Abstract
Fibronectin (FN) plays a critical role in the development and progression of keloid scarring (KS). In the present study, we hypothesized that a post-translational mechanism of microRNAs regulated the expression of FN in keloid scarring fibroblasts (KSFBs). Here, we collected 20 KS tissues and paired corresponding adjacent normal tissues from clinical patients and measured the expression of miRNA-217. First, by using PicTar, TargetScan and miRBase database, we found that miRNA-217 might be a regulator of FN in human species. Based on these hypotheses, the expression of miRNA-217 and FN in KS tissues was investigated. The results demonstrated that the expression of miRNA-217 was greatly suppressed, and FN was increased in KS tissues. Intriguingly, the expression levels of endogenous miRNA-217 negatively correlated with the FN mRNA levels (Pearson’s correlation coefficient = -0.683, P < 0.001). In vitro, miRNA-217 could regulate FN through the predicted binding sites in its 3’-UTR. miRNA-217 played an impact on cell proliferation and apoptosis, thereby regulating KSFBs growth. Moreover, miRNA-217 gain-of function decreased FN, Col-1 and Col-3 protein expression, and miRNA-217 loss-of function increased FN, Col-1 and Col-3 protein expression in KSFBs. In conclusion, overexpressed miRNA-217 could inhibit KSFBs growth, and the underlying mechanism was mediated, at least partially, through the suppression of FN expression. But above all, miRNA-217 might play a potential therapeutic avenue for the treatment of keloid fibrogenesis.
Keywords: MiRNA-217, fibronectin, keloid fibrogenesis, fibroblasts
Introduction
Keloid scarring (KS) is a benign dermal fibroproliferative disease due to abnormal wound healing process after skin injury and is characterized by overproduction of extracellular matrix (ECM) that extend beyond the area of the original skin injury in predisposed individuals [1-3]. Excess deposition of ECM such as hyalinized collagen bundles by fibroblasts is responsible for keloid [4]. Previous studies indicate that transforming growth factor-beta1 (TGF-β1) can induce collagen production and contraction in scar-derived human skin fibroblasts [5,6]. However, the underlying molecular mechanisms in keloid fibrogenesis are still poorly understood. Therefore, there is an urgent need for a better understanding of keloid pathogenesis in order to develop better prevention and treatment approaches.
Fibrogenesis is characterized by ECM remodeling and stiffening. However, the functional contribution of tissue stiffening to non-cancer pathogenesis remains largely unknown. Fibronectin (FN) is an ECM glycoprotein that exists in blood plasma in its soluble form (plasma type) and in its insoluble form (cellular type) as a part of the ECM of almost every tissue in an organism [6]. Previous studies indicate that overexpressed FN distorts the architecture of the liver and leads to hepatic cirrhosis and consequently hepatocellular cancer (HCC) [7,8]. In early phase of wound healing, FN contributes to the formation of granulation tissue, which is similar to embryonic skin [9]. In the psoriatic non-lesional skin, the expression of FN is elevated by fibroblasts, and the current findings imply that FN plays a pathophysiological roles in fibrogenesis [10]. In KS, plasma FN and then in situ FN are deposited at the site of injury. In situ FN makes by tissue cells at the injury site often contains an extra domain A (EDA) insert. Multiple wound-related signal transduction pathways control the deposition of EDA-FN, and the EDA insert can in turn trigger pathways that induce inflammation, increased extracellular matrix molecule deposition including FN and collagen, and activation of fibroblasts, which can create a vicious cycle that lead to fibrosis or keloid formation [11]. These results indicate that FN may be a key target for the development of novel therapeutic strategies for keloid fibrogenesis. However, the underlying molecular mechanisms of microRNAs by targeting FN contribute to the induction of keloid fibrogenesis remains to be determined.
In the present study, we hypothesize that a post-translational mechanism may exist for FN expression and be regulated by microRNAs in keloid tissues and keloid fibroblasts. By using PicTar, TargetScan, and miRBase database and microarray assay, we indicate that miRNA-217 is a regulator of FN in human species and decreases expression in keloid tissues and keloid fibroblasts, and its aberrant regulation has been demonstrated in a wide variety of diseases [12,13]. Furthermore, miRNA-217 will be analyzed for its potential use as therapeutic target to develop novel strategies for keloid fibrogenesis prevention and treatment.
Materials and methods
Patients’ samples
Twenty keloid scarring tissues (KS) and paired corresponding adjacent normal tissues (NC) were collected from patients who had undergone surgical excision at the Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine between June 2012 and June 2015. All collected tissue samples were immediately stored at liquid nitrogen until use. Human samples were obtained with written informed consent from all patients. The study was approved by the Ethics Committee of the Shanghai Jiao Tong University School of Medicine (Shanghai, China).
Cell culture
Human keloid scarring fibroblasts (KSFBs) and embryonic skin fibroblasts (ESF) were established as described previously [1] and cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco Life Technologies) that contained 10% fetal calf serum (Gibco Life Technologies), 10% L-glutamine, 0.5% penicillin/streptomycin, 10% nonessential amino acids and 10% pyruvate, in a 5% CO2 atmosphere and incubated at 37°C.
MTT assay
Cell proliferation was monitored by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) Cell Proliferation/Viability Assay kit (R&D SYSTEMS) in according to the guidelines.
Caspase-3 activity assay
KSFBs lysates were prepared and incubated with anti-caspase 3. Immunocomplexes were incubated with peptide substrate in assay buffer for 2 h at 37°C. Release of p-nitroaniline was measured at 405 nm using an ELISA reader (MD SpectraMax M5, USA) according to the manufacturer’s instructions.
