Abstract
Metaplastic breast carcinoma (MBC) is a rare and aggressive neoplasm. Morphologically, it is characterized by the presence of multiple cellular differentiation and heterologous elements (squamous cells, spindle cells, cartilage or bone, etc). The clinical significance, prognostic risk factors and optimal treatment modalities of MBC are limited. This study collected clinical and pathological data of 26 MBC cases in the First Affiliated Hospital of Bengbu Medical College from July 2002 to July 2012 and investigated the clinicopathological features and the prognosis data. All patients were females aged 34-76 years old. Median tumor size was 3.5 cm and 88.5% patients were triple-negative for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER-2). MBC is associated with a poor prognosis compared with conventional invasive ductal carcinoma (IDC). In our study, 5-year Overall Survival (OS) rate and 5-year Disease-Free Survival (DFS) rate were 61.5% and 53.8% respectively. Most patients in this series had high-grade, triple-negative tumors and were treated with optimal therapy.
Keywords: Breast, metaplastic breast carcinoma, clinicopathologic features, prognosis
Introduction
Invasive breast carcinoma is the most leading cause of cancer mortality in women. MBC comprise 0.2-1% of all invasive breast carcinomas [1]. They consist of a heterogeneous group of invasive carcinomas characterized by an admixture of adenocarcinoma with dominant areas of spindle cell, squamous and/or mesenchymal differentiation [2]. The current WHO classification distinguishes five subtypes: low-grade adenosquamous carcinoma, fibromatosis-like metaplastic carcinoma, squamous cell carcinoma, spindle cell carcinoma, and carcinoma with mesenchymal differentiation (chondroid differentiation, osseous differentiation, and other types of mesenchymal differentiation) [3]. Because the incidence rate of MBC is low and the pathological character is variable, the clinical characteristics, prognosis information and treatment modalities are unclear and controversial. Until now only a few large research and literature on MBC have been published. In clinical therapy, MBC is usually follows the treatment paradigm for conventional IDC. The ideal treatment guideline for MBC is unknown and the potential predictors of treatment efficacy need to be explored. We reviewed the clinicopathologic features, treatment strategies, and clinical outcomes of 26 MBC patients treated in our hospital over a 10-year period to further investigate the behavior of these neoplasms and to provide more factual evidence.
Materials and methods
Among 8973 patients who underwent surgery for breast cancer at the First Affiliated Hospital of Bengbu Medical College from July 2002 to July 2012, 26 (0.29%) patients were identified as MBC. We performed retrospective analysis of the clinical information, pathologic characteristics, treatment regimen, and follow-up details of these 26 patients. Specimens were double-blind pathological review by two pathologists. According to the 2012 WHO Classification of Tumours of Breast and Female Reproductive System: Pathology and Genetics, all patients were diagnosed as MBC and clinical staging were based on the TNM staging of breast cancer developed by the American Joint Committee on Cancer (AJCC, 7th edition) (17). All patients were females and their ages ranged from 34 to 76 years old, with a median age of 57 years. Clinical demographics and follow-up data were obtained from medical records and referring physicians.
This study was approved by the Ethics Committees of the First Affiliated Hospital of Bengbu Medical College and was conducted in accordance with the ethical guidelines of the Declaration of Helsinki.
Immunohistochemistry (IHC)
Formalin-fixed paraffin embedded tissue sections were employed in each case using a standard protocol. HE-stained sections (4 µm thickness) were re-examined to evaluate the tumor’s histological features and immunohistochemistry were performed with Elivision technique. Antibody details are given in Table 1. The threshold used for positive ER and PR expression was any nuclear labeling 1% or higher. HER-2 immunoreactivity was evaluated on a standardized scale from 0-3 based on the intensity of staining of the cell membrane and the proportion of invasive tumor cells stained. Strong complete staining of the membrane in >10% of tumor cells (score, 3+) was considered positive. Intensity patterns with scores 0-1+ were considered negative, and samples scored as 2+ were further assessed by FISH test. Fluorescence in situ hybridization ratio of 2.0 or more was considered positive for HER-2 gene amplification.
