Abstract
Objective: To observe the preventive effects of oral mucosal epithelial cells (OMECs) seeded on a decellularized human amniotic membrane (dHAM) in a rat model of intrauterine adhesion (IUA). Methods: IUAs were established by mechanical endometrial scraping in model rats. Thirty-six Sprague-Dawley rats were divided into: IUA, dHAM transplantation, and dHAM+OMECs transplantation groups (12 in each group). The oral mucosae of rats were collected, and the OMECs were cultured on the dHAM. The dHAM and dHAM+OMECs were transplanted into left scraped uteri (right uteri as controls) in the dHAM and dHAM+OMECs transplantation groups. At 3, 7, 14, and 28 days after transplantation, the uterine samples underwent histological and immunohistochemical staining. Results: After endometrial scraping, the endometria and uterine cavities were obliterated. The ratios of the fibrotic areas to the whole endometrium in the IUA and dHAM transplantation group were higher than in the control group (P<0.01). In the dHAM+OMECs transplantation group, the fibrotic area significantly decreased compared to the IUA group after 7, 14, and 28 days, and the dHAM transplantation group at 14 and 28 days (P<0.01). At 14 and 28 days after surgery in the dHAM+OMECs transplantation group, the number of endometrial glands and the endometrial thickness increased (P<0.01), and regenerated epithelia were observed. Immunohistochemical assays for cytokeratin 18 demonstrated that the newly-regenerated epithelium was endometrium. Conclusion: OMECs can enhance repair of damaged endometrium, and promise to be novel and effective in preventing IUAs. However, studies of OMECs in patients with IUA need further exploration.
Keywords: Intrauterine adhesion, oral mucosal epithelial cells, human amniotic membrane, fibrosis
Introduction
Asherman syndrome, or intrauterine adhesion (IUA), is a consequence of damage to the basal layer of the endometrium, leading to an obliteration (partial or complete) of the uterine cavity and/or the cervical canal. This condition can result in amenorrhea or hypomenorrhea, female infertility, recurrent pregnancy loss, placenta previa, and placenta accreta [1,2]. IUA occurs most commonly as a result of dilation and curettage, which injures the basal layer of the endometrium. The prevalence of IUA keepsrising due to an increasing number of artificial abortions and hysteroscopic surgeries [1,2].
Hysteroscopic adhesiolysis is considered to be the treatment of choice for IUA. However, the recurrence rate for IUAs could be up to 62.5% in patients with severe IUA [1]. Pregnancy rates with IUA are decreased, ranging from 22.5% to 33.3% [3,4]. Many adjuvant treatments have been introduced to prevent recurrences of IUA, including hormone therapy, intrauterine devices, balloons, and hyaluronic acid gel. Although some preventive effects have been achieved, in severe IUA cases, the incidence of repeat adhesion formation remains high and the prognosis is usually poor [5,6].
Human amniotic membrane (HAM) is a translucent thin membrane derived from the inner layer of the placenta that contains no blood vessels, lymphatics, or nerves. It is easily accessible, has low immunogenicity, there are minimal ethical issues with procurement, and it has both anti-inflammatory and anti-fibrotic properties [7]. HAM has been used in various medical fields to promote wound repair, especially in burns [8] and ocular surface reconstructions [9]. In order to eliminate the immunogenicity originating from epithelial and stromal cells, and to improve the biocompatibility in vivo, decellularized HAM (dHAM) was applied as a supporting framework upon which to seed other cells, which provided better cell adhesion, cell differentiation, and cell proliferation [10-12].
Oral mucosal epithelial cells (OMECs) are considered to be important seeding cells in tissue engineering and regenerative medicine. OMECs seeded on dHAM have been used to reconstruct corneal epithelium [13,14] and oral mucosa [15]. However, to our knowledge, utilization of seeded OMECs on dHAM for IUA has not been reported.
Based on the above rationale, we established IUA by mechanical endometrial scraping in a rat model, to assess the role of OMECs seeding on dHAM in the prevention of IUA and regeneration of the endometrium.
Materials and methods
Animals
Eight-week-old female Sprague-Dawley rats (Beijing Vital River Laboratory Animal Technology, China) were used in the experiments. Experimental procedures were performed according to the Institutional Animal Care and Use of Laboratory Animals guidelines of Peking University First Hospital. The procedures were approved by the Laboratory Animal Ethics Committee of Peking University First Hospital (approved number: f201242). Every effort was made to minimize animal suffering.
Preparation of dHAM
With the prior consent of the donors, fresh HAM was obtained at the time of elective cesarean section, from pregnant women who were seronegative for human hepatitis B and C, human immunodeficiency virus and syphilis. Fresh HAM was separated from the chorion, and all blood components were rinsed away using sterile 0.9% sodium chloride. Fresh HAM was then deprived of the amniotic epithelial and stromal cells by incubation with 0.02% ethylene diamine tetra-acetic acid (EDTA) solution for 2 hours at 37°C with continuous stirring. The dHAM was then lyophilized, cut into pieces, vacuum packed, and sterilized using gamma radiation (Co-60) in preparation for the experiments (Figure 1A-D).
