Abstract
Angiosarcoma of soft tissue is a group of aggressive malignancies with high mortality. However, molecular pathogenesis and therapeutic targets of angiosarcoma remain to be established. We explored the influence of M2-polarized tumor-associated macrophages (TAMs) on the formation of angiosarcoma. CD163+/CD68+ macrophages were determined by immunohistochemistry from a series of 38 samples, including 17 cases of angiosarcoma with lymphedema and 21 cases of lymphangioma. The number of CD163+/CD68+ macrophages in angiosarcoma was significantly higher than that in lymphangioma. VEGFc was universally expressed in both angiosarcoma tumor cells and CD163+/CD68+ macrophages. VEGFR3 was expressed only in angiosarcoma tumor cells. Our study indicates a potential role of TAMs in the development of angiosarcoma with lymphedema. The VEGF signaling pathway may thus serve as a potential target for treatment of angiosarcoma.
Keywords: CD68, CD163, angiosarcoma, lymphedema, tumor-associated macrophages (TAMs), VEGF
Introduction
Traditionally, malignant tumors of vascular origin (angiosarcomas) have been divided into hemangiosarcomas and lymphangiosarcomas on the basis of morphological criteria, which suggest blood or lymphatic origin of malignant endothelial cells. Studies have shown that malignant vascular tumors express mixed immunophenotypes of both lymphatic and blood endothelium [1,2]. Therefore the general term angiosarcoma is more accurate in describing lymphangiosarcomatous vs. hemangiosarcomatous origin [3,4].
Angiosarcoma of soft tissue is a very aggressive malignancy with high mortality [5]. However, the pathogenesis of angiosarcoma remains mysterious.
Malignant tumors are complex structures interacting with micro-environment for growth and invasiveness. However, the molecular mechanism in the tumor-associated immune microenvironment that drives invasion and metastasis of angiosarcoma has not been well established. These observations underscore an urgent need to identify new biomarkers with the potential to predict tumor development and progression, enable diagnosis at earlier stages of the disease, and facilitate early detection of disease recurrence or metastasis after treatment.
Macrophages are critical immune effector cells as one of the major components of tumor-infiltrating leukocytes. These cells play a key role, in carcinogenesis [6]. Macrophages that infiltrate and surround the tumor nest are defined as tumor-associated macrophages (TAMs) [7]. TAMs interact with neoplastic cells by releasing various cytokines which contribute to cancer initiation and progression. Emerging findings suggest that increased numbers of TAMs in various types of carcinomas are associated with a poor prognosis [8-10]. There are several known functional markers of TAMs. The presence of CD163 is a key factor to distinguish different TAMs. CD163, a member of the scavenger receptor cysteine-rich family, is involved in anti-inflammatory functions and predominantly expressed on M2 macrophages [11]. Accumulating evidence indicates that a high number of TAMs, as demonstrated by exclusive immunohistochemistry (IHC) with antibodies against CD163, are associated with an unfavorable prognosis in a variety of malignancies [12-14]. However, CD68, the well-established generic macrophage marker, could not distinguish M1 or M2 subtypes from other infiltrated macrophages [15]. In this study, clinical significance of macrophages in angiosarcoma was evaluated with CD163 and CD68, and the association between the number of CD163+/CD68+ macrophages and VEGF was analyzed.
Materials and methods
Tissue specimen
This retrospective study was approved by the Ethics Committee of Beijing Shijitan Hospital, Capital Medical University. Signed informed consent was obtained from each patient. Surgical tissue specimens of angiosarcoma (17 cases) and lymphangioma (21 cases) were randomly collected. Diagnosis of angiosarcoma and lymphangioma tissue was confirmed by histopathological examination.
Formalin-fixed paraffin-embedded (FFPE) specimens were used to prepare a series of tissue sections for immunohistochemical staining.
Immunohistochemistry
Primary and secondary antibodies were purchased from Zhongshan Biotechnology, China. Four-micron sections were incubated with the following primary antibodies: monoclonal mouse anti-human CD68 (KP1, 1:150), CD163 (10D6, 1:150), VEGFR3 (KLT9, 1:30), and polyclonal antibody rabbit anti-human VEGFc (1:75). The secondary antibody reagent kit PV-8000 was applied. The experimental steps were carried out according to the manufacturer’s instructions.
Histopathologic examination
The intensity of immune infiltrates was assigned a semi-quantitative score from 0-3 (16) as follows: 0 = “none” (no immune infiltrates), 1 = “focal” (mostly perivascular in tumor with some intratumoral extension), 2 = “moderate” (prominent extension of immune infiltrates away from perivascular areas and amongst tumor cells), or 3 = “severe” (immune infiltrates obscuring tumor).
The staining intensities of VEGFc in both tumor cells and macrophage, as well as VEGFR3 in tumor cells were scored based on the following criteria (17): “0” represents no staining or faint staining intensity in 10% cells; “1+” represents faint staining in >10% of cells; “2+” represents moderate staining in >10% of cells; “3+” represents strong staining in >10% of cells. The tissue specimen was considered positive for VEGFc or VEGFR3 when the staining intensity score was 1+, 2+, or 3+, and negative when the score was 0.