Table 1.
Sources of the antibodies used in the immunohistochemistry analysis
| Source | Antibody |
|---|---|
| CK | Monoclonal AE1/AE3 |
| CKH (34βE12) | Monoclonal, clone34βE12 |
| Ki-67 | Monoclonal, cloneSP6 |
| ER | Monoclonal, clone SP1 |
| PR | Monoclonal, clone1A6 |
| HER-2 | Monoclonal, cloneCB11 |
| p63 | Monoclonal, clone 4A4 |
| Vimentin | Monoclonal, cloneSP20 |
All antibodies were obtained from Maixin Biotech, Inc. (Fuzhou, China), and were ready to use.
Follow-up
For all patients, follow-up started from the date of operation. The patients were followed up at 3 months intervals for the first two year, and then at 6 months intervals for the following 2 years and then annually thereafter. All patients were followed up until mortality or the cut-off date of July 2012. All patients were followed by phone or outpatient visit. Follow-up includes patient survival, postoperative adjuvant treatment, tumor recurrence and metastasis. No lost cases.
Statistical analysis
All statistical analyses were carried out using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) for Windows. The Kaplan-Meier method was used to calculate the survival rate and draw the survival curve.
Results
Clinicopathological characteristics
The clinicopathological characteristics of 26 MBC patients are summarized in Table 2. The patient median age was 57 years. All patients were presented with a palpable, painless lump in the breast, in which 3 patients had a history of blood nipple discharge. The tumor was located in the left breast in 15 cases and in the right breast in 11 cases. Tumor sizes were determined by gross pathological examination and ranged from 2.2-6.5 cm, the median tumor size was 3.5 cm. Most patients (20/26, 76.9%) had T2 disease (tumor size 2-5 cm) and positive lymph node metastases was reported in 34.6% (9/26) of the 26 MBC patients. ER was negative in 24 (92.3%) patients and PR negative in 26 (100.0%) patients. HER-2 was negative in 23 (88.5%) patients. Most tumors were triple negative. Ki-67 was low expression in 6 cases, high expression in 17 cases, in the critical state in 3 cases.
Table 2.
Clinicopathological features of patients with MBC
| Clinicopathologic Parameter | Number (%) |
|---|---|
| Age, years | |
| <50 | 11 (42.3%) |
| ≥50 | 15 (57.7%) |
| Tumor size, cm | |
| <2 | 2 (7.7%) |
| 2-5 | 20 (76.9%) |
| >5 | 4 (15.4%) |
| Nodal status | |
| Positive | 9 (34.6%) |
| Negative | 17 (65.4%) |
| Histologic subtype | |
| Low-grade adenosquamous carcinoma | 0 (0.00%) |
| Fibromatosis-like metaplastic carcinoma | 3 (11.5%) |
| Squamous cell carcinoma | 8 (30.8%) |
| Spindle cell carcinoma | 13 (50.0%) |
| Carcinoma with mesenchymal differentiation | 2 (7.7%) |
| ER status | |
| Negative | 24 (92.3%) |
| Positive | 2 (7.7%) |
| PR status | |
| Negative | 26 (100.0%) |
| Positive | 0 (0.0%) |
| HER-2 status | |
| Negative | 23 (88.5%) |
| Positive | 3 (11.5%) |
| Ki-67 | |
| <30% | 6 (23.1%) |
| 15%-30% | 3 (11.5%) |
| >30% | 17 (65.4%) |
The histologic subtypes of the metaplastic components varied from a single metaplastic component to mixed components, consisting of any degree of squamous, chondroid, spindled, and sarcomatoid elements. (Figure 1) Among all the patients, spindle cell carcinoma was the most common histological subtype (13/26, 50%), followed by metaplastic carcinoma with squamous cell component (8/26, 30.8%), the third was fibromatosis-like metaplastic carcinoma (3/26, 11.5%), and the fourth was carcinoma with chondroid mesenchymal differentiation (2/26, 7.7%). There was no low-grade adenosquamous carcinoma in our group. Most patients (17/26, 65.4%) had an associated conventional invasive carcinomas of no special type component in association with the metaplastic component.