Quantification of apoptosis by flow cytometry
Quantitative assessment of apoptotic cells was also assessed by the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick endlabeling (TUNEL) method, which examines DNA-strand breaks during apoptosis by using BD ApoAlertTM DNA Fragmentation Assay Kit. The KSFBs were trypsinized, fixed with 4% paraformaldehyde, and permeabilized with 0.1% Triton-X-100 in 0.1% sodiumcitrate. After being washed, the cells were incubated with the reaction mixture for 60 min at 37°C. Cells were immediately analyzed using FACScan and the Cellquest program (Becton Dickinson).
Histological observation and immunohistochemical staining
Formalin-fixed and paraffin-embedded HS tissues were cut into about 4 μm section, which were stained with hematoxylin & eosin (H&E) staining, and visualized under a microscope (Leica DM 2500).
Paraffin embedded KS tissues were cut into about 4 μm section and mounted on glass slides and stained by immunoperoxidase. The paraffin sections were baked in oven at 65°C for 24 h, then dewaxing to water, rinsed with PBS 3 times. Well washed section was placed in the EDTA buffer for microwave antigen retrieval, the fire to boil, then low heat to boil after an interval of 10 min. After natural cooling, the sections were washed with PBS 3 times. The sections were put into 3% hydrogen peroxide solution and incubated at room temperature for 10 min, which was to block endogenous peroxidase, then washed with PBS 3 times, closed with 5% bovine serum albumin (BSA) for 20 min. After removal of BSA liquid, each section was added with 50 μl diluted primary antibody overnight at 4°C, then washed with PBS 3 times. After the removal of PBS liquid, each slice which added with 50-100 μl secondary antibody was incubated at 4°C for 50 min, then washed with PBS 3 times, each slice was added with 50-100 μl freshly prepared DAB solution with the help of microscope for controlling color. After being washed, sections were counterstained with hematoxylin, rinsed with tap water, dehydrated and mounted, and visualized under a microscope (Leica DM 2500).
Luciferase reporter gene activity assay
The 3’-UTR of FN gene containing the predicated target sites for miRNA-217 was obtained by PCR amplification. The fragment was inserted into the multiple cloning sites in the pMIR-REPORT luciferase microRNA expression reporter vector (Ambion, Austin, USA). HEK-293 cells were co-transfected with 0.1 μg of luciferase reporters containing FN 3’-UTR and miRNA-217 mimics by Lipofectamine 2000 (Invitrogen, CA, USA). We harvested the cell lysates after 48 h transfection and measured the luciferase activity with a dual luciferase reporter assay kit according to manufacturer’s instruction.
Transfection of miRNA-217 mimics and inhibitor
The FAM modified 2’-OMe-oligonucleotides were chemically synthesized and purified by high-performance liquid chromatography (GenePharma, Shanghai, China). The 2’-OMe-miR-217 mimics were composed of RNA duplexes with the following sequence: 5’-UACUGCAUCAGGAACUGAUUGGA-3’. The sequences of 2’-OMe-miR-217 inhibitor and 2’-Ome-scramble oligonucleotides were as follows: 5’-UCCAAUCAGUUCCUGAUGCAGUA-3’; and 5’-ACUUUCAGAGGUCUUGACCUAG-3’. Cells were transfected using Lipofectamine2000 (Invitrogen, CA, USA) at a final concentration of 50 nM. At 24 h post-transfection, the culture medium was changed. After 48 h, cells were harvested for analysis.
Real time-polymerase chain reaction
RNA extraction was performed according to the TRIzol manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). Synthesis of cDNAs was performed by reverse transcription reactions with 4 μg of total RNA using moloney murine leukemia virus reverse transcriptase (Invitrogen) with oligo dT (15) primers (Fermentas) as described by the manufacturer. miRNA-217 level was quantified by the mirVana qRT-PCR miRNA detection kit (Ambion, Austin, USA) in conjunction with real-time PCR with SYBR Green. After circle reaction, the threshold cycle (Ct) was determined and relative miRNA-217 level was calculated based on the Ct values and normalized to U6 level in each sample. PCR with the following primers: FN, Forward 5’-GCGCCGGCTGTGCTGCACAGG-3’ and Reverse 5’-GCCTGGGGACAGCGGTGCCC-3’; GAPDH, Forward 5’-GCACCGTCAAGCTGAGAAC-3’ and Reverse 5’-TGGTGAAGACGCCAGTGGA-3’.
Western blotting
KS tissues and KSFBs were homogenized and extracted in NP-40 buffer, followed by 5-10 min boiling and centrifugation to obtain the supernatant. Samples containing 30 μg of protein were separated on 10% SDS-PAGE gel, transferred to nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA, USA). After saturation with 5% (w/v) non-fat dry milk in TBS and 0.1% (w/v) Tween 20 (TBST), the membranes were incubated with primary antibodies: FN, Col-1 and Col-2 (Santa Cruz Biotechnology, CA, USA). After three washes with TBST, The membranes were next incubated with the appropriate HRP (horseradish peroxidase)-conjugated antibody visualized with chemiluminescence (Thermo, USA).
Statistical analysis
The data from these experiments were reported as mean ± standard deviation (SD) for each group. All statistical analyses were performed by using PRISM version 5.0 (GraphPad). Inter-group differences in genes expression between hypertrophic scarring tissues (KS) and paired corresponding adjacent normal tissues (NC) were assessed by the Paired-Student’s t-test. Differences with P value of < 0.05 were considered statistically significant.
