Abstract
Rectal cancer is a common malignancywith a less than 5-year postoperative survival rate. Although it often metastasizes via the lymph and blood, the detailed mechanism of this process remains unclear. This study investigated the relationship between vascular endothelial growth factor-C (VEGF-C) expression and lymphangiogenesis, as well as its relation to lymphatic metastasis of rectal cancer. To address this question, VEGF-C expression in rectal cancer and normal tissue adjacent to tumor was assessed by immunohistochemistry. The lymphatic endothelial cell-specific marker D2-40 was used to label lymphatic endothelial cells and the lymphatic vessel density (LVD) was subsequently quantified. As expected, the expression of VEGF-C in rectal cancer (75%) was significantly higher than in normal adjacent tissue (25%), and this level correlated with differentiation, Dukes stage, and lymph node metastasis, though not with sex or age. The LVD was higher in VEGF-C positive rectal cancer than in VEGF-C negative rectal cancer, and was also higher in lymphatic metastases than in non-lymphatic metastases. These results indicate that expression of VEGF-C may impact the prognosis of rectal cancer via its effect on the formation of new lymphatic vessels. This represents a significant advance in the study of the genesis and development of rectal cancer, and may have value in clinical care.
Keywords: Rectal cancer, vascular endothelial growth factor-C, lymphatic vessel density, lymphangiogenesis, and lymphatic metastasis
Introduction
Rectal cancer is a common malignancy [1-3]. Its development is a complex multi-factor, multistage process, involving numerous genes [4,5]. Though improvements in its diagnosis and treatment have led to greatly improved survival rate and functional recovery, efficacy of treatment is still unsatisfactory. The study of colorectal cancer etiology, pathogenesis, diagnosis, treatment, and prevention remains a vital topic of research [6].
The lymphatic system is one of the main routes of metastasis of rectal cancer, which correlates with a poor prognosis [7,8]. Lymphangiogenesis can promote lymph node metastasis, further facilitating distant metastasis that involves multiple genes and products [9,10]. The vascular endothelial growth factor family includes vascular endothelial growth factor-A-F (VEGF-A-F), and placental growth factor, of which VEGF-C and -D are mainly responsible for regulating lymphangiogenesis [10,11]. As an important member of the family, VEGF-C has been a major focus of research in recent years. Its C-terminus is rich in cysteine, which plays a role in maturation via protease hydrolysis. There are two receptors for VEGF-C: VEGFR-2 and VEGFR-3 [12,13]. VEGFR-2 is expressed in both blood vessels and lymphatic endothelial cells, while VEGFR-3 is only expressed in lymphatic endothelial cells [13]. VEGF-C plays a role in promoting lymphangiogenesis by binding these receptors [14,15]. VEGF-C is the most specific for induction of lymphatic vessel formation [16,17]. Many studies have shown that VEGF-C is expressed in breast cancer, gastric cancer, Hodgkin’s lymphoma, esophageal cancer, lung cancer, and bladder cancer, and is associated with lymphatic metastasis [18,19]. This study investigated the role of VEGF-C in colorectal cancer, and its relationship to lymphangiogenesis and lymph node metastasis, in order to determine new approaches for diagnosis, treatment, and prognosis of rectal cancer.
Materials and methods
Patients
A total of 40 rectal cancer patients, 20 men and 20 women hospitalized at the First Affiliated Hospital of Soochow University from August 2014 to June 2015, were enrolled based on their surgical resection and clinical diagnosis. None of the patients had received chemotherapy, radiotherapy, or biotherapy prior to surgery. The mean patient age was 59.3 ± 6.35 (range: 3087) years. Twenty patients had well-differentiated adenocarcinoma, while 20 had moderately-or poorly-differentiated adenocarcinoma. Tumors of 22 patients were classified as the Dukes stage (A + B), while 18 patients as Dukes stage (C + D); 16 patients had lymph node metastasis, while 24 did not have metastasis. The normal tissue adjacent to carcinoma (> 8 cm from the tumor edge) was selected as control. The study protocol was approved by the Research Ethics Committee of Children’s Hospital of Soochow University and all patients provided written informed consent prior to their enrollment into the study.
