Abstract
Autoimmune pancreatitis (AIP) is a rare chronic pancreatitis and the incidence is increasing recently. However, the formal report of this disease is still rare in literatures. Here, we reported a rare case of IgG4 positive autoimmune pancreatitis to make the awareness of this type of disease. The patient was a 58-year-old Chinese male who was suffered from epigastric pain accompanied by nausea and vomiting. An occupying lesion was detected in the body of the pancreas tail with the ultrasound examination. The serum IgG4 levels, white blood cells, blood amylase and the γ-globulin fraction were all increased. After operation, the following pathological detection with immunochemistry test confirmed the diagnosis of autoimmune pancreatitis.
Keywords: Autoimmune pancreatitis, IgG4 positive, immunohistochemistry
Introduction
The AIP is a kind of idiopathic chronic pancreatitis accompanied with hypergammaglobulinemia and thus is considered as an autoimmune disease [1,2]. In 1961, Sarles H first reported this type of disease and was then defined by Kawaguchi K in 1995 [1]. The diagnosis of AIP mainly depends on a comprehensive examination of clinical manifestations, serological analysis and morphological characteristics [1,3-6]. Patients usually exhibited signs of epigastria discomfort, loss of weight, icterus obstructivus and glycuresis, which unfortunately also often appeared in patients with common chronic pancreatitis [7-11]. The serological analysis often reveals the abnormal increases of γ-globulin, IgG or IgG4, serum trypsin and autoantibodies [5,7-13]. The morphological characteristics often include atrophy of the acinar cells and fibrosis, periductal and interlobular fibrosis, IgG4 positive plasmacytes and vasculopathy [5,7-13].
Since AIP shares many clinical and radiological features with other types of chronic pancreatitis as well as pancreatic cancer [1,2]. It is difficult to make an accurate diagnosis during clinical setting, especially in the intraoperative pathological diagnosis. Thus, more information about this disease will greatly help more accurate diagnosis of AIP.
Case report
The patient was a 58-year-old Chinese male who was diagnosed with hypertension and type 2 diabetes several years ago. He was suffered from epigastric pain accompanied with nausea and vomiting for three days before admission. He was not a smoker or alcohol drinker with a healthy family history. Physical examination revealed jaundice of the bulbar conjunctiva with soft abdomen and normal body temperature. Laboratory analysis showed: peripheral total white blood cells was 10.9×109/L (3.97×109/L-9.15×109/L), the serum amylase was 73 IU/L (28-100 IU/L), the glomerular filtration rate was 167.09 ml/min (>80 ml/min), the CEA was 1.04 ng/ml (0-5 ng/ml) and the carbohydrate antigen 199 was 13.97 U/ml (0-37 U/ml). However, the serum IgG was elevated at 1938 mg/dL (700-1600 mg/dL) with a serum IgG4 level of 422 mg/dL (3-201 mg/dL). The CT and ultrasound examination showed an occupying lesion located in the body of the pancreas tail (Figure 1). The primary diagnosis of CT examination indicated a thicken pancreatic body and tail, reduction of density, blurred peripheral fat clearance. Ultrasound examination also confirmed that the pancreatic body and tail harbored an unknown disease (Figure 1).
Figure 1.
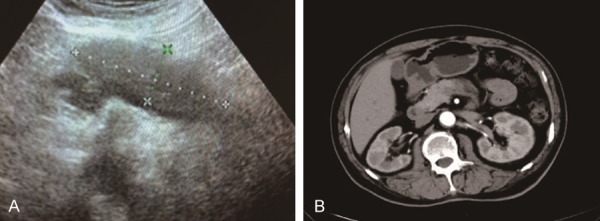
A. The ultrasound result showed that a hypoechoic group of size 7.5×2.5 cm at the tail of pancreas with clear boundary and uniform internal echo. The blood flow signal was obvious, and the main pancreatic duct was not dilated. B. The CT examination revealed that the pancreatic body and tail became thicken with density reduction and slightly blurred peripheral fat clearance.
Surgical operation revealed an 8×6×2.5 cm lesion in the pancreatic body and tail. The lesion was hard with unclear boundary, and was attached to the surrounding tissues and wrapped by spleen artery.
Gross pathological examination of the frozen specimen showed that there were multiple gray white nodular lesions in the pancreas; ranging from 0.5 cm to 2 cm in diameters with relatively clear boundaries and hard texture. The microscopic examination indicated that the sections from the lesion were nodular without significant proliferation of glandular tubular structures in the fibrous tissue, which was often misdiagnosed as invasive carcinoma (Figure 2A). The lesion also displayed slightly disordered arrangement in the glandular tubular tissue that was infiltrated with, inflammatory cells, mainly lymphocytes (Figure 2A).