Results
Histological observation
By H&E staining, we observed that keloid tissues had a dense and excessive collagen deposition, and the collagen bundles were running parallel to the epidermis in a large fraction of the dermis. However, the thickness and complexity of the collagen bundles were diminished in paired corresponding adjacent normal tissues compared with keloid tissues (Figure 1A). To further investigate induction of fibrogenic in KS, α-SMA and E-cad expression levels in lesional tissues were measured by immunohistochemical staining. α-smooth muscle actin (α-SMA), a marker of myofibroblasts, and E-cadherin (E-cad), an epithelial marker, are closely related to fibrotic diseases [14]. In our study, α-SMA was significantly increased in KS group compared with NC group, in contrast, the lesional keloid tissues showed very dramatic reduction of E-cad as compared to NC group (Figure 1A). Moreover, the immunohistochemical staining was measured by integral optical density (IOD) assay (Figure 1B).
Figure 1.
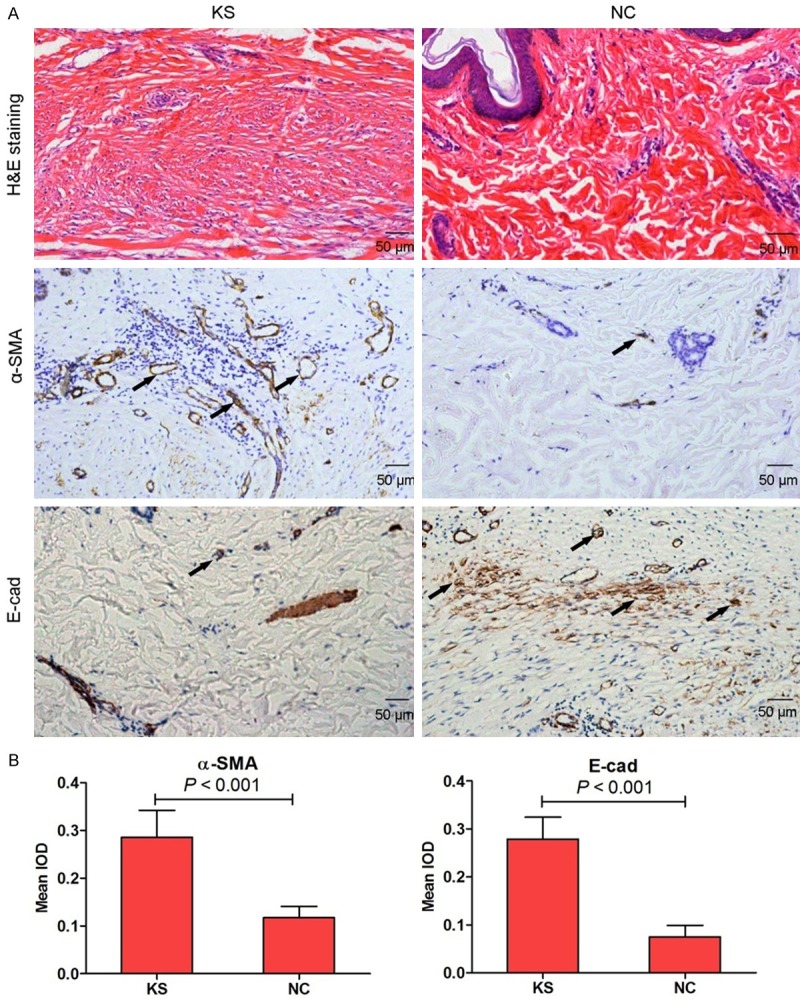
The histological observation on keloid scarring tissues (KS) and paired corresponding adjacent normal tissues (NC) from clinical patients by hematoxylin and eosin (H&E) staining. α-SMA and E-cad expression was measured by immunohistochemical staining in KS and NC tissues (A, 100×). Histogram represented the mean integral optical density (IOD) of α-SMA and E-cad in KS and NC tissues (B).
Keloid fibrogenesis-related proteins expression
To investigate the expression of TGF-β1, collagen types I and III in KS, we used immunohistochemical staining to confirm this conclusion in our study. As expected, the expression of collagen types I (Col-1) and III (Col-3) were significantly increased in KS group compared with NC group (Figure 2B and 2C). However, the expression of TGF-β1 had no significant differences between keloid tissues and corresponding adjacent normal tissues (Figure 2A and 2C).
Figure 2.