Figure 1.
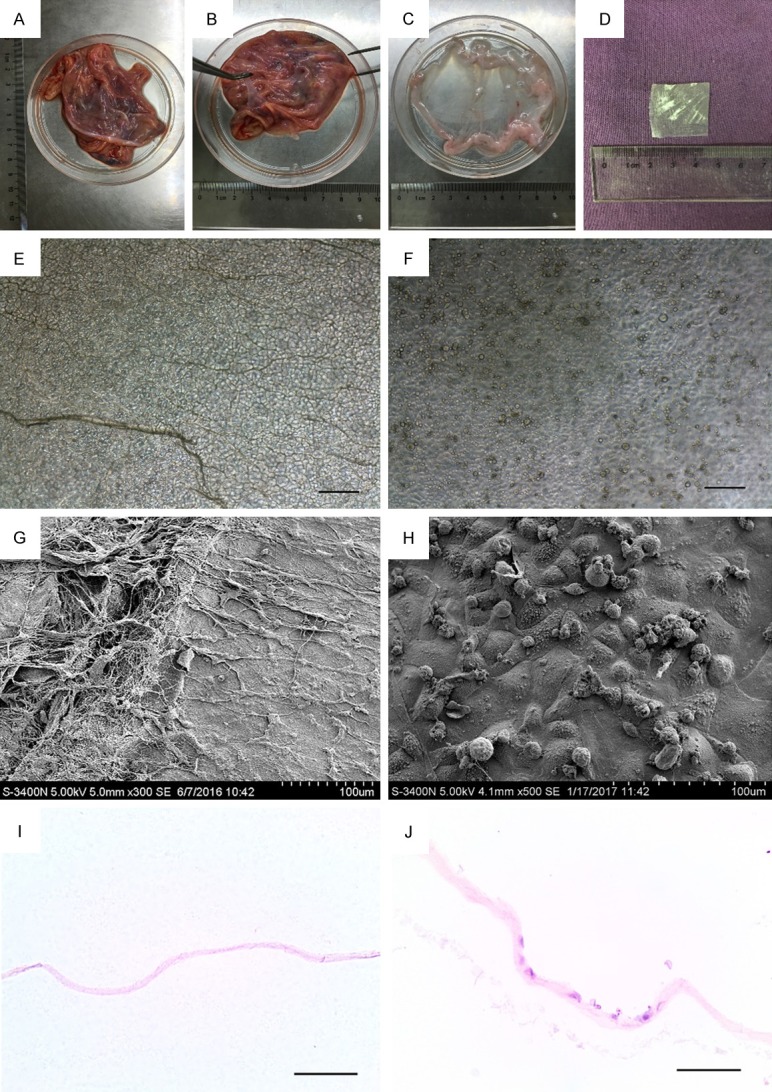
Human amniotic membrane (HAM) decellularization and oral mucosal epithelial cells (OMECs) seeded on decellularized HAM. (A-C) Separation of HAM from fetal membranes. (D) Decellularized and lyophilized amniotic membrane. (E, F) Decellularized HAM (E) and decellularized HAM seeded OMECs (F) under light microscopy. (G, H) Decellularized HAM (G) and decellularized HAM seeded OMECs (H) under scanning electron microscopy. (I, J) Hematoxylin and eosin (H&E) staining of decellularized HAM (I) and decellularized HAM seeded OMECs (J). Magnification: ×100. Bar = 100 μm.
OMECs culture and seeding
Oral mucosal tissues were obtained under anesthesia from the 8-week-old female Sprague-Dawley rats. The oral mucosal tissues were immersed in sterile phosphate-buffered saline (PBS), containing 5% antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin), for 30 minutes at room temperature. The oral mucosal tissues were then incubated with dispase (1 mg/ml, Gibco, USA) for 16-18 hours at 4°C, and treated them with 0.05% trypsin-EDTA at 37°C for 3 minutes to separate the OMECs. All enzymatic activity was eliminated by adding 10% fetal calf serum. We centrifuged the OMECs at 1000 revolutions per minute for 5 min, re-suspended the OMECs, then seeded them onto dHAM with oral keratinocyte medium (ScienCell, USA). The tissues were kept at 37°C in a 5% CO2: 95% O2 air incubator, with the medium changed every two days.
