Abstract
Objective: To investigate the expression of PD-L1 and STING in colorectal cancer and analyze their correlation with disease prognosis. Methods: The colorectal tissue chip was taken as the research object. Immunohistochemical methods were used to detect expression of PD-L1 and STING of 87 cases of colorectal cancer tissue and corresponding adjacent tissue in the tissue chip. Chi-square test or Fisher’s test were used to analyze the relationship of expression of PD-L1 and STING and with the clinical pathological features. Kaplan-Meier curves were used to analyze the relationship between expression of PD-L1 and STING and the prognosis of colorectal cancer. Results: The positive expression rate of PD-L1 in 87 cases of colorectal cancer tissue and corresponding adjacent tissue was 79.3% and 42.5% respectively, and the difference was significant (P < 0.05). The positive expression rate of PD-L1 in colorectal carcinoma tissues was not related with age, gender, tumor size, differentiation, TNM clinical stage, or depth of invasion (P > 0.05). The positive expression rate of STING in 87 cases colorectal cancer tissues and corresponding adjacent tissue was 9.2% and 40.2% respectively and the difference was significant (P < 0.05). The positive expression rate of STING in colorectal carcinoma tissue was correlated with gender, age, and differentiation (P < 0.05), but not associated with tumor size, depth of invasion, and TNM clinical stage (P > 0.05). The expression rate of PD-L1 was related to the prognosis of colorectal cancer (P=0.018). The expression rate of STING was not correlated with prognosis of colorectal cancer (P=0.784). In four independent groups (PD-L1+STING+ group, PD-L1-STING- group, PD-L1-STING+ group, PD-L1+STING- group), there were significant differences in 5-year survival (P=0.047 < 0.05), the 5-year survival rate of the PD-L1-STING+ group was significantly higher than that of the other three groups. In the STING positive group, the PD-L1+STING+ group had a worse prognosis than the PD-L1-STING+ group. In the STING negative group, the PD-L1-STING-group had a higher survival rate than the PD-L1+STING- group. Conclusion: PD-L1 might promote the occurrence of disease and STING might play an important role in anti-tumor immunity. PD-L1 was related with the prognosis of patients with colorectal cancer but the expression of STING was not obviously associated with the prognosis. Survival rates of patients with colorectal cancer were higher in patients with PD-L1 negative expression or STING positive expression. When PD-L1 expression was negative and STING expression was positive, the 5-year survival rate of patients with colorectal cancer was highest.
Keywords: Colorectal cancer, PD-L1, STING, immunohistochemical method, prognosis
Introduction
Colorectal cancer is one of the most common malignant tumors in the world. The incidence of colorectal cancer is third and second place in male and female tumors, respectively. The estimated mortality rate is fourth and third in men’s and women’s tumors, respectively [1]. Cancer immunotherapy is envisioned as a new approach to achieve the purpose of controlling and killing tumor cells by stimulating and enhancing the immune function of the body. Immunotherapy acts as a complementary therapy combined with conventional therapies such as surgery, chemotherapy, and radiotherapy, and has become a promising strategy to treat various types of cancer. The antibody targeting PD-1/PD-L1 has shown very promising efficacy in the clinic in recent years. Studies have shown that PD-1/PD-L1 can negatively regulate the immune response by inhibiting activation and proliferation of T cells, and it also can participate in regulating immune tolerance, microbial infection, and tumor cell immune escape [2].