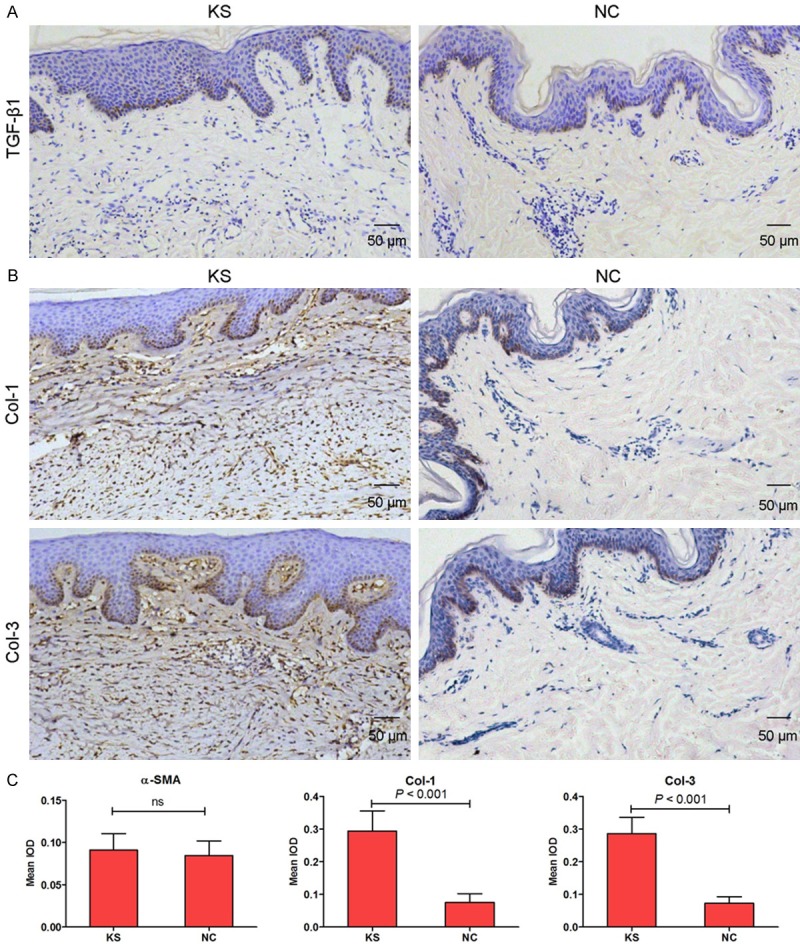
TGF-β1 (A, 100×), Col-1 and Col-3 (B, 100×) expression was measured by immunohistochemical staining in KS and NC tissues. Histogram represented the mean integral optical density (IOD) of TGF-β1, Col-1 and Col-3 in KS and NC tissues (C).
Fibronectin (FN) expression in keloid tissues
We results demonstrated that immunohistochemical staining (Figure 3A), RT-PCR (Figure 3B) and western blotting (Figure 3C) showed a marked increase in FN levels in the keloid tissues compared with the corresponding adjacent normal tissues. However, the underlying molecular mechanisms of miRNAs via targeting FN contribute to the induction of KS remains to be determined. A follow-up study will try to identify the miRNAs expression profiles in keloid tissues.
Figure 3.
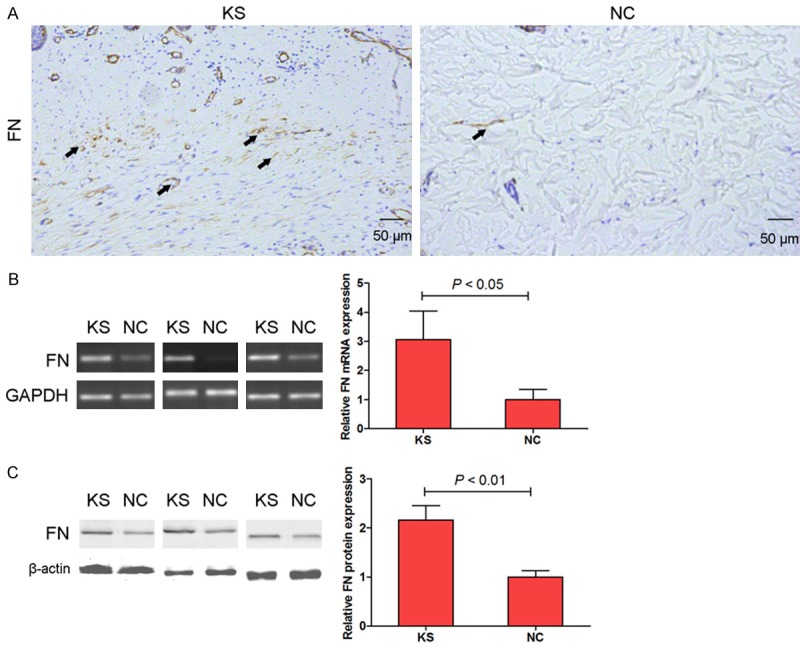
FN expression was measured by immunohistochemical staining in KS and NC tissues (A, 100×). mRNA (B) and protein (C) expression of FN were measured by RT-PCR and western blotting assay respectively.
miRNAs expression in keloid tissues
To explore the miRNAs expression profiles in KS tissues, we compared miRNAs expression between KS tissues and adjacent normal tissues using microarray assay. The results showed that 13 miRNAs were found to be significantly down-regulated, and 15 miRNAs were significantly up-regulated in the KS tissues as compared to the corresponding adjacent normal tissues, and we finally focused on miRNA-217 in our study. miRNA-217 was significantly lowly expressed in KS tissues as compared to adjacent normal tissues (Figure 4A). Moreover, by using PicTar, TargetScan, and miRBase database, we indicated that miR-217 was a potential regulator of FN. Based on these results, the expression of miRNA-217 was investigated in 20 KS tissues and paired corresponding adjacent normal tissues. Subsequent investigation led to an intriguing conclusion that the expression of miRNA-217 was greatly suppressed in KSFBs and KS tissues (Figure 4B and 4C). To validate the correlation between miRNA-217 and FN, the FN mRNA and miRNA-217 expression levels were analyzed in 20 KS tissue samples by real-time PCR. As shown in Figure 4D, the expression levels of endogenous miRNA-217 negatively correlated with the FN mRNA levels (Pearson’s correlation coefficient = -0.683; P < 0.001). These data provide further evidence of a functional link between miRNA-217 and FN in KS tissues.
Figure 4.