IUA rat model
Exfoliated vaginal cells were observed on vaginal smears prior to surgery and tissue collection in order to determine the estrous cycle. All rats were operated and sacrificed in the anestrus period. Thirty-six rats were randomly allocated to groups as follows: the IUA group (n=12), dHAM transplantation group (n=12), and the dHAM+OMECs transplantation group (n=12). For all groups, the left uterus of each rat was kept as control group. The right uterus of each rat was cut and the endometrium was scraped by surgical scalpel blade. In the IUA group, the previously scraped uteri were sutured carefully after hemostasis was achieved. In the dHAM transplantation and dHAM+OMECs transplantation groups, the dHAM and dHAM seeding OMECs were transplanted and sutured onto the previously scraped uteri, and then the uterine incisions were closed. After the postoperative days 3, 7, 14, and 28 (n=3 in each), tissues were collected for histological evaluation and immunohistochemical staining.
Histological evaluation
The uteri were fixed in 4% paraformaldehyde, embedded in paraffin wax and cut into 5 μm slices. Sirius red staining was performed to evaluate the degree of fibrosis. The fibrotic area rate was defined as the ratio of endometrial fibrosis area to the total endometrial area in each high-power field (HPF) (×100). These values were calculated using the Image Pro Plus (IPP) v6.0 and used to assess fibrosis. Four HPFs in each section, and two sections fromeach rat were selected for the calculation of the fibrotic area rate, the endometrial gland number (Number/HPF), the endometrial thickness (the average vertical thickness of stromal layer), and the proportion of areas of collagen type I and III. Slices were then observed under polarized light microscopy to differentiate collagen types I and III according to their color. Collagen type I ranged from orange to red, while collagen type III ranged from green to yellow. These different colored collagen fibers were quantified by a morphometrical analysis using IPP. The proportion of the area of each color in each section was calculated.
Immunohistochemical staining
After dewaxing and rehydration, the slices were incubated in 0.3% Triton X-100 at 37°C for 30 minutes to initiate perforation. A citrate buffer (pH 6.0) at 95~100°C for 15 min was used to retrieve the antigens. The slices were immersed in 3% hydrogen peroxide solution for 15 min to block endogenous peroxidase before being incubated overnight at 4°C with primary antibodies including anti-cytokeratin 18 (CK-18) (bsm-33103M, Bioss) and anti-high molecular weight cytokeratin (anti-HMW-CK) (bs-1494R, Bioss). Sections were then incubated with secondary antibody at room temperature for 2 hours and with 3-3’-diaminobenzidine (DAB) solution for visualization.
Scanning electron microscopy
The specimens were fixed in 1% glutaraldehyde for 24 h at 4°C. They were then treated with 1% osmium tetroxide for 2 h, then dehydrated, dried, and coated with gold before visualization. All specimens were observed under a scanning electron microscope (S-3400N, Hitachi, Japan).
Statistical analysis
The statistical analyses were performed using the Statistical Package of Social Science 22.0 (SPSS 22.0). The data were expressed as mean ± standard deviation (SD). We also used the Student’s t-test and one-way ANOVA in our analyses. P<0.05 was considered to be statistically significant.
Results
HAM decellularization and OMECs seeding
The dHAM seeding OMECs were observed by light microscopy (Figure 1E, 1F), scanning electron microscopy (Figure 1G, 1H), and hematoxylin and eosin (H&E) staining (Figure 1I, 1J). These methods confirmed the absence of epithelial cells in the dHAM, suggestive of a successful decellularization procedure. After transplantation of the OMECs, the cells were found on the surface of the dHAM.
Evaluation of endometrial fibrosis
For Sirius red staining, the sections were first imaged using light microscopy, under which all collagen types would stain red, while non-collagen types would stain purple. Sirius red staining of the control group demonstrated that the single columnar endometrial epithelial cells and endometrial glands both stained purple. At 3, 7, 14, and 28 days after the rat operations, the uteri had no evidence of epithelial cells and a minimal number of endometrial glands, but there was evidence of many fibers that stained red. The fibrotic area rate was defined as the ratio of fibrotic area to the endometrial area (including the stromal layer). In the IUA group, the fibrotic area rate was significantly higher than in the control group (P<0.05). In the dHAM transplantation group, the firotic area rate was lower when compared to the IUA group at all time-points except for 3 days after transplantation (P<0.05), but it was still higher than in the control group (P<0.05). In the dHAM+OMECs transplantation group, the fibrotic area rate was significantly decreased compared to the IUA group after 7, 14, and 28 days, as well as compared to the dHAM transplantation group at 14 and 28 days after transplantation (P<0.05). Furthermore, no significant differences infibrotic area rate were observed between the control group and the dHAM+OMECs transplantation group at 28 days after surgery (P>0.05) (Figure 2).
Figure 2.