STING (stimulator of interferon genes), which mediates cytosolic DNA-induced signaling events, has recently been implicated as a mediator of processes in cancer immunotherapy. Exogenous or endogenous DNA can lead to STING dimerization, activation of Tank binding kinase 1 (TBK1), activation of interferon regulatory factor 3 (interferon regulatory 3 factor, IRF3) and nuclear factor kappa B (nuclear factor kappa-B kinase, NF-κB), and eventually generation of interferon [3,4]. It is known that interferon is widely used in cancer treatment because it has the effect of regulating immune function and therefore inhibiting tumor cell proliferation. Recent studies have found that IFN can induce expression of PD-L1 in ovarian cancer, hepatoma, gastric cancer, and so on [5-7]. Moreover, it has been found that downregulation of STING could decrease cisplatin induced upregulation of PD-L1 in breast cancer [8], and the STING signaling pathway is essential for the anti-tumor efficacy of PD-L1 antibody blockage [9]. Furthermore, the combination of a STING agonist and PD-1 pathway blockage could reduce the tumor growth in tolerized mice [10], therefore, STING and PD-L1 have important interactions that control the cancer prognosis. However, few studies have provided information regarding a correlation of PD-L1 and STING expression with clinicopathological factors and disease prognosis in colorectal cancer. This study aims to analyze the expression of PD-L1 and STING in colorectal cancer, and compare the clinical pathological features to investigate their expression in colorectal carcinoma and its relation with prognosis in colorectal cancer, which might provide some insights for the immunotherapy of colorectal cancer in the near future.
Materials and methods
Materials and agents
The PD-L1 monoclonal antibody purchased from the Cell Signaling Technology Company (USA), the STING monoclonal antibody purchased from the Proteintech Company (China). The Goat anti-rabbit antibody and Diaminobenzidine (DAB) reagent purchased from the Shanghai Genetech Biotech Co., Ltd (China). Other reagents used were obtained from the Shanghai Beyotime Biotech Co., Ltd (China).
The patients’ tissue chip of colorectal cancer was purchased from Shanghai Outdo Biotech Co., Ltd (Product number: HCol-Ade180Sur-07), all patients did not receive any treatment before operation, and had complete clinical data. The chip included 87 colorectal cancer tissues and its paired adjacent tissue. The detailed clinical information of colorectal cancer patients were shown in Table 2.
Table 2.
The relationship between PD-L1 expression and clinicopathological features in colorectal cancer
| Parameters | Cases | PD-L1 expression | Positive rate (%) | P value | |
|---|---|---|---|---|---|
|
| |||||
| Positive | Negative | ||||
| Gender | 0.798 | ||||
| Male | 46 | 36 | 10 | 78.3 | |
| Female | 41 | 33 | 8 | 80.5 | |
| Age | 0.863 | ||||
| ≤ 60 | 28 | 23 | 5 | 82.1 | |
| > 60 | 59 | 46 | 13 | 77.9 | |
| Tumor size | 0.209 | ||||
| ≤ 5 | 50 | 42 | 8 | 84 | |
| > 5 | 37 | 27 | 10 | 72.9 | |
| Differentiation | 0.029 | ||||
| High | 21 | 13 | 8 | 61.9 | |
| Medium | 52 | 35 | 17 | 67.3 | |
| Low | 14 | 10 | 4 | 71.4 | |
| Lymph node metastasis | 0.261 | ||||
| Yes | 29 | 21 | 8 | 72.4 | |
| No | 58 | 48 | 10 | 82.8 | |
| TNM stage | 0.993 | ||||
| I | 10 | 7 | 3 | 70 | |
| II | 49 | 39 | 10 | 79.6 | |
| III | 28 | 25 | 3 | 89.3 | |
| Depth of invasion | 0.375 | ||||
| T1-T2 | 10 | 6 | 4 | 60 | |
| T3-T4 | 77 | 60 | 17 | 77.9 | |
Immunohistochemistry
To retrieve antigen, the purchased tissue chips were routinely dewaxed, rehydrated, and immunohistochemistry was performed in accordance with the standard operation process. The anti-human antibody PD-L1 (1:500 dilution) and STING (1:500 dilution) were used as primary antibody, and HRP-labeled anti-mouse antibody used as secondary antibody to incubate with the chips and Diaminobenzidine (DAB) reagent was used to visualize the expression of PD-L1 and STING on the colorectal cancer tissue. PBS was used as a negative control. A double blind method was used for the determination of positive staining results, and 2 senior pathologists read the film independently. Expression of PD-L1 and STING was mainly located in the cytoplasm and cell membrane, and the yellow or yellow brown granules were indicated as positive expression. Five visual fields from different areas of each specimen were chosen randomly.