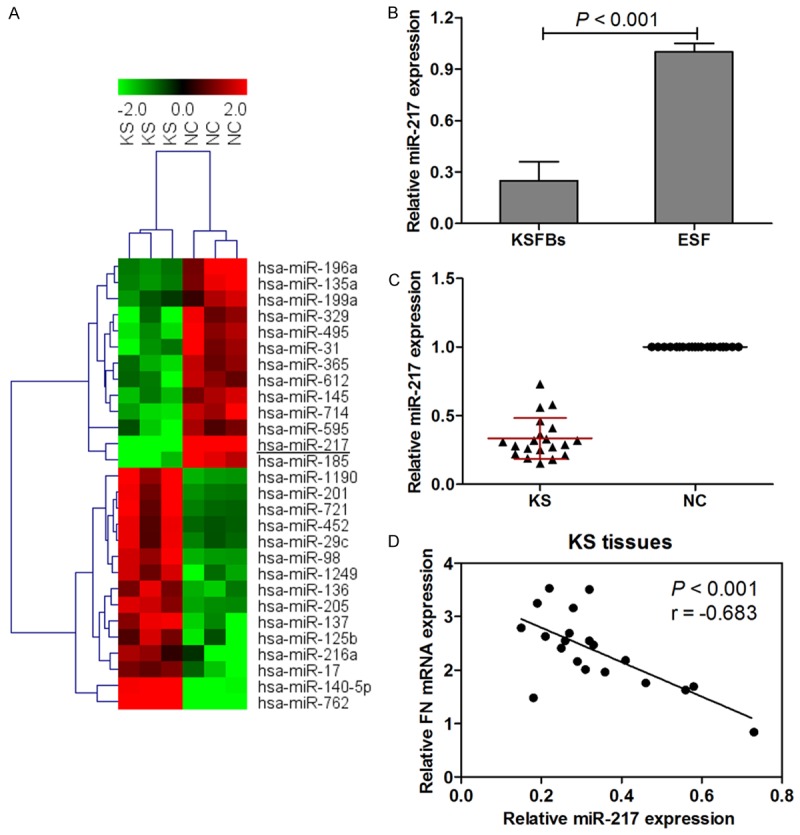
Microarray and hierarchical cluster analysis of the significantly regulated miRNAs in patients with keloid scarring. The figure is drawn by MeV software (version 4.2.6). Correlation similarity matrix and average linkage algorithms are used in the cluster analysis. Each row represents an individual miRNA, and each column represents a sample. The dendrogram at the left side and the top displays similarity of expression among miRNAs and samples individually. The color legend at the top represents the level of miRNA expression, with red indicating high expression levels and green indicating low expression levels (A). miRNA-217 expression was examined by real-time PCR and normalized to U6 expression in KSFBs (B) and 20 keloid scarring tissues and paired corresponding adjacent normal tissues (C). Inverse correlation of miRNA-217 and FN mRNA expression in KS tissues (D).
miRNA-217 is a regulator of FN
The miRNAs were initially proposed to mediate post-translational repression of their target mRNAs [15]. To further validate the miRNA microarray results, we selected miRNA-217 from the down-regulated miRNAs and evaluated the alignment predicted by the TargetScan algorithm. We found the potential miR-217 binding sites within the 3’-UTR of FN in human sapiens (Figure 5A). The complementarity between the seed region of miRNA and 3’-UTR of mRNAs is the most important determinant for target specificity, and miRNA-mediated post-translational repression often depends on perfect or near-perfect base pairing of a seed region to its target [1]. As shown in Figure 5A, FN is a strong candidate that is regulated by miRNA-217. To further investigate whether miRNA-217 directly regulates the expression of FN, we generated luciferase reporter plasmids containing the 3’-UTR of the wild-type or mutant-type FN gene in HEK293 cells. Cotransfection with this luciferase construct containing wild-type 3’-UTR and miR-185 mimics resulted in a lower luciferase activity than control group, which resulted in more than 50% decline in the luciferase activity compared with control group. Cotransfection of HEK293 cells with this recombination structure containing mutant-type 3’-UTR of FN, and the reporter activity did not show significant difference compared with control group (Figure 5B).
Figure 5.
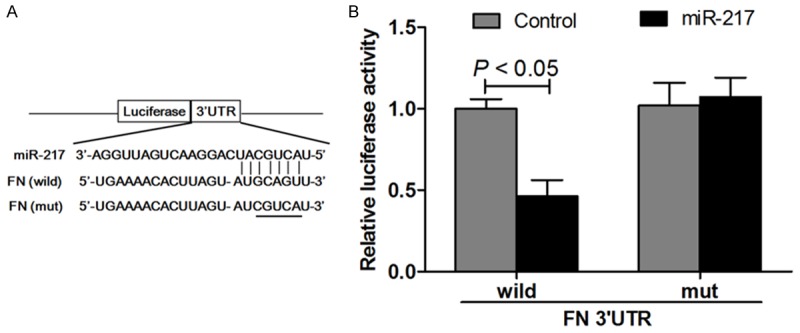
Schematic representation of the putative miRNA-217 binding site in the FN 3’UTR as in Targetscan (A) and luciferase activity assay (B).