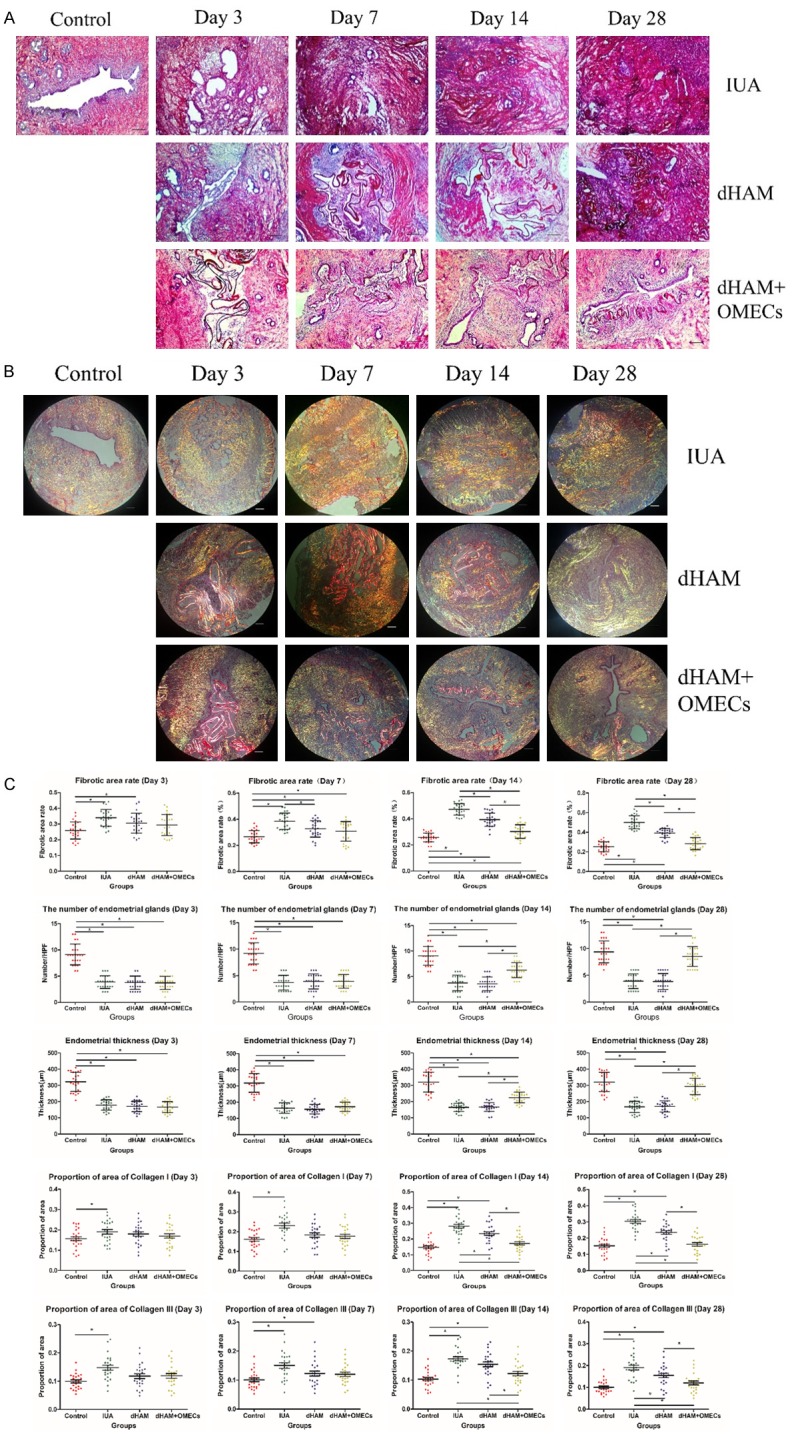
Histological evaluation of dHAM+OMECs after transplantation on IUA. A: Sirius red staining of the control, IUA, dHAM transplantation, and dHAM+OMECs transplantation groups at 3, 7, 14, and 28 days after surgery. B: Images of all groups under polarized light microscopy in sections stained with Sirius red. Collagen type I was visualized in the orange to red range, while collagen type III was visualized in the green to yellow range. C: Scatter plot of the fibrotic area rate, the number of endometrial glands, the endometrial thicknesses, and the proportion of areas of collagen type I and III in all groups. After scraping for 3, 7, 14, and 28 days, the percentage of the area containing collagenous fibers and the proportion of areas of collagen type I and III in the endometrial layer increased (P<0.05) with the development of fibrosis. In the dHAM transplantation group, the fibrotic area rate and the proportion of areas of collagen type I and III were lower compared to the IUA group (P<0.05), but still higher than that of the control group (P<0.05) at 7, 14, and 28 days after transplantation. In the dHAM+OMECs transplantation group, the fibrotic area rate and the proportion of areas of collagen type I and III were significantly decreased compared to the IUA group after 7, 14, and 28 days, and in the dHAM transplantation group at 14 and 28 days after transplantation (P<0.05). Furthermore, there were no significant differences in the fibrotic area rate and the proportion of areas of collagen type I and III between the control group and the dHAM+OMECs transplantation group at 28 days after surgery (P>0.05).