PD-L1 positivity was evaluated by modified H-Score [11], a combination of staining intensity and percentage of tumor cell staining. The score of PD-L1 expression was in two steps, first, the PD-L1 slices were scored according to the percentage of positive cells: < 5% was 0, 5% to 25% was 1, 26% to 50% was 2, 51% to 75% was 3, and > 75% was 4. At the same time, the staining intensity was scored as 0 (negative), 1 (weak), 2 (moderate), or 3 (strong) based on membranous localization. After that, the final score of each slice was calculated by multiplying the score of which based on the percentage of positive cells in first step and the score of staining intensity. Therefore, the modified H-scores ranged from 0 to 12. Based on the above score, the PD-L1 expression was divided into 4 groups: Negative (-): score 0~1; weak positive (+): score 2~4; medium positive (++): score 5~8; strong positive (+++): score 9~12. The ‘+~+++’ was defined as positive. For STING positivity, the positive/negative was considered as staining with/without yellow (or yellow brown) granules (Figures 1 and 2).
Figure 1.
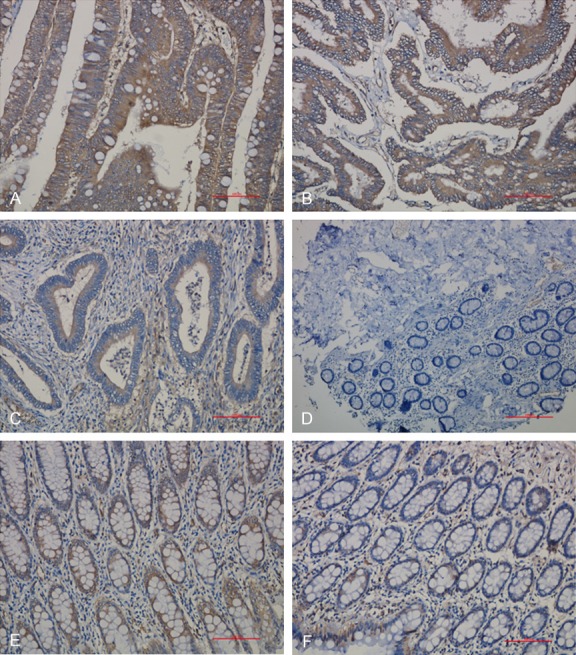
PD-L1 expression in colorectal cancer and its adjacent tissue. A: PD-L1 positive expression (+++); B: PD-L1 positive expression (++); C: PD-L1 positive expression (+); D: PD-L1 negative expression; E: PD-L1 positive expression in normal adjacent tissue; F: PD-L1 negative expression in normal adjacent tissue. (Magnification: 200×).
Figure 2.
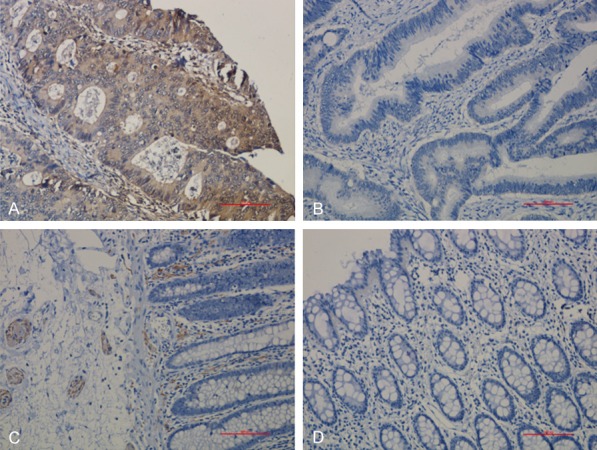
STING expression in colorectal cancer and its adjacent tissue. A: STING positive expression; B: STING negative expression; C: STING positive expression in normal adjacent tissue; D: STING negative expression in normal adjacent tissue. (Magnification: 200×).
Statistical method
SPSS 13 statistical software was used for data analysis. Chi square test or Fisher’s exact test were used for comparison between groups. Kaplan-Meier method and parallel log-rank test were used to compare survival status. P < 0.05 was considered as statistically significant difference.