miRNA-217 regulates FN expression in keloid scarring fibroblasts
miRNA-217 levels were measured when the KSFBs were transfected with miRNA-217 mimics or inhibitor. As expected, KSFBs transfected with miRNA-217 mimics increased miRNA-217 levels, and KSFBs transfected with miRNA-217 inhibitor decreased miRNA-217 levels in KSFBs (Figure 6A). Next, in KSFBs, the miRNA-217 mimics or inhibitor was transfected to evaluate the potential effect on cell proliferation and apoptosis of miRNA-217 in vitro. KSFBs cell viability was measured by MTT assay when cells were transfected with miRNA-217 mimics or inhibitor for 24 h, 48 h and 72 h. The viabilities of KSFBs transfected with miRNA-217 mimics was significantly lower than those of untreatment group. However, miRNA-217 loss-of function increased the growth of KSBFs as compared to the scramble cells (Figure 6B). Moreover, we examined whether miRNA-217 mimics or inhibitor induced apoptosis in KSFBs through an apoptotic mechanism. Caspase-3 activity assay and Triphosphate nick-end labeling (TUNEL) staining were measured after KSFBs transfected with miRNA-217 mimics or inhibitor for 48 h. The results indicated that the caspase-3 activity in miRNA-217 mimics group were significantly higher than that of the control group. In the contrast to that KSFBs transfected with miRNA-217 inhibitor suppressed caspase-3 activity (Figure 6C). TUNEL staining showed that miRNA-217 gain-of function induced cell apoptosis, and miRNA-217 loss-of function suppressed cell apoptosis in KSFBs (Figure 6D). These results suggested that miRNA-217 played an impact on cell proliferation and apoptosis, thereby regulating KSFBs growth. In addition, the protein expression of Col-1 and Col-3 was measured by western blotting in KSFBs transfected with miRNA-217 mimics or inhibitor for 48 h. The results showed that miRNA-217 gain-of function decreased Col-1 and Col-3 protein expression, and miRNA-217 loss-of function increased Col-1 and Col-3 protein expression in KSFBs (Figure 6E-G). Furthermore, miRNA-217 gain-of function decreased, and miRNA-217 loss-of function increased FN expression in KSFBs (Figure 6H).
Figure 6.
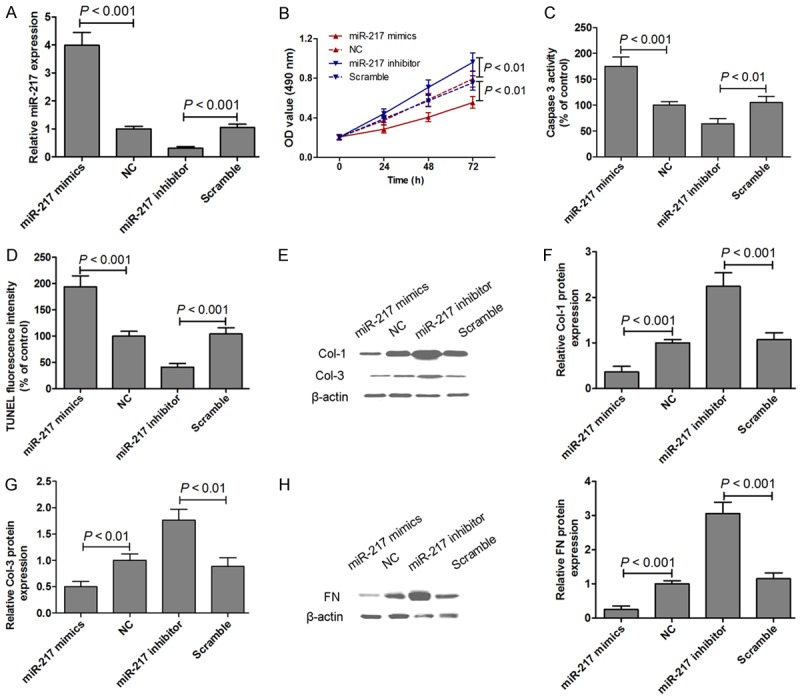
miRNA-217 expression was examined by real-time PCR and normalized to U6 expression in KSFBs transfected with miRNA-217 mimics or inhibitor for 48 h (A). MTT assays were performed on KSFBs transfected with miRNA-217 mimics or inhibitor for 24 h, 48 h and 72 h (B). Caspase-3 activity assay (C) and Triphosphate nick-end labeling (TUNEL) staining (D) were measured after KSFBs transfected with miRNA-217 mimics or inhibitor for 48 h. The protein expression of Col-1 and Col-3 was measured by western blotting in KSFBs transfected with miRNA-217 mimics or inhibitor for 48 h (E-G). The protein expression of FN was measured by western blotting in KSFBs transfected with miRNA-217 mimics or inhibitor for 48 h (H).
Discussion
Downregulation of miRNA-217 is a frequent event in various cancers, such as gastric cancer [16], colorectal cancer [12], pancreatic cancer [17] and hepatocellular carcinoma [18]. Moreover, miRNA-217 was demonstrated to modulate glomerular mesangial cell survival and hypertrophy [19]. However, the role of miRNA-217 in keloid fibrogenesis remains to be elucidated. Keloid is benign skin tumors characterized by collagen accumulation and hyperproliferation of fibroblasts and represents the end-point of a spectrum of abnormal wound healing [20]. Previous studies demonstrate that miRNA-29a [21], miRNA-21 [22] and miRNA-196a [1] are involved in the fibrogenesis of keloid. In the present study, we found that the expression of miRNA-217 was greatly suppressed in KSFBs and KS tissues. miRNA-217 could directly regulate the expression of FN via targeting its 3’-UTR. Moreover, the correlation between miRNA-217 and FN showed that endogenous miRNA-217 negatively correlated with the FN mRNA levels in KS tissues. These data provide further evidence of a functional link between miRNA-217 and FN in KS tissues, and miRNA-217 via targeting FN as a post-translational mechanism might provide new insights into the underlying mechanism and therapeutic strategies for keloid fibrogenesis.