Immunohistochemistry (IHC)
The aforementioned tissues were fixed and sectioned in order to perform IHC staining according to the manufacturer’s instructions. Endogenous peroxidase was inactivated by incubating the sections in 3% H2O2 for 30 min. The sections were subjected to sequential incubations with 10% normal goat serum in 0.01 M PBS for 30 min at room temperature, and then respectively incubated in rabbit-derived anti-VEGFC antibody (ab135506, Abcam, USA), and mouse-derived anti-D2-40 antibody [D2-40] (ab77854, Abcam, USA) in PBS containing 0.3% Triton X-100 overnight at 4°C. The sections were washed 3 times (5 min each) with PBS, and then incubated in peroxidase-conjugated goat anti-rat IgG (1:200; Zymed, South San Francisco, CA, USA) for 1 hr at room temperature. Finally, the sections were developed with diaminobenzidine (DAB) in 0.1 M Tris-buffered saline (TBS) containing 0.001% H2O2 for 30 min. The sections were observed by microscopy (Olympus, Tokyo, Japan), images were captured, and the VEGF-C and D2-40 expression levels were detected as described below.
VEGF-C was identified in the cytoplasm and cell membrane as tan/brown particles, and the VEGF-C signal was evaluated according to the staining intensity: 0: No color, 1: Light yellow, 2: Claybank, 3: Tawny, and the positive cells number: 0: < 5%; 1: 5%-25%; 2: 26%-50%; 3: 51%-75%; 4: > 75%, and defined as the immunohistochemical score: -: 0-2 score; +: 3-5 score; ++: 6-8 score; +++: > 8 score.
D2-40 was specifically expressed in the cytoplasm of lymphatic vessel endothelial cells and stained brown, and the lymphatic vessel density (LVD), was quantified according to the average number of lymphatic vessels per area, and a histogram was generated using Origin 9.0 software (http://www.originlab.com/; Northampton, MA, USA).
Statistical analysis
All data were expressed as the mean ± standard deviation (SD). Statistical analyses were performed via one-way ANOVA using SPSS software (version 21.0, http://spss.en.softonic.com/), and Student’s t-tests were performed in a group of two samples, with P < 0.05 and P < 0.01 indicated significant differences and highly significant differences, respectively.
Results
VEGF-C expression was increased in rectal cancer compared to normal adjacent tissue
As shown in Figure 1A, VEGF-C is localized to the cytoplasm and cell membrane, with the brown particles representing positive signal. VEGF-C was weak in normal tissue (Figure 1B). The VEGF-C positive rate in rectal cancer (72.5%) was significantly higher than in normal adjacent tissue (12.5%, Table 1, **: P < 0.01).
Figure 1.
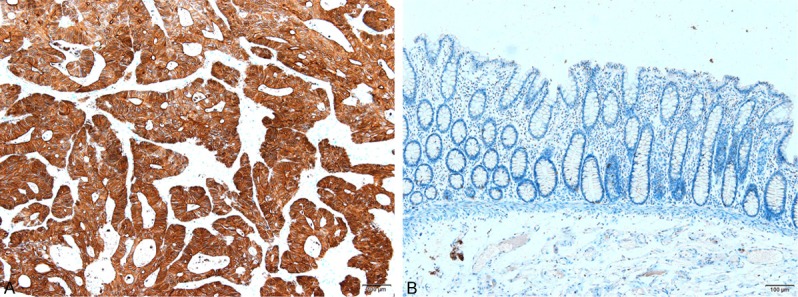
VEGF-C expression level assay by immunohistochemistry (IHC) in rectal cancer and normal adjacent tissue. A: VEGF-C is expressed in rectal cancer; B: VEGF-C is negative in normal adjacent tissue. VEGF-C expression in rectal cancer was higher than that of the normal adjacent tissue (**: P < 0.01, 100×).
Table 1.
VEGF-C* expression by immunostaining in rectal cancer and normal adjacent tissue
| Types | Cases | VEGF-C | Positive rate (%) | P-value | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| - | + | ++ | +++ | ||||
| Rectal cancer | 40 | 11 | 12 | 9 | 8 | 72.5 | 0.00025 |
| Para-carcinoma tissue | 40 | 35 | 4 | 1 | 0 | 12.5 | |
Vascular endothelial growth factor-C.