Results
PD-L1 expression in colorectal cancer tissue and its adjacent tissue
In 87 cases of colorectal cancer, 69 cases were positive for PD-L1 expression at a rate of 79.3%. In the corresponding adjacent tissues, PD-L1 expression was positive in 37 cases, and the positive rate was 42.5%. There was a significant difference between the colorectal cancer tissue and its adjacent tissue on PD-L1 expression (P < 0.05). The data are summarized in Table 1.
Table 1.
Expression of PD-L1 and STING in colorectal cancer and corresponding para-cancerous tissues
| Groups | n | PD-L1 expression | STING expression | ||
|---|---|---|---|---|---|
|
|
|
||||
| Positive | Negative | Positive | Negative | ||
| Colorectal cancer tissue | 87 | 69 | 18 | 8 | 79 |
| Para-cancerous tissue | 87 | 37 | 50 | 35 | 52 |
| P value | 0.000 | 0.000 | |||
STING expression in colorectal cancer tissue and its adjacent tissue
In 87 colorectal cancer tissues, 8 cases had positive expression of STING with the positive rate being 9.2%. However, there were 35 cases with positive expression in the adjacent tissues, which was significantly higher than that in the corresponding colorectal cancer tissues (P < 0.05), as shown in Table 1.
The relationship between PD-L1 expression and clinicopathological features in colorectal cancer
In 87 cases of colorectal carcinoma, the positive rate in low differentiation group was higher than that of medium and high differentiation group (71.4% vs. 67.3% vs. 61.9%). For TNM clinical stage (I, II, III), the late of clinical stage, the higher expression of PD-L1 (I: 70%, II: 79.6%, III: 89.3%). Considering the factor of tumor invasion depth, it was found that positive rate in T3-T4 group was higher than that of T1-T2 group (77.9% vs. 60%). However, it was found that there was no statistical significance between them, which indicated the no correlation of the PD-L1 protein expression with the differentiation, clinical stage, and depth of tumor invasion. Also, it was found that PD-L1 expression was unrelated with the patients’ age, gender, tumor size, and whether they had lymph node metastasis or not (P > 0.05). (Data are summarized in Table 2).
The relationship between STING expression and clinicopathological features in colorectal cancer
In 87 cases of colorectal carcinoma, the positive ratio of STING expression in high differentiation group was higher than that of medium and low differentiation group (38.1% vs. 11.5% vs. 14.3%), and it was also found that the positive rate of STING in women was higher than that of men (17.1% vs. 2.17%) with the difference being significant (P < 0.05). Also, the positive rate in age less than 60 was higher than that age larger than 60, all of which illustrated the positive expression of STING was related with the tumor differentiation status, gender, and age. For TNM clinical stage (I, II, III), the later of clinical stage, the low expression of STING (20% vs. 8.2% vs. 7.1%). Even though the positive ratio of STING in T1-T2 group was higher than that of T3-T4 group (20% vs. 7.8%), there was no significant difference between them (P > 0.05), which suggested that the positive expression of STING was unrelated with patients’ clinical TNM stage, tumor invasion depth. Moreover, the positive expression of STING was also unrelated with tumor size and whether lymph node metastasis was present or not (P > 0.05), as shown in Table 3.
Table 3.