Figure 1.
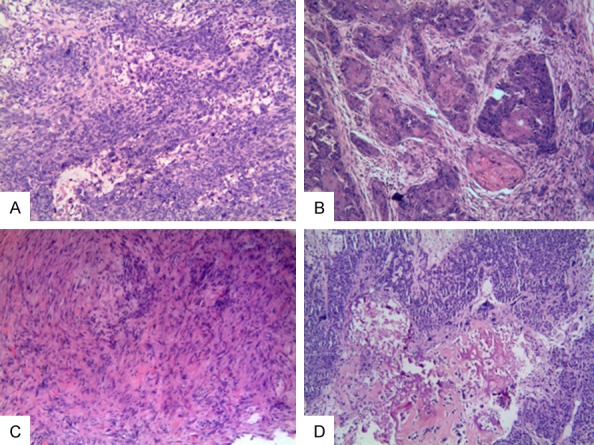
A: Microscopic examination revealed tumor composed of malignant spindle cells and sarcomatoid elements. B: Microscopic examination revealed tumor composed of squamous cell component. C: Microscopic examination revealed low-grade fibromatosis-like spindle cells and displayed tapering nuclei with mild anisonucleosis. D: Microscopic examination revealed tumor cells in a background of osseous stromal matrix with spindle component. (magnification, × 100) (Hematoxylin and Eosin Stained).
Treatment modalities
All patients received surgical treatment. The most common surgical procedure was modified radical mastectomy (MRM), which was performed in 24 patients. The other 2 cases received segmental mastectomy and sentinel lymph node biopsy. All cases had received chemotherapy, 14 cases had received radiotherapy, and 2 cases with positive ER expression had received adjuvant hormonal therapy. Only 1 patient in 3 cases with HER-2 positive expression had received targeted treatment.
Outcome, recurrence and prognosis
Up to the cut-off date, 14 patients were still alive without recurrence. Twelve patients experienced locoregional recurrence, distant metastasis, in which 10 patients died due to disease progression. Among the 12 cases with local recurrence and distant metastasis, 4 cases of local chest wall recurrence; 2 cases of contralateral breast metastasis. The most common organs involved were the lung (n=5), liver (n=2), bone (n=1), supraclavicular LNs (n=1) and brain (n=1). The 5-year overall survival rate and 5-year disease-free survival rate were 61.5% and 53.8% respectively (Figure 2).
Figure 2.
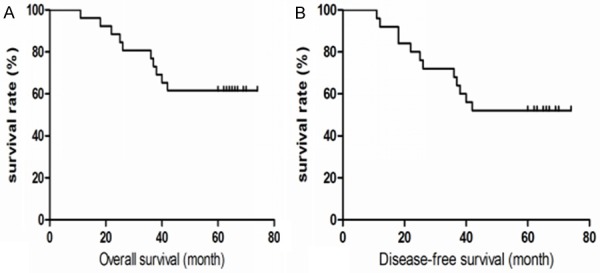
Overall survival curves (A) and Disease-free survival curves (B) of 26 MBC patients.
Discussion
MBC was first described in 1973 by Huvos et al. and was defined as a mammary carcinoma with mixed epithelial and sarcomatoid components [4]. The histologic classification of MBC was primarily based on the morphologic findings of tumor cell types: purely epithelial (squamous, adenosquamous and spindle cell carcinomas) or mixed epithelial and mesenchymal (carcinoma with chondroid/osseous metaplasia and carcinosarcoma) components [5]. It may arise with or without an accompanying conventional in situ or invasive mammary carcinoma. Pathological assessment remains the gold standard for diagnosing MBC. Understanding of MBC is relatively late and it has different pathological subtypes, so there are no specific guidelines for diagnosis and treatment of MBC at present.