VEGF-C expression is closely associated with differentiation, Dukes staging, and lymph node metastasis
VEGF-C was associated with the differentiation, Dukes staging, and lymph node metastasis (Table 2, **: P < 0.01), but not with either sex or age.
Table 2.
Relationship of VEGF-C* with clinical features
| Parameters | VEGF-C | Positive rate (%) | P-value | |
|---|---|---|---|---|
|
| ||||
| Positive | Negative | |||
| Gender | 0.658 | |||
| Male | 18 | 2 | 90.0 | |
| Female | 16 | 4 | 80.0 | |
| Age | 1.000 | |||
| ≥ 60 | 20 | 4 | 83.3 | |
| < 60 | 14 | 2 | 87.5 | |
| Differentiation | 0.027 | |||
| Well | 20 | 0 | 100.0 | |
| Moderate-poor | 14 | 6 | 70.0 | |
| Dukes stage | 0.015 | |||
| A + B | 13 | 9 | 59.1 | |
| C + D | 16 | 2 | 88.9 | |
| Lymphatic metastasis | 0.00014 | |||
| With | 15 | 1 | 93.8 | |
| Without | 8 | 16 | 33.3 | |
VEGF-C: Vascular endothelial growth factor-C.
D2-40 is specifically expressed in lymphatic endothelial cells
As shown in Figure 2A and 2B, the D2-40 protein is specifically expressed in lymphatic endothelial cells of rectal cancer. The expression of D2-40 was significantly higher in the lymphatic endothelial cells of rectal cancer than in the normal adjacent tissue (**: P < 0.01).
Figure 2.
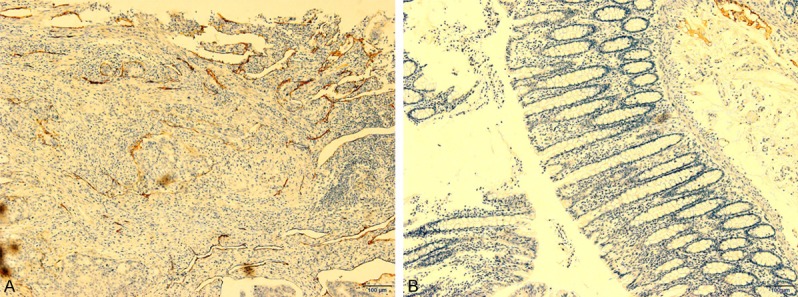
D2-40 expression level assay by immunohistochemistry (IHC) in rectal cancer and normal adjacent tissue. A: The D2-40 expressed in rectal cancer; B: The D2-40 expressed in normal adjacent tissue. D2-40 expression in rectal cancer was obviously higher than in normal adjacent tissue (**: P < 0.01, 100×).
LVD is higher in VEGF-C positive rectal cancer than in normal adjacent tissue
LVD of rectal cancer tissue demonstrated a significantly greater proportion of VEGF-C positive cells than VEGF-C negative cells (Figure 3). Similarly, in normal adjacent tissue, increased VEGF-C expression was noted with more LVD. In comparing the two LVD expressions, significantly higher VEGF-C expression was present in the rectal cancer tissue than that of normal adjacent tissue.
Figure 3.
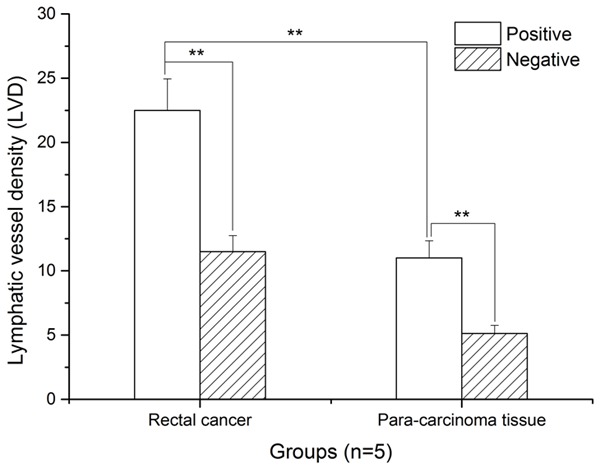
Histogram analysis of LVD in rectal cancer and normal adjacent tissue. LVD was higher in rectal cancer than in normal adjacent (Para-carcinoma) tissue (**: P < 0.01).