Figure 2.
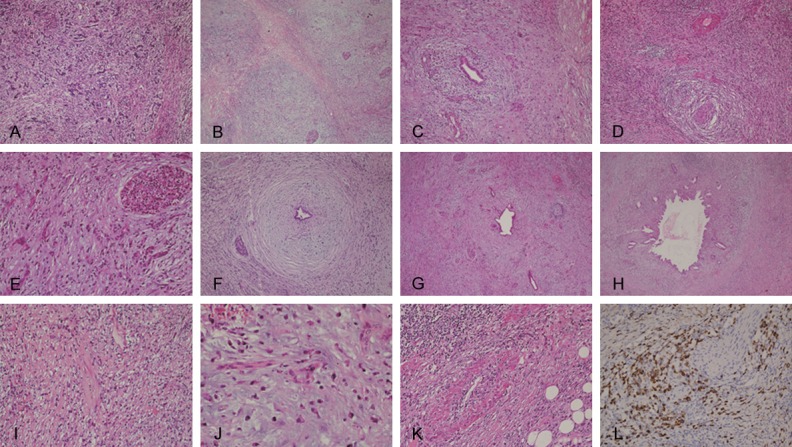
The typical lesions of autoimmune pancreatitis. A. A number of small ducts of connective tissue atrophy was observed in the frozen section, which might be easily misdiagnosed as invasive carcinoma (HE, ×100); B. The nodular lesion was compared with diffuse fibrosis, which looks like a residual tumor and easily misdiagnosed as neoplastic lesion (HE, ×20); C. A large number of plasma cells were infiltrated into lesions with extruded duct around the dilated duct (HE, ×40); D. Massive plasma cell infiltration and vasculitis in the lestions (HE, ×40); E. Residual islet, extruded and hyperplastic small ducts, diffuse fibrosis and inflammatory cell infiltration (HE, ×100); F. Fibrosis and hyaline degeneration and inflammatory cell infiltration around the duct (HE, ×20); G. Diffuse fibrosis with hyaline degeneration, residual dilatation of the catheter (HE, ×40); H. Dilatation of the catheter and fibrosis, and infiltration of inflammatory cells (HE, ×100); I. Infiltration of a large number of plasma cell (HE, ×100); J. Infiltration of a large number of plasma cells (HE, ×200); K. Infiltration of a large number of plasma cells and vasculitis (HE, ×100); L. Infiltration of IgG4 positive plasma cells (IHC staining, ×100).
The further examination of the specimen also showed a reduced pancreatic volume with hard texture and thickened coat. The specimen section displayed multiple gray white nodules. Microscopic examination showed that pancreatic tissue was significantly atrophic, and the nodular lesions contained hyperplastic nodules and residual pancreatic tissue as well as disappeared pancreatic ductal and glandular atrophy (Figure 2B). In addition, plasma cell infiltration and vasculitis in the lesions and extruded small ducts were also observed around the dilated small ducts (Figure 2C-E). The fibrous tissue hyperplasia with storiform arrangement was visible in the other pancreatic tissues (Figure 2F-H). Massive plasma cells infiltrated into the tissues (Figure 2I and 2J). Small vessel vasculitis and occlusive vasculitis were obvious (Figure 2K). Immunohistochemical (IHC) staining revealed diffuse infiltration of IgG4 positive plasma cells (>30 per high field) in the tissues (Figure 2L).
Discussion
Although there were various criteria to help accurate diagnosis of AIP according to the Japanese Pancreas Society guidelines, Mayo HISORt criteria, and the International Consensus Diagnostic Criteria for AIP [1]. AIP is divided into AIP type 1 and AIP type 2 based on clinical, radiological, and histological features [4,14-17]. The major differences were pathological morphology [4,14-17]. The AIP type 1, also known as lymphoplasmacytic sclerosing pancreatitis, is the more common than the AIP type 2 [1]. The AIP type 1 is characterized histopathologically by the infiltration of lymphoplasmacytic cells without granulocytes and IgG4-positive plasma cells, as well as the storiform fibrosis around the pancreatic duct and vein and obliterating phlebitis [11,16-19]. However, the histopathology of AIP type 2 is characterized by infiltration of more granulocytes without IgG4-positive plasma cells [1,3-6]. In this case, there was infiltration of many lymphocytes and diffused infiltration of IgG4 positive cells with few granulocytes, suggesting a AIP type 1 classification.