MBC is a very rare form of breast tumor and its incidence is <1% of all breast malignancies [6]. Patients with MBC were most commonly found to be older, with tumors of larger size and more advanced stage, they usually tested triple negative [7]. In our series, the incidence rate was 0.29%, which was compatible with the literature. The median age was 57 and the median tumor size was 3.5 cm. 92.3% of patients tested negative for ER, PR negative in 100.0% patients and HER-2 negative in 88.5% patients. As previously reported, MBC usually presents as a palpable mass that grows rapidly [8] and axillary LN metastases in 22.0-31.0% of patients [9]. Despite this low rate, MBC patients with LN metastasis had a greater risk of developing metastatic disease and a poorer prognosis than IDC patients [10]. In the current study, all patients were presented with a palpable, painless lump at stage T2-4 and 34.6% of patients had axillary LN metastases, which was consistent with the majority of previous reports. Because MBC patients with large tumor size, LN positivity had a poor survival outcome, early diagnosis, and treatment is critical to patient prognosis.
The presence of certain metaplastic elements has been associated with varying prognoses. For example, the presence of high-grade spindled or pleomorphic components is associated with aggressive behavior such as metastases with a worse prognosis [11] whereas the low-grade, fibromatosis-like metaplastic carcinomas (FLMC) with bland spindled cells have a high risk of local recurrence but minimal risk of metastatic spread [12]. There were three patients with the low-grade, fibromatosis-like histologic subtype in our series, where a chest wall mass appeared in one case, and pathology prompted tumor recurrence but no distant metastasis. The other two patients were good until now. In contrast, the ten patients who died were pathologically high-grade cases. An important factor in determining the patient prognosis is the type and spread of the metaplastic component [13]. However, there is currently no widely accepted prognostic value to either metaplastic subtype or histologic grading in MBC.
As MBC patients typically present with large tumors, >70% of patients with MBC present with AJCC stage II [14] and basically all the patients with MBC receive mastectomy rather than lumpectomy [15]. In our study, 24 cases were received MRM and 2 cases received segmental mastectomy and sentinel lymph node biopsy. Although the majority of previous studies have revealed an ineffective response of MBC to chemotherapy compared with the response rates of stage-matched female patients with IDC [16] and those with MBC receiving chemotherapy had lower response rates to the chemotherapy regimens [17]. We still use chemotherapy for all cases follow the treatment paradigm for conventional IDC. But other studies have shown that among the different chemotherapy regimens, CMF and cisplatin-based regimens may be effective to certain subgroups of MBC patients. These findings would suggest that a subset of MBC patients may potentially experience a curative benefit from systemic chemotherapy [18].
In patients undergoing mastectomy, radiotherapy (RT) is recommended for those with 4 or more lymph node metastases and tumors larger than 5 cm [19]. Another study suggested that RT, regardless of the type of surgery, should be considered as a part of the therapy for patients with MBC [20]. Tseng and Martinez [21] described patients with MBC who had received RT and experienced a benefit in terms of OS and DFS. In our study, 14 cases had received radiotherapy. Because the present study is a small sample retrospective analysis, we cannot compare the differences between the risk of recurrence for those patients treated with adjuvant RT and not treated with RT. We need to further accumulate samples to clarify the impact of radiotherapy on the prognosis of patients.
Due to the high incidence of triple-negative in MBC, most patients cannot benefit from hormonal therapy and HER-2-targeted treatment [22]. In our group, only 2 cases with positive ER expression had received adjuvant hormonal therapy. The tumors in 3 patients were HER-2 positive, however, only 1 patient was treated with trastuzumab due to financial reasons.
In conclusion, MBC is a rare and heterogeneous breast cancer which can be subcategorized mainly based on the pathologic findings and considered to be clinically aggressive. The 26 patients in our group were of older age with large size tumors, they had high incidence of triple-negative and their histologic stage were moderate to high. In clinical therapy we followed the treatment paradigm for conventional IDC at present. This study is only a singlecenter, retrospective study with a small number of cases. Therefore, prospective multi-center wide-scale studies should be carried out in the future to confirm the clinicopathological features and prognosis.