Next, we visualized the specimens using polarized light microscopy. Collagen typeI fiber colors ranged from orange to red, while collagen type III fiber colors ranged from green to yellow. The proportion of the area of collagen type Ito collagen type III in the control group was low, but the value was higher in the IUA group (P<0.05). The proportions of the areas of collagen type I to collagen type III were lower in the uteri of the dHAM transplantation group compared with the IUA group, but still higher than in the control group. In the dHAM+OMECs transplantation group, the proportion of area of collagen type I and collagen type III was lower than in the IUA and dHAM transplantation groups (P<0.05), but no significant difference was observed at 28 days after surgery compared to the control group (P>0.05) (Figure 2).
Reconstruction of endometrial epithelium
The number of endometrial glands (Number/HPF) in the IUA and dHAM transplantation groups was significantly lower than in the control group (P<0.05) and no statistically significant difference was found between these groups (P>0.05). However, 14 and 28 days after dHAM+OMECs transplantation, the number of endometrial glands and endometrial thickness were remarkably increased compared to both the IUA and the dHAM transplantation groups (P<0.05), but still remained lower and thinner compared to the control group (P<0.05) at 14 after transplantation. Twenty-eight days after scraping, no significant difference was observed between the control and the dHAM+OMECs transplantation groups, with respect to the number of endometrial glands and endometrial thickness (P>0.05) (Figure 2).
Immunohistochemical staining of CK-18 in the control group showed positive reactivity in both the endometrial epithelium and the endometrial glands. In the IUA and the dHAM transplantation groups, the uteri were deprived of endometrium and most endometrial glands. While the remaining endometrial glands were positive for CK-18. However, 14 and 28 days after surgery in the dHAM+OMECs transplantation group, the uterine cavity had re-formed, and the regenerated endometrial epithelium was observed. Further immunohistochemical staining of CK-18 of these sections confirmed that the newly-generated epithelial cells were positive for CK-18. Immunohistochemical staining using HMW-CK also led to a positive reaction to OMECs, but not in the endometrium or the endometrial glands. HMW-CK-positive OMECs were also observed in all uteri in the dHAM+OMECs transplantation group (Figure 3).
Figure 3.
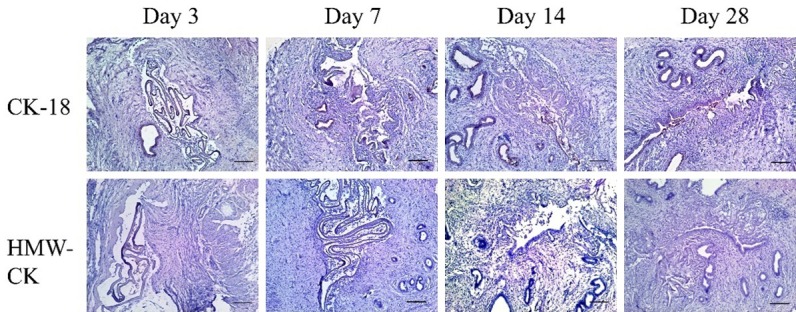
Immunohistochemical staining of CK-18 and HMW-CK in the dHAM+OMECs group. Endometrial epithelium and endometrial glands were positive for CK-18. However, 14 and 28 days after surgery in the dHAM+OMECs transplantation group, the regenerated epithelium was also observed and was positive for CK-18. Immunohistochemical staining of HMW-CK also revealed a positive expression of OMECs, but not endometrium and endometrial glands in the dHAM+OMECs transplantation group.
Discussion
Trauma to the basal layer of the endometrium has been considered to be the most common reason for IUA. The inability to regenerate endometrium is associated with the loss of the endometrial basalis. Although clinical treatment for IUA can restore the structure of the uterine cavity, it remains difficult to repair injured endometrium and restore endometrial physiological function, especially in patients with moderate to severe IUA. Thus, it was essential to establish an animal model of IUA to further discover the mechanisms leading to the development of IUA, and also to assess for novel treatment options. In the present study, we successfully induced a physiologically and clinically relevant IUA rat model by mechanical scraping. Following this procedure, we confirmed the presence of IUA based on findings of an absence of endometrium, loss of most of the endometrial glands, the presence of a thinner endometrium, and increased levels of endometrial fibrosis. The level of endometrial fibrosis was demonstrated by findings of a higher fibrotic area rate, and greater expression of collagen type I and collagen type III (two predominant types of collagen in the development of fibrosis) [16,17].