LVD was higher in lymphatic metastasis than that of non-lymphatic metastasis
As shown in Figure 4, LVD is higher in lymphatic metastasis than non-lymphatic metastasis.
Figure 4.
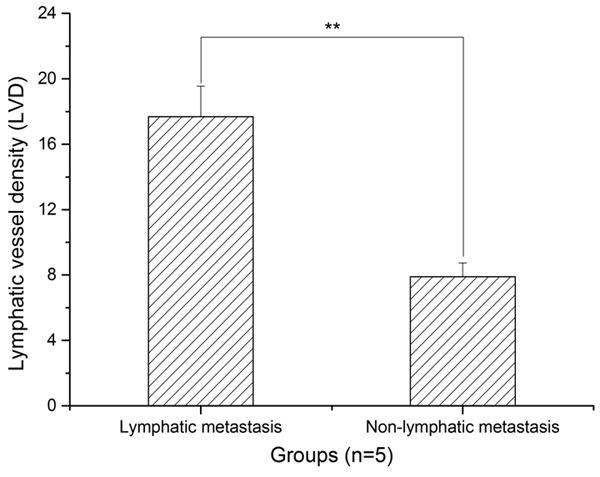
Histogram analysis of the LVD in lymphatic metastasis and non-lymphatic metastasis. LVD was higher in lymphatic metastasis than in non-lymphatic metastasis (**: P < 0.01).
Discussion
This study demonstrated that enhanced VEGF-C expression is located in the cytoplasm and cell membrane of rectal cancer tissue, and these levels were significantly higher than that of normal adjacent tissue. In addition, VEGF-C expression was closely associated with differentiation, Dukes staging, and lymph node metastasis, but not with patient sex or age. Moreover, D2-40 protein was specifically expressed in lymphatic endothelial cells of rectal cancer, at a higher level than lymphatics of normal adjacent tissue. The LVD was higher in VEGF-C positive tissue than in VEGF-C negative tissue in rectal cancer, and correlated with the expression of VEGF-C in rectal cancer but not normal tissue.
VEGF is a known regulatory factor family in lymphangiogenesis. After specifically binding with endothelial cells, the family activates the tyrosine kinase signaling system, thereby promoting tumor lymphangiogenesis [11,20]. VEGF-C was first isolated from prostate cancer cells by Joukov in 1996. It has 419 amino acid residues with a molecular weight of 46.9 kDa [21,22]. After proteolytic hydrolysis, the VEGF-C precursor forms the mature dimer that activates tyrosine kinase signaling. It further activates serine protease and plasminogen activator, eventually producing collagen enzyme which results in lymphangiogenesis. VEGF-C plays an important role in regulating lymphangiogenesis through binding with its specific receptor [23]. D2-40 is a connective sialic acid glycoprotein that is detected by IgG1 type monoclonal antibody with molecular weight as 40 kDa [24,25]. As with podoplanin and LYVE-1, it is also a specific marker of lymphatic vessels. This study identified D2-40 as a specific marker for lymphatic vessels and a means to tag lymphatic vessels for LVD assessment [26,27].
Lymphatic metastasis is an important predictor of clinical prognosis, in which lymphangiogenesis is an essential step. Many studies have shown that VEGF-C can promote lymphangiogenesis in tumors and increase tumor invasiveness, thus facilitating distant metastasis [28,29]. Yang et al. applied immunohistochemistry to investigate VEGF-C expression in 64 patients with esophageal cancer, and found that VEGF-C expression in esophageal squamous carcinoma tissue was 59.4%, which was higher than in adjacent normal tissue [30]. Furthermore, its positive expression was related to lymph node metastasis and TNM stage, suggesting that VEGF-C is associated with lymph node metastasis in esophageal cancer and is a potential marker of esophageal cancer lymphatic metastasis. Kostis et al. identified overexpression of VEGF-C expression in prostate cancer tissue. They also found that its expression was associated with lymphatic metastasis. High LVD counts showed elevated VEGF-C, indicating that VEGF-C played an important role in prostate cancer lymphatic metastasis [31]. Liu et al. explored VEGF-C expression in 109 cases of primary non-small cell lung cancer patients. VEGF-C expression was found in 65.14% of cases, significantly higher than in normal tissue. LVD counting was higher in VEGF-C positive cells than in VEGF-C negative cells, suggesting that VEGF-C can promote non-small cell lung cancer growth and lymph node metastasis. This provides a new direction for non-small cell lung cancer targeted therapy [32].