Acknowledgements
We would like to thank Editage for English language editing. Thanks to Jun Qian and Qun Xie (Department of Surgical Oncology and Department of Pathology, the First Affiliated Hospital of Bengbu Medical College) for their assistance with the clinical information and histopathological evaluations. This study was supported by the Natural Science Foundation of Anhui Province (No. 1608085-QH207), Key Supporting Project of Prominent Youth in Universities of Anhui (No. gxyqZD20-16161) and the Natural Science Foundation of Bengbu Medical College (No. BYKF1712, No. BYKF1707).
Disclosure of conflict of interest
None.
References
- 1.Al Sayed AD, El Weshi AN, Tulbah AM, Rahal MM, Ezzat AA. Metaplastic carcinoma of the breast clinical presentation, treatment results and prognostic factors. Acta Oncol. 2006;45:188–195. doi: 10.1080/02841860500513235. [DOI] [PubMed] [Google Scholar]
- 2.Fayaz S, Demian GA, Eissa HE, Amanguno H, Abuzalouf S. Metaplastic breast carcinoma: Analysis of 31 cases from a single institute. J Egypt Natl Canc Inst. 2017;29:141–145. doi: 10.1016/j.jnci.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, Van De Vijer MJ, editors. WHO classification of tumours of the breast. Lyon: IACR Press; 2012. [Google Scholar]
- 4.McKinnon E, Xiao P. Metaplastic carcinoma of the breast. Arch Pathol Lab Med. 2015;139:819–822. doi: 10.5858/arpa.2013-0358-RS. [DOI] [PubMed] [Google Scholar]
- 5.Cimino-Mathews A, Verma S, Figueroa-Magalhaes MC, Jeter SC, Zhang Z, Argani P, Stearns V, Connolly RM. A clinicopathologic analysis of 45 patients with metaplastic breast carcinoma. Am J Clin Pathol. 2016;145:365–372. doi: 10.1093/ajcp/aqv097. [DOI] [PubMed] [Google Scholar]
- 6.Beatty JD, Atwood M, Tickman R, Reiner M. Metaplastic breast cancer: clinical significance. Am J Surg. 2006;191:657–664. doi: 10.1016/j.amjsurg.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 7.Lai HW, Tseng LM, Chang TW, Kuo YL, Hsieh CM, Chen ST, Kuo SJ, Su CC, Chen DR. The prognostic significance of metaplastic carcinoma of the breast (MCB)-a case controlled comparison study with infiltrating ductal carcinoma. Breast. 2013;22:968–973. doi: 10.1016/j.breast.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Lee H, Jung SY, Ro JY, Kwon Y, Sohn JH, Park IH, Lee KS, Lee S, Kim SW, Kang HS, Ko KL, Ro J. Metaplastic breast cancer: clinicopathological features and its prognosis. J Clin Pathol. 2012;65:441–446. doi: 10.1136/jclinpath-2011-200586. [DOI] [PubMed] [Google Scholar]
- 9.Park HS, Park S, Kim JH, Lee JH, Choi SY, Park BW, Lee KS. Clinicopathologic features and outcomes of metaplastic breast carcinoma: comparison with invasive ductal carcinoma of the breast. Yonsei Med J. 2010;51:864–869. doi: 10.3349/ymj.2010.51.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bae SY, Lee SK, Koo MY, Hur SM, Choi MY, Cho DH, Kim S, Choe JH, Lee JE, Kim JH, Kim JS, Nam SJ, Yang JH. The prognoses of metaplastic breast cancer patients compared to those of triple-negative breast cancer patients. Breast Cancer Res Treat. 2011;126:471–478. doi: 10.1007/s10549-011-1359-8. [DOI] [PubMed] [Google Scholar]