As a traditional natural biomaterial, HAM has been proven to have anti-fibrotic properties and has been successfully applied in the prevention of IUA reformation, leading to improvements in menstruation [18,19]. However, sufficient evidence of histological findings with respect to IUA prevention and endometrium regeneration were still lacking in HAM. Decellularized HAM is used to seed other cells for tissue repair because of its high biocompatibility and low immunogenicity. Whether decellularized HAM is effective in the prevention of IUA and endometrium regeneration has been unknown [10-12]. In our study, we found that the fibrotic area rate in the IUA group was significantly higher than the control group. After dHAM transplantation, the degree of fibrosis improved compared with the IUA group, but was still higher than the control group. There was no statistically significant difference in the number of endometrial glands and the endometrial thickness measurements between the IUA and the dHAM transplantation groups. Furthermore, we found no evidence of endometrium regeneration, suggesting that the preventive effects of dHAM in IUA were limited. These results provide evidence as to the level of effectiveness of dHAM in the prevention of IUA. However, other methods might also be introduced to regenerate endometrium.
In the field of tissue engineering and regenerative medicine, cell seedingis of great importance. Cell transplantation has been widely used in tissue repair. For IUA, bone marrow-derived mesenchymal stem cells have been proven to achieve some preventive effects and improve endometrium regeneration [20,21]. OMECs have characteristics of sufficient availability, minimal invasiveness, and high proliferation, and have been widely used in tissue repair methods such as the reconstruction of corneal [22,23], bladder [24] urethral [25], and esophageal ulcerations [26], and the prevention of esophageal strictures [27]. Preventive effects of OMECs on IUA have also been reported [28], however, long-term preventive effect of OMECs on IUA, and whether OMECs could improve regeneration of the endometrium, remained unknown. In our study, we transplanted OMECs seeded on the dHAM, which had anti-fibrotic potential, to observe the long-term preventive effect of OMECs on IUA and endometrial repair.
Immunohistochemical staining using CK-18, and histological staining, suggested that the regenerated epithelium was endometrium. However, it remained unknown as to where the regenerated epithelium came from. Regeneration of endometrium relies on epithelial progenitor cells and mesenchymal stem/stromal cells [29]. It has been confirmed by the markers of epithelial stem cells such as p63 [7,30] and integrin β1 [31], that based on the results of colony-forming assays and immunohistochemistry, cultured OMECs contain epithelial stem cells/progenitor cells. The question remains as to whether cultured OMECs can differentiate into endometrial epithelial cells or improve regeneration of remaining epithelial progenitor cells and the stromal cells in endometrium. In the present study, the regenerated epithelium did not express HMW-CK, which was positive in the OMECs, suggesting that the regenerated epithelium did not have the same characteristics as the OMECs. However, the above method could not prove the origin of regenerated epithelium. It is still controversial as to whether the OMECs can differentiate into other cells. Studies on the differentiation of OMECs have mainly focused on ocular reconstruction. Kolli et al. [32] reported that cultured OMECs looked morphologically like corneal epithelium, while their gene expression remained related to oral but not corneal tissue. Hayashida et al. [33] and Bardag-Gorce et al. [34] reported that the keratin expression of OMECs changed, but transplanted OMECs might not have fully differentiated into corneal epithelial cells. However, it is worth noting that endometrial epithelium is a simple columnar type of epithelium, while OMECs and corneal epithelial cellsare of the stratified squamous epithelial type. Previous research of Kuramoto et al. [28] reported the finding that cultured OMECs sustained the characteristic of OMECs in keratin expression 8 days after transplantation, but no regenerative epithelium was observed. The mechanism of action of OMECs in the regeneration of endometrium will need to be explored further.
Although the present study indicates that OMECs have some effects on preventing IUA formation and improving the regeneration of the endometrium in a rat model, there are some additional and unique problems applicable to humans. It was difficult for both the dHAM and OMECs to tightly adhere to the uterine wall. Decellularized HAM also could not be easily degraded in 28 days, and OMECs might be shed with menstruation. It remains unknown whether regenerative endometrium is functional endometrium, and whether pregnancy outcomes can be improved. More effort will be needed to further the research on OMECs in IUA.
In conclusion, we confirmed that OMECs carried on dHAM are effective in preventing IUA formation and promoting endometrial regeneration in a rat model. Transplantation of OMECs seeded on dHAM represents a new therapy for the prevention of IUA formation after hysteroscopic operations and artificial abortions,as well as the prevention of re-adhesion formation after synechiotomy to treat IUA. However, more comprehensive research related to transplantation of OMECs should be performed prior to any clinical application in the treatment and prevention of IUA.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81270666).
Disclosure of conflict of interest
None.