Xu et al. found that VEGF-C expression in colorectal cancer tissue was 78.2%, which was associated with Dukes staging and lymph node metastasis [33]. Our study confirmed that VEGF-C was highly expressed in colorectal cancer (positive rate 72.5%) and related to differentiation, Dukes stage, and lymph node metastasis, in accordance with the results of Xu et al. We further illustrated that VEGF-C had a critical role in colorectal cancer and lymphatic metastasis and can be used as anindicator of poor prognosis. Liu et al. found that LVD counting in VEGF-C positive lung cancer cells was clearly higher than that in VEGF-C negative cancers. Our investigation revealed a similar result, further illustrating that VEGF-C is an important factor in lymphangiogenesis. LVD counting was higher in patients with lymph node metastasis than in patients without lymph node metastasis, indicating that VEGF-C is closely related to lymphangiogenesis and lymphatic metastasis in rectal cancer. These results provide new pathways for early diagnosis, treatment, and prognosis of rectal cancer [32]. Limits of our study include limited sample size, warranting an increase the sample size and use of multiple methods for systemic investigation in the future. Our study provides a significant advance on the genesis and development of rectal cancer and represents a significant advance in clinical practice.
Acknowledgements
This research project was sponsored by the National Natural Science Foundation of China (Grant No. 81420108002).
Disclosure of conflict of interest
None.
References
- 1.Buchs NC, Koessler T, Vitali GC, Zilli T, Puppa G, Breguet R, Bichard P, Roth A, Morel P, Ris F. [Multidisciplinary management of localized rectal cancer] . Rev Med Suisse. 2017;13:1229–1235. [PubMed] [Google Scholar]
- 2.Fu C, Han J. [Strategy and technique for simultaneous resection of rectal cancer and liver metastasis] . Zhonghua Wei Chang Wai Ke Za Zhi. 2017;20:618–620. [PubMed] [Google Scholar]
- 3.Xu P, Xu J. [Application of robotic surgical system in sphincter-preserving surgery for low rectal cancer] . Zhonghua Wei Chang Wai Ke Za Zhi. 2017;20:606–609. [PubMed] [Google Scholar]
- 4.Joye I, Debucquoy A, Deroose CM, Vandecaveye V, Cutsem EV, Wolthuis A, D’Hoore A, Sagaert X, Zhou M, Gevaert O, Haustermans K. Quantitative imaging outperforms molecular markers when predicting response to chemoradiotherapy for rectal cancer. Radiother Oncol. 2017;124:104–109. doi: 10.1016/j.radonc.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korphaisarn K, Loree JM, Nguyen V, Coulson R, Holla V, Litzenburger BC, Chen K, Mills GB, Maru DM, Meric-Bernstan F, Mills Shaw KR, Kopetz S. Genomic analysis of exceptional responder to regorafenib in treatment-refractory metastatic rectal cancer: a case report and review of the literature. Oncotarget. 2017;8:57882–57888. doi: 10.18632/oncotarget.18357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papp G, Besznyak I, Porneczi B, Saftics G, Bursics A. [Advantages of transanal approach in low rectal cancer resections] . Magy Seb. 2017;70:119–124. doi: 10.1556/1046.70.2017.2.2. [DOI] [PubMed] [Google Scholar]
- 7.Hoshino A, Lyden D. Metastasis: lymphatic detours for cancer. Nature. 2017;546:609–610. doi: 10.1038/546609a. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Han C, Teng F, Bai Z, Tian W, Xue F. Predictive value of serum HE4 and CA125 concentrations for lymphatic metastasis of endometrial cancer. Int J Gynaecol Obstet. 2017;136:58–63. doi: 10.1002/ijgo.12010. [DOI] [PubMed] [Google Scholar]