IgG4-related disease (IgG4-RD) is a new type of human disease affecting many organs that is characterized often by increased serum IgG4 concentration [20,21]. IgG4-RD has been described in virtually all organs, but the common diseases include IgG4-related AIP, retroperitoneal fibrosis, chronic periaortitis, autoimmune hypophysitis, sclerosing cholangitis Riedel thyroiditis, Mikulicz’s disease [2]. IgG4-related AIP, which is the first identified IgG4 RD, typically exhibiting epigastria discomfort, loss of weight, icterus obstructivus and glycuresis [2].
The clinical symptoms of the pancreas diseases usually shows obstructive jaundice, lose weight, diabetes mellitus, epigastric discomfort, yellow stain of skin [1,3-6]. The clinical also shows IgG4-associated cholangitis, dacryoadenitis, sialadenitis, retroperitoneal fibrosis, lymphadenopathy, interstitial pneumonia, tubulointerstitial nephritis [1,3-6]. However, the symptom of AIP in clinical often was not obvious and the common chronic pancreatitis and pancreatic cancer often share the same symptom [1]. Thus, many cases of AIP were misdiagnosed as pancreatic cancer [2]. In this case, the CT and ultrasound examination indicated the existence of an occupying lesion, suggesting a possible pancreatic cancer. However, the close examination revealed the massive infiltration of lymphoplasmacytic cells without granulocytes indicating its AIP diagnosis. Thus, IgG4 IHC analysis is critical for accurate diagnosis.
In conclusion, the AIP diagnose is difficult and requires a comprehensive examination with different methods. The clinical symptom-epigastric pain accompanied with nausea and vomiting, indicated the patients should be detected in the serological analysis, which supply important clue for the pathology reference. The IgG4 IHC results are critical and should be included in the pathological examination. The pathologists need to closely examine the infiltration of lymphoplasmacytic cells in the pancreas.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 30870981 and No. 81272754 and No. 81470110). We acknowledge to Professor Zhaoyi Wang, for providing critical reading of our manuscript.
Disclosure of conflict of interest
None.
References
- 1.Karimi S, Bharill P. Autoimmune pancreatitis: a case of atypical radiographic findings. Case Rep Gastroenterol. 2016;10:581–588. doi: 10.1159/000448988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Della-Torre E, Lanzillotta M, Doglioni C. Immunology of IgG4-related disease. Clin Exp Immunol. 2015;181:191–206. doi: 10.1111/cei.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heo WG, Kim TH, Kim YJ, Chon HK, Woo YS, Sohn YW. Autoimmune pancreatitis complicated with pancreatic ascites, pancreatic ductal leakage, and multiple pseudocyst. Pancreas. 2017;46:e10–e11. doi: 10.1097/MPA.0000000000000687. [DOI] [PubMed] [Google Scholar]
- 4.Ennazk L, Mghari GE, Ansari NE. Association of newly diagnosed type 1 diabetes and autoimmune pancreatitis. Endocrinol Diabetes Metab Case Rep. 2016;2016 doi: 10.1530/EDM-16-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buijs J, Cahen DL, van Heerde MJ, Hansen BE, van Buuren HR, Peppelenbosch MP, Fuhler GM, Bruno MJ. Testing for Anti-PBP antibody is not useful in diagnosing autoimmune pancreatitis. Am J Gastroenterol. 2016;111:1650–1654. doi: 10.1038/ajg.2016.241. [DOI] [PubMed] [Google Scholar]
- 6.Hart PA, Chari ST. Preventing disease relapses in autoimmune pancreatitis with maintenance steroids: are we there yet? Gut. 2017;66:394–396. doi: 10.1136/gutjnl-2016-312738. [DOI] [PubMed] [Google Scholar]