The relationship between STING expression and clinicopathological features in colorectal cancer
| Parameters | Cases | STING expression | Positive rate (%) | P value | |
|---|---|---|---|---|---|
|
| |||||
| Positive | Negative | ||||
| Gender | 0.016 | ||||
| Male | 46 | 1 | 45 | 2.17 | |
| Female | 41 | 7 | 34 | 17.1 | |
| Age | 0.007 | ||||
| ≤ 60 | 28 | 6 | 22 | 21.4 | |
| > 60 | 59 | 2 | 57 | 3.4 | |
| Tumor size | 0.051 | ||||
| ≤ 5 | 50 | 2 | 48 | 4 | |
| > 5 | 37 | 6 | 31 | 16.2 | |
| Differentiation | 0.234 | ||||
| High | 21 | 8 | 13 | 38.1 | |
| Medium | 52 | 6 | 46 | 11.5 | |
| Low | 14 | 2 | 12 | 14.3 | |
| Lymph node metastasis | 0.6 | ||||
| Yes | 29 | 2 | 27 | 6.8 | |
| No | 58 | 6 | 52 | 10.3 | |
| TNM stage | 0.449 | ||||
| I | 10 | 2 | 8 | 20 | |
| II | 49 | 4 | 45 | 8.2 | |
| III | 28 | 2 | 26 | 7.1 | |
| Depth of invasion | 0.209 | ||||
| T1-T2 | 10 | 2 | 8 | 20 | |
| T3-T4 | 77 | 6 | 71 | 7.8 | |
Correlation between the expression of PD-L1 and STING in colorectal cancer and the prognosis of patients
The expression of PD-L1 in colorectal cancer was associated with prognosis of colorectal cancer (P=0.018), and expression of STING was not correlated with the prognosis of colorectal cancer (P=0.784), as shown in Figures 3 and 4, respectively.
Figure 3.
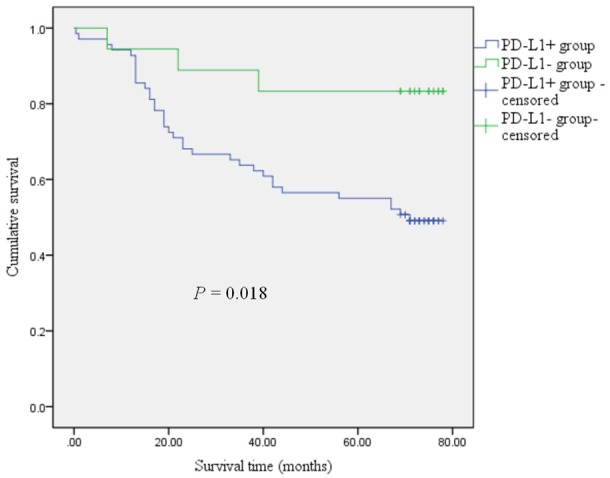
Kaplan-Meier curves comparing 5-year survival outcome of colorectal cancer patients with positive and negative PD-L1 expression. The log-rank test was used to calculate P value.
Figure 4.
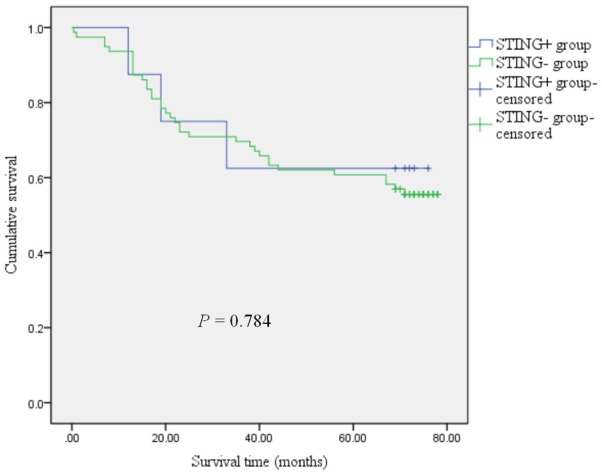
Kaplan-Meier curves comparing 5-year survival outcome of colorectal cancer patients with positive and negative for STING expression (log-rank test).
The correlation of PD-L1 and STING expression with colorectal cancer patients’ survival
To explore the relationship of the PD-L1 and STING with colorectal cancer survival, as shown in Figure 5, 87 cases of colorectal cancer samples were divided into 4 groups (PD-L1+STING+ group, PD-L1-STING- group, PD-L1-STING+ group, PD-L1+STING- group), the analyzed results showed that there were significant differences between the 4 groups (P=0.047, Figure 5), the 5 year survival rate in the PD-L1-STING+ group was significantly higher than that in the other three groups (PD-L1+STING+ group, PD-L1-STING- group, PD-L1+STING- group). In contrast, the 5 year survival rate in the PD-L1+STING+ group was worst. In the STING positive group, the PD-L1+STING+ group had a worse prognosis than that of the PD-L1-STING+ group. In the STING negative group, the 5 year survival rate in the PD-L1-STING- group was higher than that in the PD-L1+STING- group (Figure 5).
