/0008-5472.CAN-16-0357. [DOI] [PubMed] [Google Scholar]
- 15.Posimo JM, Unnithan AS, Gleixner AM, Choi HJ, Jiang Y, Pulugulla SH, Leak RK. Viability assays for cells in culture. J Vis Exp. 2014:e50645. doi: 10.3791/50645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer K, Andreesen R, Mackensen A. An improved flow cytometric assay for the determination of cytotoxic T lymphocyte activity. J Immunol Methods. 2002;259:159–69. doi: 10.1016/s0022-1759(01)00507-5. [DOI] [PubMed] [Google Scholar]
- 17.So EC, Lin YX, Tseng CH, Pan BS, Cheng KS, Wong KL, Hao LJ, Wang YK, Huang BM. Midazolam induces apoptosis in MA-10 mouse Leydig tumor cells through caspase activation and the involvement of MAPK signaling pathway. Onco Targets Ther. 2014;7:211–21. doi: 10.2147/OTT.S56084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohno S, Kobayashi K, Uchida S, Amano O, Sakagami H, Nagasaka H. Cytotoxicity and type of cell death induced by midazolam in human oral normal and tumor cells. Anticancer Res. 2012;32:4737–47. [PubMed] [Google Scholar]
- 19.Dou YL, Lin JP, Liu FE, Wang LY, Shu HH, Jiang N, Xie Y, Duan Q. Midazolam inhibits the proliferation of human head and neck squamous carcinoma cells by downregulating p300 expression. Tumour Biol. 2014;35:7499–504. doi: 10.1007/s13277-014-1991-0. [DOI] [PubMed] [Google Scholar]
- 20.Hong JQ, Wu SH, Chen ZY, Zhuang WH, Gao HZ. [Effect of midazolam on mantle cell lymphoma JeKo-1 cell line and its relevant mechanisms] . Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013;21:1460–3. doi: 10.7534/j.issn.1009-2137.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Makino Y, Yoon JH, Bae E, Kato M, Miyazawa K, Ohira T, Ikeda N, Kuroda M, Mamura M. Repression of Smad3 by Stat3 and c-Ski/SnoN induces gefitinib resistance in lung adenocarcinoma. Biochem Biophys Res Commun. 2017;484:269–77. doi: 10.1016/j.bbrc.2017.01.093. [DOI] [PubMed] [Google Scholar]
- 22.Yan L, Yu J, Tan F, Ye GT, Shen ZY, Liu H, Zhang Y, Wang JF, Zhu XJ, Li GX. SP1-mediated microRNA-520d-5p suppresses tumor growth and metastasis in colorectal cancer by targeting CTHRC1. Am J Cancer Res. 2015;5:1447–59. [PMC free article] [PubMed] [Google Scholar]
- 23.Park YY, Kim SB, Han HD, Sohn BH, Kim JH, Liang J, Lu Y, Rodriguez-Aguayo C, Lopez-Berestein G, Mills GB, Sood AK, Lee JS. Tat-activating regulatory DNA-binding protein regulates glycolysis in hepatocellular carcinoma by regulating the platelet isoform of phosphofructokinase through microRNA 520. Hepatology (Baltimore, Md) 2013;58:182–91. doi: 10.1002/hep.26310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsukerman P, Yamin R, Seidel E, Khawaled S, Schmiedel D, Bar-Mag T, Mandelboim O. MiR-520d-5p directly targets TWIST1 and downregulates the metastamiR miR-10b. Oncotarget. 2014;5:12141–50. doi: 10.18632/oncotarget.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao D, Besser AH, Wander SA, Sun J, Zhou W, Wang B, Ince T, Durante MA, Guo W, Mills G, Theodorescu D, Slingerland J. Cytoplasmic p27 promotes epithelial-mesenchymal transition and tumor metastasis via STAT3-mediated Twist1 upregulation. Oncogene. 2015;34:5447–59. doi: 10.1038/onc.2014.473. [DOI] [PMC free article] [PubMed] [Google Scholar]