References
- 1.Yu D, Wong YM, Cheong Y, Xia E, Li TC. Asherman syndrome-one century later. Fertil Steril. 2008;89:759–779. doi: 10.1016/j.fertnstert.2008.02.096. [DOI] [PubMed] [Google Scholar]
- 2.March CM. Management of Asherman’s syndrome. Reprod Biomed Online. 2011;23:63–76. doi: 10.1016/j.rbmo.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Yu D, Li TC, Xia E, Huang X, Liu Y, Peng X. Factors affecting reproductive outcome of hysteroscopic adhesiolysis for Asherman’s syndrome. Fertil Steril. 2008;89:715–722. doi: 10.1016/j.fertnstert.2007.03.070. [DOI] [PubMed] [Google Scholar]
- 4.Roy KK, Baruah J, Sharma JB, Kumar S, Kachawa G, Singh N. Reproductive outcome following hysteroscopic adhesiolysis in patients with infertility due to Asherman’s syndrome. Arch Gynecol Obstet. 2010;281:355–361. doi: 10.1007/s00404-009-1117-x. [DOI] [PubMed] [Google Scholar]
- 5.Lin X, Wei M, Li TC, Huang Q, Huang D, Zhou F, Zhang S. A comparison of intrauterine balloon, intrauterine contraceptive device and hyaluronic acid gel in the prevention of adhesion reformation following hysteroscopic surgery for Asherman syndrome: a cohort study. Eur J Obstet Gynecol Reprod Biol. 2013;170:512–516. doi: 10.1016/j.ejogrb.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Xiao S, Wan Y, Xue M, Zeng X, Xiao F, Xu D, Yang X, Zhang P, Sheng W, Xu J, Zhou S. Etiology, treatment, and reproductive prognosis of women with moderate-to-severe intrauterine adhesions. Int J Gynaecol Obstet. 2014;125:121–124. doi: 10.1016/j.ijgo.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Fairbairn NG, Randolph MA, Redmond RW. The clinical applications of human amnion in plastic surgery. J Plast Reconstr Aesthet Surg. 2014;67:662–675. doi: 10.1016/j.bjps.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 8.Fraser JF, Cuttle L, Kempf M, Phillips GE, Hayes MT, Kimble RM. A randomised controlled trial of amniotic membrane in the treatment of a standardised burn injury in the merino lamb. Burns. 2009;35:998–1003. doi: 10.1016/j.burns.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes M, Sridhar MS, Sangwan VS, Rao GN. Amniotic membrane transplantation for ocular surface reconstruction. Cornea. 2005;24:643–653. doi: 10.1097/01.ico.0000151501.80952.c5. [DOI] [PubMed] [Google Scholar]
- 10.Shi P, Gao M, Shen Q, Hou L, Zhu Y, Wang J. Biocompatible surgical meshes based on decellularized human amniotic membrane. Mater Sci Eng C Mater Biol Appl. 2015;54:112–119. doi: 10.1016/j.msec.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Koizumi N, Rigby H, Fullwood NJ, Kawasaki S, Tanioka H, Koizumi K, Kociok N, Joussen AM, Kinoshita S. Comparison of intact and denuded amniotic membrane as a substrate for cellsuspension culture of human limbal epithelial cells. Graefes Arch Clin Exp Ophthalmol. 2007;245:123–134. doi: 10.1007/s00417-005-0095-3. [DOI] [PubMed] [Google Scholar]
- 12.Koizumi N, Fullwood NJ, Bairaktaris G, Inatomi T, Kinoshita S, Quantock AJ. Cultivation of corneal epithelial cells on intact and denuded human amniotic membrane. Invest Ophthalmol Vis Sci. 2000;41:2506–2513. [PubMed] [Google Scholar]
- 13.Nakamura T, Inatomi T, Sotozono C, Amemiya T, Kanamura N, Kinoshita S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br J Ophthalmol. 2004;88:1280–1284. doi: 10.1136/bjo.2003.038497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi F, Yoshida T, Koike T, Aizawa H, Shimane T, Li Y, Yamada S, Okabe M, Nikaido T, Kurita H. Construction and characterization of human oral mucosa equivalent using hyper-dry amniotic membrane as a matrix. Arch Oral Biol. 2016;65:26–34. doi: 10.1016/j.archoralbio.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Amemiya T, Nakamura T, Yamamoto T, Kinoshita S, Kanamura N. Autologous transplantation of oral mucosal epithelial cell sheets cultured on an amniotic membrane substrate for intraoral mucosal defects. PLoS One. 2015;10:e0125391. doi: 10.1371/journal.pone.0125391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanta J. Collagen matrix as a tool in studying fibroblastic cell behavior. Cell AdhMigr. 2015;9:308–316. doi: 10.1080/19336918.2015.1005469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown SR, Cleveland EM, Deeken CR, Huitron SS, Aluka KJ, Davis KG. Type I/type III collagen ratio associated with diverticulitis of the colon in young patients. J Surg Res. 2017;207:229–234. doi: 10.1016/j.jss.2016.08.044. [DOI] [PubMed] [Google Scholar]