Fibroblasts are the effector cells in fibrotic diseases including keloid and hypertrophic scars which are characterized by their over-proliferation and over-production of collagen and ECM. In addition to the suppression of fibroblast proliferation, the inhibition of collagen and ECM production and deposition by fibroblasts is an important goal for pharmaceutical agents used in the treatment of these fibroproliferative conditions [23]. Massive collagen synthesis and changes has been regarded as a main character of hypertrophic scar formation. Excess deposition of ECM, such as type I and III collagen, by fibroblasts is responsible for keloid and hypertrophic scarring [24]. Consistent with previous data, we found that upregulations of Col-1 and Col-3 were observed in KS tissues. Moreover, miRNA-217 gain-of function decreased Col-1 and Col-3 protein expression, and miRNA-217 loss-of function increased Col-1 and Col-3 protein expression in KSFBs. Contributing to the pathogenesis of keloid fibrogenesis are both a genetic predisposition and various local factors. Previous studies indicate that collagen types I and III are the most abundant components within the abnormal ECM deposits in keloids [4,25]. Previous studies indicate that miRNA-196a and miRNA-200b display the highest altered expression in KSFBs compared with normal fibroblast and inhibit the expression of type I and III collagens, whose deposition is a major manifestation in keloid pathology [1,26].
There is increasing evidence to show that the FN signalling is involved in keloid pathogenesis [6,9,27]. Early in healing, fibroblasts migrate into the wound area and rapidly produce a transient matrix of FN. However, as healing progresses, collagen content increases, the collagen is organized into bundles, and eventually the fibronectin matrix diminishes [9]. These results suggest that overexpressed FN may be resulting in abnormal physiology in keloid fibrogenesis. Consistent with these previous reports, we results demonstrated that immunohistochemical staining, RT-PCR and western blotting showed a marked increase in FN levels in the keloid tissues compared with the corresponding adjacent normal tissues. Based on these studies, we conclude that FN may be a key target for the development of novel therapeutic strategies for KS. Intriguingly, we also found that miRNA-217 affected KSFBs growth and apoptosis through regulating FN expression. Thus, our study provided evidence to determine that miRNA-217 might be a useful target for management of keloid fibrogenesis.
In conclusion, the data reported herein indicate that overexpressed miRNA-217 can inhibit KSFBs growth, and the underlying mechanism was mediated, at least partially, through the suppression of FN expression. But above all, miRNA-217 may play a potential therapeutic avenue for the treatment of keloid fibrogenesis.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81272109).
Disclosure of conflict of interest
None.
References
- 1.Kashiyama K, Mitsutake N, Matsuse M, Ogi T, Saenko VA, Ujifuku K, Utani A, Hirano A, Yamashita S. miR-196a downregulation increases the expression of type I and III collagens in keloid fibroblasts. J Invest Dermatol. 2012;132:1597–1604. doi: 10.1038/jid.2012.22. [DOI] [PubMed] [Google Scholar]
- 2.Lee WJ, Ahn HM, Roh H, Na Y, Choi IK, Lee JH, Kim YO, Lew DH, Yun CO. Decorin-expressing adenovirus decreases collagen synthesis and upregulates MMP expression in keloid fibroblasts and keloid spheroids. Exp Dermatol. 2015;24:591–597. doi: 10.1111/exd.12719. [DOI] [PubMed] [Google Scholar]
- 3.Harn HI, Wang YK, Hsu CK, Ho YT, Huang YW, Chiu WT, Lin HH, Cheng CM, Tang MJ. Mechanical coupling of cytoskeletal elasticity and force generation is crucial for understanding the migrating nature of keloid fibroblasts. Exp Dermatol. 2015;24:579–584. doi: 10.1111/exd.12731. [DOI] [PubMed] [Google Scholar]
- 4.Aoki M, Miyake K, Ogawa R, Dohi T, Akaishi S, Hyakusoku H, Shimada T. siRNA knockdown of tissue inhibitor of metalloproteinase-1 in keloid fibroblasts leads to degradation of collagen type I. J Invest Dermatol. 2014;134:818–826. doi: 10.1038/jid.2013.396. [DOI] [PubMed] [Google Scholar]