Figure 5.
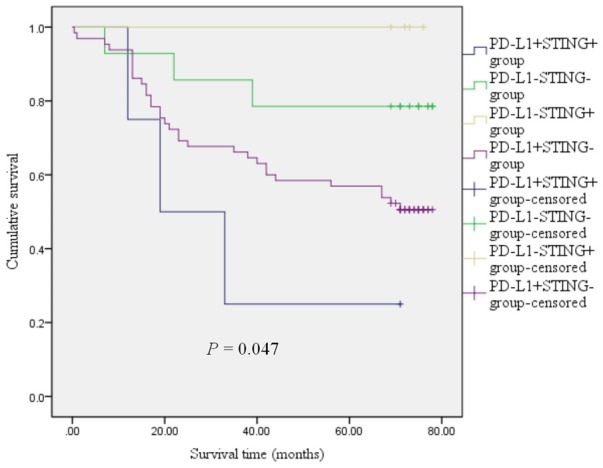
Kaplan-Meier survival curves of patients with colorectal cancer according to the combination status with PD-L1 and STING. The log-rank test was used to calculate P value.
Discussion
Immunotherapy has become one of the choices for the treatment of various cancers [12]. More and more evidence suggests that the immunological checkpoint plays an important role in the anti-tumor immune response which is mediated by T cells in the tumor microenvironment [12]. Although T cells are present in almost all tumors, most cancers are not naturally identified by the immune system. Preclinical and clinical data indicated the key function of type I interferon in connecting tumor immune rejection with the innate and adaptive immune responses [13-15].
PD-L1 is a member of the B7 family which was found by Dong et al. from a placenta cDNA Library in 1999 [16]. After binding with its receptor PD-1, can mediate inhibitory signals for activation of T cells and cytokine secretion, and thus promotes immune tolerance, microbial infection, and immune escape of tumor cells. At present, the effect of PD-1/PD-L1 inhibitor on the anti-tumor efficacy has been greatly recognized [17].
Interferon stimulated gene (STING) acts as a sensor, which can recognize the exogenous and endogenous cyclic dinucleotide (CDNs), and then activates the downstream genes TBK1/IRF3 (interferon regulatory factor 3), nuclear factor κB, and STAT6 (signal transducer and activator of transcription 6), which induce type I interferons and pro-inflammatory cytokines [18]. STING signals are critical for innate immune response. It causes the production of type I IFN in tumor microenvironment, and promotes the production of tumor antigen specific CD8 +T cells via the dendritic cells, thereby playing a role in tumor inhibition [13,18].
In the microenvironment of colorectal cancer, the antitumor activity of T cells could be inhibited by the PD-L1 expressed on the tumor cell. Usually, STING plays an active role in the antitumor immune via the promoting the generation of CD8+ T cells, however, some studies have shown that STING can induce the formation of an inhibitory tumor microenvironment, indirectly promoting tumor growth and metastasis [19,20]. Therefore, STING play a complicated role in signaling in tumor immune responses. Recently, some studies have shown a close connection of the STING signaling pathway with PD-L1 [8-10,21], and in our study we also found that downregulation of STING expression could decrease the expression level of PD-L1 in colon cancer (data not shown). In order to investigate the role of STING and PD-L1 in colorectal cancer, the IHC method was used to detect the STING and PD-L1 expression in 87 cases of colorectal cancer and its peri-tumor tissue. The results showed that PD-L1 was expressed in 69 cases and the positive rate was 79.3%, the positive rate of PD-L1 in peri-tumor tissue was 42.5%, which was significantly lower than that of tumor tissues. For STING expression, only 8 cases in 87 cases were positive and the positive rate was 9.2%, however, the positive rate in the peri-tumor tissue was 40.2%, which was significantly higher than that of colorectal cancer tissues (P < 0.05).