- 9.Kinashi H, Falke LL, Nguyen TQ, Bovenschen N, Aten J, Leask A, Ito Y, Goldschmeding R. Connective tissue growth factor regulates fibrosis-associated renal lymphangiogenesis. Kidney Int. 2017;92:850–863. doi: 10.1016/j.kint.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Guc E, Briquez PS, Foretay D, Fankhauser MA, Hubbell JA, Kilarski WW, Swartz MA. Local induction of lymphangiogenesis with engineered fibrin-binding VEGF-C promotes wound healing by increasing immune cell trafficking and matrix remodeling. Biomaterials. 2017;131:160–175. doi: 10.1016/j.biomaterials.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 11.Yang GH, Zhou X, Ji WJ, Liu JX, Sun J, Dong Y, Jiang TM, Li YM. VEGF-C-mediated cardiac lymphangiogenesis in high salt intake accelerated progression of left ventricular remodeling in spontaneously hypertensive rats. Clin Exp Hypertens. 2017;39:740–747. doi: 10.1080/10641963.2017.1324478. [DOI] [PubMed] [Google Scholar]
- 12.Peng FW, Liu DK, Zhang QW, Xu YG, Shi L. VEGFR-2 inhibitors and the therapeutic applications thereof: a patent review (2012-2016) Expert Opin Ther Pat. 2017;27:987–1004. doi: 10.1080/13543776.2017.1344215. [DOI] [PubMed] [Google Scholar]
- 13.Lee S, Rho SS, Park H, Park JA, Kim J, Lee IK, Koh GY, Mochizuki N, Kim YM, Kwon YG. Carbohydrate-binding protein CLEC14A regulates VEGFR-2- and VEGFR-3-dependent signals during angiogenesis and lymphangiogenesis. J Clin Invest. 2017;127:457–471. doi: 10.1172/JCI85145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang XL, Zhao J, Qin L, Qiao M. Promoting inflammatory lymphangiogenesis by vascular endothelial growth factor-C (VEGF-C) aggravated intestinal inflammation in mice with experimental acute colitis. Braz J Med Biol Res. 2016;49:e4738. doi: 10.1590/1414-431X20154738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franc M, Kachel-Flis A, Michalski B, Fila-Danilow A, Mazurek U, Michalski M, Michalska A, Kuczerawy I, Skrzypulec-Plinta V. Lymphangiogenesis in cervical cancer evaluated by expression of the VEGF-C gene in clinical stage IB-IIIB. Prz Menopauzalny. 2015;14:112–117. doi: 10.5114/pm.2015.49397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao G, Zhu G, Huang Y, Zheng W, Hua J, Yang S, Zhuang J, Ye J. IL-6 mediates the signal pathway of JAK-STAT3-VEGF-C promoting growth, invasion and lymphangiogenesis in gastric cancer. Oncol Rep. 2016;35:1787–1795. doi: 10.3892/or.2016.4544. [DOI] [PubMed] [Google Scholar]
- 17.Starek I, Salzman R, Kucerova L, Skalova A, Hauer L. Expression of VEGF-C/-D and lymphangiogenesis in salivary adenoid cystic carcinoma. Pathol Res Pract. 2015;211:759–765. doi: 10.1016/j.prp.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Ji H, Cao R, Yang Y, Zhang Y, Iwamoto H, Lim S, Nakamura M, Andersson P, Wang J, Sun Y, Dissing S, He X, Yang X, Cao Y. TNFR1 mediates TNF-alpha-induced tumour lymphangiogenesis and metastasis by modulating VEGF-C-VEGFR3 signalling. Nat Commun. 2014;5:4944. doi: 10.1038/ncomms5944. [DOI] [PubMed] [Google Scholar]
- 19.Hall MA, Robinson H, Chan W, Sevick-Muraca EM. Detection of lymphangiogenesis by near-infrared fluorescence imaging and responses to VEGF-C during healing in a mouse full-dermis thickness wound model. Wound Repair Regen. 2013;21:604–615. doi: 10.1111/wrr.12063. [DOI] [PubMed] [Google Scholar]
- 20.Xu Z, Han K, Chen J, Wang C, Dong Y, Yu M, Bai R, Huang C, Hou L. VEGF is neuroprotective against ischemic brain injury by inhibiting scavenger receptor A expression on microglia. J Neurochem. 2017;142:700–709. doi: 10.1111/jnc.14108. [DOI] [PubMed] [Google Scholar]