- 5.Fan C, Dong Y, Xie Y, Su Y, Zhang X, Leavesley D, Upton Z. Shikonin reduces TGF-beta1-induced collagen production and contraction in hypertrophic scar-derived human skin fibroblasts. Int J Mol Med. 2015;36:985–991. doi: 10.3892/ijmm.2015.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang CJ, Yen YH, Hung LY, Wang SH, Pu CM, Chien HF, Tsai JS, Lee CW, Yen FL, Chen YL. Thalidomide inhibits fibronectin production in TGF-beta1-treated normal and keloid fibroblasts via inhibition of the p38/Smad3 pathway. Biochem Pharmacol. 2013;85:1594–1602. doi: 10.1016/j.bcp.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 7.Zeng C, Wang YL, Xie C, Sang Y, Li TJ, Zhang M, Wang R, Zhang Q, Zheng L, Zhuang SM. Identification of a novel TGF-beta-miR-122-fibronectin 1/serum response factor signaling cascade and its implication in hepatic fibrogenesis. Oncotarget. 2015;6:12224–12233. doi: 10.18632/oncotarget.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 9.Babu M, Diegelmann R, Oliver N. Fibronectin is overproduced by keloid fibroblasts during abnormal wound healing. Mol Cell Biol. 1989;9:1642–1650. doi: 10.1128/mcb.9.4.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guban B, Vas K, Balog Z, Manczinger M, Bebes A, Groma G, Szell M, Kemeny L, Bata-Csorgo Z. Abnormal regulation of fibronectin production by fibroblasts in psoriasis. Br J Dermatol. 2016;174:533–41. doi: 10.1111/bjd.14219. [DOI] [PubMed] [Google Scholar]
- 11.Kelsh RM, McKeown-Longo PJ, Clark RA. EDA Fibronectin in Keloids Create a Vicious Cycle of Fibrotic Tumor Formation. J Invest Dermatol. 2015;135:1714–1718. doi: 10.1038/jid.2015.155. [DOI] [PubMed] [Google Scholar]
- 12.Wang B, Shen ZL, Jiang KW, Zhao G, Wang CY, Yan YC, Yang Y, Zhang JZ, Shen C, Gao ZD, Ye YJ, Wang S. MicroRNA-217 functions as a prognosis predictor and inhibits colorectal cancer cell proliferation and invasion via an AEG-1 dependent mechanism. BMC Cancer. 2015;15:437. doi: 10.1186/s12885-015-1438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vychytilova-Faltejskova P, Kiss I, Klusova S, Hlavsa J, Prochazka V, Kala Z, Mazanec J, Hausnerova J, Kren L, Hermanova M, Lenz J, Karasek P, Vyzula R, Slaby O. MiR-21, miR-34a, miR-198 and miR-217 as diagnostic and prognostic biomarkers for chronic pancreatitis and pancreatic ductal adenocarcinoma. Diagn Pathol. 2015;10:38. doi: 10.1186/s13000-015-0272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- 15.Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Dong X, Gu X, Qin R, Jia H, Gao J. The microRNA-217 functions as a potential tumor suppressor in gastric cancer by targeting GPC5. PLoS One. 2015;10:e0125474. doi: 10.1371/journal.pone.0125474. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Zhao WG, Yu SN, Lu ZH, Ma YH, Gu YM, Chen J. The miR-217 microRNA functions as a potential tumor suppressor in pancreatic ductal adenocarcinoma by targeting KRAS. Carcinogenesis. 2010;31:1726–1733. doi: 10.1093/carcin/bgq160. [DOI] [PubMed] [Google Scholar]
- 18.Su J, Wang Q, Liu Y, Zhong M. miR-217 inhibits invasion of hepatocellular carcinoma cells through direct suppression of E2F3. Mol Cell Biochem. 2014;392:289–296. doi: 10.1007/s11010-014-2039-x. [DOI] [PubMed] [Google Scholar]
- 19.Kato M, Putta S, Wang M, Yuan H, Lanting L, Nair I, Gunn A, Nakagawa Y, Shimano H, Todorov I, Rossi JJ, Natarajan R. TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol. 2009;11:881–889. doi: 10.1038/ncb1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jumper N, Paus R, Bayat A. Functional histopathology of keloid disease. Histol Histopathol. 2015;30:1033–1057. doi: 10.14670/HH-11-624. [DOI] [PubMed] [Google Scholar]
- 21.Zhang GY, Wu LC, Liao T, Chen GC, Chen YH, Zhao YX, Chen SY, Wang AY, Lin K, Lin DM, Yang JQ, Gao WY, Li QF. A novel regulatory function for miR-29a in keloid fibrogenesis. Clin Exp Dermatol. 2016;41:341–5. doi: 10.1111/ced.12734. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Liu Y, Chen X, Zhang M, Xiao Z. Impact of MiR-21 on the expression of FasL in the presence of TGF-beta1. Aesthet Surg J. 2013;33:1186–1198. doi: 10.1177/1090820X13511969. [DOI] [PubMed] [Google Scholar]
- 23.Phan TT, Lim IJ, Sun L, Chan SY, Bay BH, Tan EK, Lee ST. Quercetin inhibits fibronectin production by keloid-derived fibroblasts. Implication for the treatmcent of excessive scars. J Dermatol Sci. 2003;33:192–194. doi: 10.1016/j.jdermsci.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Syed F, Ahmadi E, Iqbal SA, Singh S, McGrouther DA, Bayat A. Fibroblasts from the growing margin of keloid scars produce higher levels of collagen I and III compared with intralesional and extralesional sites: clinical implications for lesional site-directed therapy. Br J Dermatol. 2011;164:83–96. doi: 10.1111/j.1365-2133.2010.10048.x. [DOI] [PubMed] [Google Scholar]
- 25.Al-Attar A, Mess S, Thomassen JM, Kauffman CL, Davison SP. Keloid pathogenesis and treatment. Plast Reconstr Surg. 2006;117:286–300. doi: 10.1097/01.prs.0000195073.73580.46. [DOI] [PubMed] [Google Scholar]
- 26.Li P, He QY, Luo CQ. Overexpression of miR-200b inhibits the cell proliferation and promotes apoptosis of human hypertrophic scar fibroblasts in vitro. J Dermatol. 2014;41:903–911. doi: 10.1111/1346-8138.12600. [DOI] [PubMed] [Google Scholar]
- 27.Chua AW, Gan SU, Ting Y, Fu Z, Lim CK, Song C, Sabapathy K, Phan TT. Keloid fibroblasts are more sensitive to Wnt3a treatment in terms of elevated cellular growth and fibronectin expression. J Dermatol Sci. 2011;64:199–209. doi: 10.1016/j.jdermsci.2011.09.008. [DOI] [PubMed] [Google Scholar]