- 18.Amer MI, Abd-El-Maeboud KH, Abdelfatah I, Salama FA, Abdallah AS. Human amnion as a temporary biologic barrier after hysteroscopic lysis of severe intrauterine adhesions: pilot study. J Minim Invasive Gynecol. 2010;17:605–611. doi: 10.1016/j.jmig.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Lu G, Hua D, Sun FQ, Xu Q, Tang YQ, Wang S. Efficacy of freeze-dried amnion graft following hysteroscopicadhesiolysis of severe intrauterine adhesions. Int J Gynaecol Obstet. 2017;137:116–122. doi: 10.1002/ijgo.12112. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Ju B, Pan C, Gu Y, Zhang Y, Sun L, Zhang B, Zhang Y. Application of bone marrowderived mesenchymal stem cells in the treatment of intrauterine adhesions in rats. Cellular Physio Biochem. 2016;39:1553–1560. doi: 10.1159/000447857. [DOI] [PubMed] [Google Scholar]
- 21.Alawadhi F, Du H, Cakmak H, Taylor HS. Bone marrow-derived stem cell (BMDSC) transplantation improves fertility in a murine model of Asherman’s syndrome. PLoS One. 2014;9:e96662. doi: 10.1371/journal.pone.0096662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, Nagai S, Kikuchi A, Maeda N, Watanabe H, Okano T, Tano Y. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 23.Prabhasawat P, Ekpo P, Uiprasertkul M, Chotikavanich S, Tesavibul N, Pornpanich K, Luemsamran P. Long-term result of autologous cultivated oral mucosal epithelial transplantation for severe ocular surface disease. Cell Tissue Bank. 2016;17:491–503. doi: 10.1007/s10561-016-9575-4. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe E, Yamato M, Shiroyanagi Y, Tanabe K, Okano T. Bladder augmentation using tissue-engineered autologous oral mucosal epithelial cell sheets grafted on demucosalized gastric flaps. Transplantation. 2011;91:700–706. doi: 10.1097/TP.0b013e31820e0170. [DOI] [PubMed] [Google Scholar]
- 25.Li C, Xu YM, Liu ZS, Li HB. Urethral reconstruction with tissue engineering and RNA interference techniques in rabbits. Urology. 2013;81:1075–1080. doi: 10.1016/j.urology.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 26.Ohki T, Yamato M, Murakami D, Takagi R, Yang J, Namiki H, Okano T, Takasaki K. Treatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut. 2006;55:1704–1710. doi: 10.1136/gut.2005.088518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohki T, Yamato M, Ota M, Takagi R, Murakami D, Kondo M, Sasaki R, Namiki H, Okano T, Yamamoto M. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology. 2012;143:582–588. doi: 10.1053/j.gastro.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 28.Kuramoto G, Takagi S, Ishitani K, Shimizu T, Okano T, Matsui H. Preventive effect of oral mucosal epithelial cell sheets on intrauterine adhesions. Hum Reprod. 2015;30:406–416. doi: 10.1093/humrep/deu326. [DOI] [PubMed] [Google Scholar]
- 29.Gargett CE, Schwab KE, Deane JA. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update. 2016;22:137–163. doi: 10.1093/humupd/dmv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugiyama H, Yamato M, Nishida K, Okano T. Evidence of the survival of ectopically transplanted oral mucosal epithelial stem cells after repeated wounding of cornea. Mol Ther. 2014;22:1544–1555. doi: 10.1038/mt.2014.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soma T, Hayashi R, Sugiyama H, Tsujikawa M, Kanayama S, Oie Y, Nishida K. Maintenance and distribution of epithelial stem/progenitor cells after corneal reconstruction using oral mucosal epithelial cell sheets. PLoS One. 2014;9:e110987. doi: 10.1371/journal.pone.0110987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolli S, Ahmad S, Mudhar HS, Meeny A, Lako M, Figueiredo FC. Successful application of ex vivo expanded human autologous oral mucosal epithelium for the treatment of total bilateral limbal stem cell deficiency. Stem Cells. 2014;32:2135–2146. doi: 10.1002/stem.1694. [DOI] [PubMed] [Google Scholar]
- 33.Hayashida Y, Nishida K, Yamato M, Watanabe K, Maeda N, Watanabe H, Kikuchi A, Okano T, Tano Y. Ocular surface reconstruction using autologous rabbit oral mucosal epithelial sheets fabricated ex vivo on a temperature-responsive culture surface. Invest Ophthalmol Vis Sci. 2005;46:1632–1639. doi: 10.1167/iovs.04-0813. [DOI] [PubMed] [Google Scholar]
- 34.Bardag-Gorce F, Oliva J, Wood A, Hoft R, Pan D, Thropay J, Makalinao A, French SW, Niihara Y. Carrier-free cultured autologous oral mucosa epithelial cell sheet (CAOMECS) for corneal epithelium reconstruction: a histological study. Ocul Surf. 2015;13:150–163. doi: 10.1016/j.jtos.2014.12.003. [DOI] [PubMed] [Google Scholar]