- 7.Adler JM, Gardner TB. Fine-needle aspiration for autoimmune pancreatitis-not ready for prime time. Gastrointest Endosc. 2016;84:249–251. doi: 10.1016/j.gie.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 8.Akamatsu M, Makino N, Ikeda Y, Matsuda A, Ito M, Kakizaki Y, Saito Y, Ishizawa T, Kobayashi T, Furukawa T, Ueno Y. Specific MAPK-associated MicroRNAs in serum differentiate pancreatic cancer from autoimmune pancreatitis. PLoS One. 2016;11:e0158669. doi: 10.1371/journal.pone.0158669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Notohara K, Nishimori I, Mizuno N, Okazaki K, Ito T, Kawa S, Egawa S, Kihara Y, Kanno A, Masamune A, Shimosegawa T. Clinicopathological features of Type 2 autoimmune pancreatitis in japan: results of a multicenter survey. Pancreas. 2015;44:1072–1077. doi: 10.1097/MPA.0000000000000438. [DOI] [PubMed] [Google Scholar]
- 10.Bi Y, Hart PA, Law R, Clain JE, Farnell MB, Gleeson FC, Kendrick ML, Levy MJ, Pearson RK, Petersen BT, Pisney LD, Smyrk TC, Takahashi N, Topazian MD, Vege SS, Chari ST. Obstructive jaundice in autoimmune pancreatitis can be safely treated with corticosteroids alone without biliary stenting. Pancreatology. 2016;16:391–396. doi: 10.1016/j.pan.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Bolia R, Chong SY, Coleman L, MacGregor D, Hardikar W, Oliver MR. Autoimmune pancreatitis and IgG4 related disease in three children. ACG Case Rep J. 2016;3:e115. doi: 10.14309/crj.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borufka L, Volmer E, Muller S, Engelmann R, Nizze H, Ibrahim S, Jaster R. In vitro studies implicate an imbalanced activation of dendritic cells in the pathogenesis of murine autoimmune pancreatitis. Oncotarget. 2016;7:42963–42977. doi: 10.18632/oncotarget.10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chebli JM, Chebli LA, Gaburri PD, de Almeida Delgado AA, Costa TM. Severe malabsorption refractory to pancreatic enzyme supplementation unmasking autoimmune enteropathy in a chronic pancreatitis patient. Pancreas. 2016;45:e43–44. doi: 10.1097/MPA.0000000000000611. [DOI] [PubMed] [Google Scholar]
- 14.Chiang AL, Hornick JL, Sahni VA, Clancy TE, Ryou M. Autoimmune pancreatitis presenting as multifocal masses, diagnosed on ampullary biopsy. Pancreas. 2016;45:e25–27. doi: 10.1097/MPA.0000000000000581. [DOI] [PubMed] [Google Scholar]
- 15.De Marchi G, Paiella S, Luchini C, Capelli P, Bassi C, Frulloni L. Very high serum levels of CA 19-9 in autoimmune pancreatitis: report of four cases and brief review of literature. J Dig Dis. 2016;17:697–702. doi: 10.1111/1751-2980.12403. [DOI] [PubMed] [Google Scholar]
- 16.Donet JA, Czul F, Pena NA, Barkin JS. Type 1 autoimmune pancreatitis: case scenario and review of the disease. Rev Gastroenterol Peru. 2016;36:252–255. [PubMed] [Google Scholar]
- 17.Felix K, Hauck O, Schnolzer M, Kempf T, Warnken U, Schneider K, Bergmann F, Fritz S, Werner J. Identification of novel serum autoantibodies for differential diagnosis of autoimmune pancreatitis and pancreatic ductal adenocarcinoma. Pancreas. 2016;45:1309–1319. doi: 10.1097/MPA.0000000000000647. [DOI] [PubMed] [Google Scholar]
- 18.Serifoglu I, Oz II, Ustundag Y, Ilikhan SU, Tokgoz O. Sequential evaluation of pancreato-biliary findings in a case with IgG4-associated cholangiopathy and autoimmune pancreatitis during corticosteroid treatment. Balkan Med J. 2016;33:458–461. doi: 10.5152/balkanmedj.2016.15420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yonenaga Y, Kushihata F, Watanabe J, Tohyama T, Inoue H, Sugita A, Takada Y. Localized 18F-fluorodeoxyglucose uptake at the pancreatic head during remission phase of autoimmune pancreatitis: a case report. Oncol Lett. 2016;12:1801–1805. doi: 10.3892/ol.2016.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katabathina VS, Khalil S, Shin S, Lath N, Menias CO, Prasad SR. Immunoglobulin G4-related disease: recent advances in pathogenesis and imaging findings. Radiol Clin North Am. 2016;54:535–551. doi: 10.1016/j.rcl.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Takamura S, Suyama T, Teraki Y. Immunoglobulin G4-related disease presenting with prurigo: circulating T-helper 2 cells may be involved in the pathogenesis. J Dermatol. 2016;43:1067–1070. doi: 10.1111/1346-8138.13372. [DOI] [PubMed] [Google Scholar]