- 21.Matsui T, Shigeta T, Umeda M, Komori T. Vascular endothelial growth factor C (VEGF-C) expression predicts metastasis in tongue cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:436–442. doi: 10.1016/j.oooo.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Visuri MT, Honkonen KM, Hartiala P, Tervala TV, Halonen PJ, Junkkari H, Knuutinen N, Yla-Herttuala S, Alitalo KK, Saarikko AM. VEGFC and VEGF-C156S in the pro-lymphangiogenic growth factor therapy of lymphedema: a large animal study. Angiogenesis. 2015;18:313–326. doi: 10.1007/s10456-015-9469-2. [DOI] [PubMed] [Google Scholar]
- 23.Xu L, Sun K, Xia M, Li X, Lu Y. MMP13 regulates aggressiveness of pediatric multiple myeloma through VEGF-C. Cell Physiol Biochem. 2015;36:509–516. doi: 10.1159/000430116. [DOI] [PubMed] [Google Scholar]
- 24.Tan Y, Hu Y, Wu J, Cheng Q, Lei X. Massive retiform hemangioendothelioma that expresses D2-40. Indian J Dermatol Venereol Leprol. 2017;83:360–363. doi: 10.4103/ijdvl.IJDVL_373_16. [DOI] [PubMed] [Google Scholar]
- 25.Tajima S, Fukayama M. Possibility of D2-40 as a diagnostic and tumor differentiation-suggestive marker for some of phosphaturic mesenchymal tumors. Int J Clin Exp Pathol. 2015;8:9390–9396. [PMC free article] [PubMed] [Google Scholar]
- 26.Herwig MC, Munstermann K, Klarmann-Schulz U, Schlereth SL, Heindl LM, Loeffler KU, Muller AM. Expression of the lymphatic marker podoplanin (D2-40) in human fetal eyes. Exp Eye Res. 2014;127:243–251. doi: 10.1016/j.exer.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Gao T, Wang G. Expression of Prox1, D2-40, and WT1 in spindle cell hemangioma. J Cutan Pathol. 2014;41:447–450. doi: 10.1111/cup.12309. [DOI] [PubMed] [Google Scholar]
- 28.Chitale S, Al-Mowallad AF, Wang Q, Kumar S, Herrick A. High circulating levels of VEGF-C suggest abnormal lymphangiogenesis in systemic sclerosis. Rheumatology (Oxford) 2008;47:1727–1728. doi: 10.1093/rheumatology/ken372. [DOI] [PubMed] [Google Scholar]
- 29.Sun P, Gao J, Liu YL, Wei LW, Wu LP, Liu ZY. RNA interference (RNAi)-mediated vascular endothelial growth factor-C (VEGF-C) reduction interferes with lymphangiogenesis and enhances epirubicin sensitivity of breast cancer cells. Mol Cell Biochem. 2008;308:161–168. doi: 10.1007/s11010-007-9624-1. [DOI] [PubMed] [Google Scholar]
- 30.Yang Z, Wang YG, Su K. VEGF-C and VEGFD expression and its correlation with lymph node metastasis in esophageal squamous cell cancer tissue. Asian Pac J Cancer Prev. 2015;16:271–274. doi: 10.7314/apjcp.2015.16.1.271. [DOI] [PubMed] [Google Scholar]
- 31.Kostis G, Ioannis L, Helen K, Helen P. The expression of vascular endothelial growth factor-C correlates with lymphatic microvessel density and lymph node metastasis in prostate carcinoma: an immunohistochemical study. Urol Ann. 2014;6:224–230. doi: 10.4103/0974-7796.134275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Liu C, Qiu L, Li J, Zhang P, Sun Y. Overexpression of both platelet-derived growth factor-BB and vascular endothelial growth factor-C and its association with lymphangiogenesis in primary human non-small cell lung cancer. Diagn Pathol. 2014;9:128. doi: 10.1186/1746-1596-9-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu CJ, Xu F. MMP-11 and VEGF-C expression correlate with clinical features of colorectal adenocarcinoma. Int J Clin Exp Med. 2014;7:2883–2888. [PMC free article] [PubMed] [Google Scholar]