After that, the correlation of PD-L1 and STING expression in colorectal cancer with the clinicopathological data were analyzed. It was found that the positive expression of PD-L1 was not correlated with the patients’ age, gender, tumor size, differentiation, TNM clinical stage, depth of invasion (P > 0.05). In contrast, the STING expression was correlated with the patients’ gender, age, differentiation (P < 0.05), and was not correlated with tumor size, depth of invasion, and TNM clinical stage (P > 0.05). The STING positive expression had a linear correlation with differentiation, higher differentiation, and a higher positive expression of STING. It is known that the higher differentiation of tumor tissue usually means better prognosis, therefore, positive expression of STING might be a potential biomarker for the evaluation of patients’ prognosis in colorectal cancer.
Studies have shown that PD-1/PD-L1 (programmed cell death receptor 1/programmed cell death ligand 1) inhibitors are effective in many types of cancer [17]. Recent studies showed that mice lacking STING gene have poor responses on radiotherapy and immunotherapy, such as blocking immunosuppressant molecules, including PD-1, PD-L1, CTLA4, and CD47 [8-10,22]. Therefore, STING and PD-L1 might have a close connection in disease progression. Survival analysis found that the expression of PD-L1 was correlated with prognosis of colorectal cancer (P=0.018), and expression of STING was not correlated with the prognosis of colorectal cancer (P=0.784). The difference between the 4 independent groups (PD-L1+STING+ group, PD-L1-STING- group, PD-L1-STING+ group, PD-L1+STING- group) was statistically significant (P=0.047), the PD-L1+STING+ group had worse prognosis than the PD-L1-STING+ group in the STING positive group, and the PD-L1-STING+ group had higher survival rate than the PD-L1-STING- group in the PD-L1 negative group. This would provide a theoretical basis for the application of PD-L1 and STING related immunotherapy in colorectal cancer.
In summary, this study confirmed that expression of PD-L1 in colorectal cancer tissues was higher than that of adjacent tissues and that the expression of STING in colorectal cancer was lower than the corresponding adjacent tissues. The expression of PD-L1 in colorectal cancer and the clinical indicators were not correlated, suggesting that PD-L1 expression might be an independent prognostic factor. Expression of STING in colorectal cancer was related to gender, age and degree of differentiation. Therefore, PD-L1 may promote the occurrence of tumors, and STING may play an important role in anti-tumor immunity. Expression of PD-L1 correlated with the prognosis of colorectal cancer patients, with the PD-L1-STING+ group having the highest survival rate, thus providing a theoretical basis for PD-L1 and STING related immunotherapy in colorectal cancer. Furthermore, STING agonist combined with PD-1/PD-L1 inhibitors could achieve better therapeutic effects than that of single treatment. Of course, there are many inadequacies in this study, and large sample studies and long-term follow-up are also warranted in future studies.
Acknowledgements
This study was supported by the Key Scientific Research Projects for Higher Education of Henan Province (grant No. 17A310022; 15A320063), the Young Backbone Teachers Fellowship in Henan Province (2016GGJS-105) and the young scientist project in the First Affiliated Hospital of Xinxiang Medical University (QN-2017-A010), the Disciplinary group of Psychology and Neuroscience, Xinxiang Medical University (2016PN-KFKT-12).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Teng F, Meng X, Kong L, Yu J. Progress and challenges of predictive biomarkers of anti PD-1/PD-L1 immunotherapy: a systematic review. Cancer Lett. 2017;414:166–173. doi: 10.1016/j.canlet.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 3.He L, Xiao X, Yang X, Zhang Z, Wu L, Liu Z. STING signaling in tumorigenesis and cancer therapy: a friend or foe? Cancer Lett. 2017;402:203–212. doi: 10.1016/j.canlet.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Pepin G, Gantier MP. cGAS-STING activation in the tumor microenvironment and its role in cancer immunity. Adv Exp Med Biol. 2017;1024:175–194. doi: 10.1007/978-981-10-5987-2_8. [DOI] [PubMed] [Google Scholar]