- 11.Carter MR, Hornick JL, Lester S, Fletcher CD. Spindle cell (sarcomatoid) carcinoma of the breast: a clinicopathologic and immunohistochemical analysis of 29 cases. Am J Surg Pathol. 2006;30:300–309. doi: 10.1097/01.pas.0000184809.27735.a1. [DOI] [PubMed] [Google Scholar]
- 12.Nonnis R, Paliogiannis P, Giangrande D, Marras V, Trignano M. Low-grade fibromatosis-like spindle cell metaplastic carcinoma of the breast: a case report and literature review. Clin Breast Cancer. 2012;12:147–150. doi: 10.1016/j.clbc.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Dave G, Cosmatos H, Do T, Lodin K, Varshney D. Metaplastic carcinoma of the breast: a retrospective review. Int J Radiat Oncol Biol Phys. 2006;64:771–775. doi: 10.1016/j.ijrobp.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 14.Tse GM, Tan PH, Putti TC, Lui PC, Chaiwun B, Law BK. Metaplastic carcinoma of the breast: a clinicopathological review. J Clin Pathol. 2006;59:1079–1083. doi: 10.1136/jcp.2005.030536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson GR, Qian D, Ku JK, Lai LL. Metaplastic breast cancer: clinical features and outcomes. Am Surg. 2005;71:725–730. [PubMed] [Google Scholar]
- 16.Moulder S, Moroney J, Helgason T, Wheler J, Booser D, Albarracin C, Morrow PK, Koenig K, Kurzrock R. Responses to liposomal doxorubicin, bevacizumab, and temsiro-limus in metaplastic carcinoma of the breast: biologic rationale and implications for stem-cell research in breast cancer. J. Clin. Oncol. 2011;29:e572–575. doi: 10.1200/JCO.2010.34.0604. [DOI] [PubMed] [Google Scholar]
- 17.Chen IC, Lin CH, Huang CS, Lien HC, Hsu C, Kuo WH, Lu YS, Cheng AL. Lack of efficacy to systemic chemotherapy for treatment of metaplastic carcinoma of the breast in the modern era. Breast Cancer Res Treat. 2011;130:345–351. doi: 10.1007/s10549-011-1686-9. [DOI] [PubMed] [Google Scholar]
- 18.Hennessy BT, Giordano S, Broglio K, Duan Z, Trent J, Buchholz TA, Babiera G, Hortobagyi GN, Valero V. Biphasic metaplastic sarcomatoid carcinoma of the breast. Ann Oncol. 2006;17:605–613. doi: 10.1093/annonc/mdl006. [DOI] [PubMed] [Google Scholar]
- 19.Weigelt B, Ng CK, Shen R, Popova T, Schizas M, Natrajan R, Mariani O, Stern MH, Norton L, Vincent-Salomon A, Reis-Filho JS. Metaplastic breast carcinomas display genomic and transcriptomic heterogeneity [corrected] . Mod Pathol. 2015;28:340–351. doi: 10.1038/modpathol.2014.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luini A, Aguilar M, Gatti G, Fasani R, Botteri E, Brito JA, Maisonneuve P, Vento AR, Viale G. Metaplastic carcinoma of the breast, an unusual disease with worse prognosis: the experience of the European institute of oncology and review of the literature. Breast Cancer Res Treat. 2007;101:349–353. doi: 10.1007/s10549-006-9301-1. [DOI] [PubMed] [Google Scholar]
- 21.Tseng WH, Martinez SR. Metaplastic breast cancer: to radiate or not to radiate? Ann Surg Oncol. 2011;18:94–103. doi: 10.1245/s10434-010-1198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim KH, Oh DY, Chie EK, Han W, Im SA, Kim TY, Park IA, Noh DY, Ha SW, Bang YJ. Metaplastic breast carcinoma: clinicopathologic features and prognostic value of triple negativity. Jpn J Clin Oncol. 2010;40:112–118. doi: 10.1093/jjco/hyp139. [DOI] [PubMed] [Google Scholar]