- 5.Li N, Wang J, Zhang N, Zhuang M, Zong Z, Zou J, Li G, Wang X, Zhou H, Zhang L, Shi Y. Cros-talk between TNF-α and IFN-γ signaling in induction of B7-H1 expression in hepatocellular carcinoma cells. Cancer Immunol Immunother. 2018;67:271–283. doi: 10.1007/s00262-017-2086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abiko K, Matsumura N, Hamanishi J, Horikawa N, Murakami R, Yamaguchi K, Yoshioka Y, Baba T, Konishi I, Mandai M. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112:1501–1509. doi: 10.1038/bjc.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mimura K, The JL, Okayama H, Shiraishi K, Kua LF, Koh V, Smoot DT, Ashktorab H, Oike T, Suzuki Y, Fazreen Z, Asuncion BR, Shabbir A, Yong WP, So J, Soong R, Kono K. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. 2018;109:43–53. doi: 10.1111/cas.13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parkes EE, Walker SM, Taggart LE, McCabe N, Knight LA, Wilkinson R, McCloskey KD, Buckley NE, Savage KI, Salto-Tellez M, McQuaid S, Harte MT, Mullan PB, Harkin DP, Kennedy RD. Activation of STING-dependent innate immune signaling by S-phase-specific DNA damage in breast cancer. J Natl Cancer Inst. 2016;109 doi: 10.1093/jnci/djw199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, Hu S, Chen X, Shi H, Chen C, Sun L, Chen ZJ. cGAS is essential for the antitumor effect of immune checkpoint blockage. Proc Natl Acad Sci U S A. 2017;114:1637–1642. doi: 10.1073/pnas.1621363114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foote JB, Kok M, Leatherman JM, Armstrong TD, Marcinkowski BC, Ojalvo LS, Kanne DB, Jaffee EM, Dubensky TW Jr, Emens LA. A STING agonist given with OX40 receptor and PD-L1 modulators primes immunity and reduces tumor growth in tolerized mice. Cancer Immunol Res. 2017;5:468–479. doi: 10.1158/2326-6066.CIR-16-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tessier-Cloutier B, Kalloger SE, AI-Kandari M, Milne K, Gao D, Nelson BH, Renouf DJ, Sheffield BS, Schaeffer DF. Programmed cell death ligand 1 cut-point is associated with reduced disease specific survival in resected pancreatic ductal adenocarcinoma. BMC Cancer. 2017;17:618. doi: 10.1186/s12885-017-3634-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208:2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demaria O, De Gassart A, Coso S, Gestermann N, Di Domizio J, Flatz L, Gaide O, Michielin O, Hwu P, Petrova TV, Martinon F, Modlin RL, Speiser DE, Gilliet M. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc Natl Acad Sci U S A. 2015;112:15408–15413. doi: 10.1073/pnas.1512832112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, Murphy KM, Schreiber RD. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 17.Liu B, Song Y, Liu D. Recent development in clinical applications of PD-1 and PD-L1 antibodies for cancer immunotherapy. J hematol Oncol. 2017;10:174. doi: 10.1186/s13045-017-0541-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng KW, Marshall EA, Bell JC, Lam WL. cGAS-STING and cancer: dichotomous roles in tumor immunity and development. Trends Immunol. 2018;39:44–54. doi: 10.1016/j.it.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Lemos H, Mohamed E, Huang L, Ou R, Pacholczyk G, Arbab AS, Munn D, Mellor AL. STING promotes the growth of tumors characterized by low antigenicity via IDO activation. Cancer Res. 2016;76:2076–2081. doi: 10.1158/0008-5472.CAN-15-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaston J, Cheradame L, Yvonnet V, Deas O, Poupon MF, Judde JG, Cairo S, Goffin V. Intracellular STING inactivation sensitizes breast cancer cells to genotoxic agents. Oncotarget. 2016;7:77205–77224. doi: 10.18632/oncotarget.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E, Mechette K, Leong JJ, Lauer P, Liu W, Sivick KE, Zeng Q, Soares KC, Zheng L, Portnoy DA, Woodward JJ, Pardoll DM, Dubensky TW Jr, Kim Y. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockage. Sci Transl Med. 2015;7:283ra53. doi: 10.1126/scitranslmed.aaa4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, Huang X, Gajewski TF, Chen ZJ, Fu YX, Weichselbaum RR. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41:843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]