Statistical methods
Statistical data were analyzed using SPSS version 20.0 software. Associations between tumor types and different biomarkers were examined by χ2-test (2-sided). The significance level was set at a P<0.05.
Results
Clinicopathologic characteristics of angiosarcoma with lymphedema
This study was conducted in a cohort of 17 patients diagnosed angiosarcoma with lymphedema (Table 1) and 21 patients diagnosed lymphangioma (Table 2).
Table 1.
Clinicopathologic features of patients with angiosarcoma
| ID | Gender | Age (years) | Histo-pathological diagnosis |
|---|---|---|---|
| 1 | Female | 21 | (Left lower extremities) angiosarcoma, FNCLCC, grade 1 |
| 2 | Male | 23 | (Left hand) angiosarcoma, FNCLCC, grade 2 |
| 3 | Female | 60 | (Left upper limb) angiosarcoma, FNCLCC, grade 3 |
| 4 | Female | 52 | (Left chest wall) angiosarcoma, FNCLCC, grade 2 |
| 5 | Female | 66 | (Right foot) angiosarcoma, FNCLCC, grade 2 |
| 6 | Female | 38 | (Right calf) angiosarcoma, FNCLCC, grade 2 |
| 7 | Male | 39 | (Left calf) angiosarcoma, FNCLCC, grade 2 |
| 8 | Female | 65 | (Right upper limb) angiosarcoma, FNCLCC, grade 2 |
| 9 | Female | 51 | (Right upper arm) angiosarcoma, FNCLCC, grade 1 |
| 10 | Female | 62 | (Left shoulder and back) angiosarcoma, FNCLCC, grade 2 |
| 11 | Female | 54 | (Right arm) angiosarcoma, FNCLCC, grade 2 |
| 12 | Male | 63 | (Right lower extremities) angiosarcoma, FNCLCC, grade 2 |
| 13 | Female | 15 | (Left ankle) angiosarcoma, FNCLCC, grade 1 |
| 14 | Female | 65 | (Left calf) angiosarcoma, FNCLCC, grade 1 |
| 15 | Female | 43 | (Left upper arm) angiosarcoma, FNCLCC, grade 3 |
| 16 | Female | 47 | (Left lower extremities) angiosarcoma, FNCLCC, grade 2 |
| 17 | Female | 58 | (Left calf) angiosarcoma, FNCLCC, grade 2 |
Table 2.
Clinicopathologic features of patients with lymphangioma
| ID | Gender | Age (years) | Histo-pathological diagnosis |
|---|---|---|---|
| 1 | Male | 1 | (Left and right pleural) lymphangioma |
| 2 | Female | 62 | (Thymus) lymphangioma |
| 3 | Male | 40 | (Left axilla) cystic lymphangioma |
| 4 | Female | 14 | (Right neck) lymphangioma |
| 5 | Male | 37 | (The penis) lymphangioma |
| 6 | Male | 10 | (Right thigh) lymphangioma |
| 7 | Female | 14 | (Left neck) lymphangioma |
| 8 | Female | 16 | (Left neck) lymphangioma |
| 9 | Male | 47 | (Left neck) lymphangioma |
| 10 | Female | 43 | (Thigh) lymphangioma |
| 11 | Male | 12 | (Retroperitoneal) lymphangioma |
| 12 | Female | 49 | (Right supraclavicular) lymphangioma |
| 13 | Male | 50 | (Left neck) lymphangioma |
| 14 | Female | 48 | (Left neck) lymphangioma |
| 15 | Male | 36 | (Thigh) lymphangioma |
| 16 | Male | 62 | (Neck and supraclavicular) lymphangioma |
| 17 | Male | 9 | (Right upper limb) lymphangioma |
| 18 | Male | 26 | (Left and right perididymis ) lymphangioma |
| 19 | Female | 33 | (Left thigh) lymphangioma |
| 20 | Male | 14 | (Right axilla ) lymphangioma |
| 21 | Female | 35 | (Left neck) lymphangioma |
The patients diagnosed angiosarcoma with lymphedema age ranging from 15 to 66 years with a median age of 48.4 years. The lesion located in the extremities with primary or secondary lymphedema. Four, 11, and 2 patients, respectively, were diagnosed with Federation Nationale des Centers de Lutte Contre le Cancer (FNCLCC), grade 1, grade 2, grade 3 angiosarcoma. The patient diagnosed lymphangioma age ranging from 1 to 62 years with a median age of 31.3 years. The lesions were located in the extremities, neck, supraclavicular, axilla, pleural, penis, and retroperitoneal.
Upregulation of CD68+ macrophages in angiosarcoma
CD68 was localized within the cytoplasm of the macrophages and exhibited granular, brownish staining in angiosarcoma specimens (Figure 1A). There were no or very few CD68+ macrophages in the lymphangioma (Figure 1A). The levels of total CD68+ macrophages in angiosarcoma tissues were significantly higher than those in the lymphangioma tissue (P<0.05, Table 3).
Figure 1.
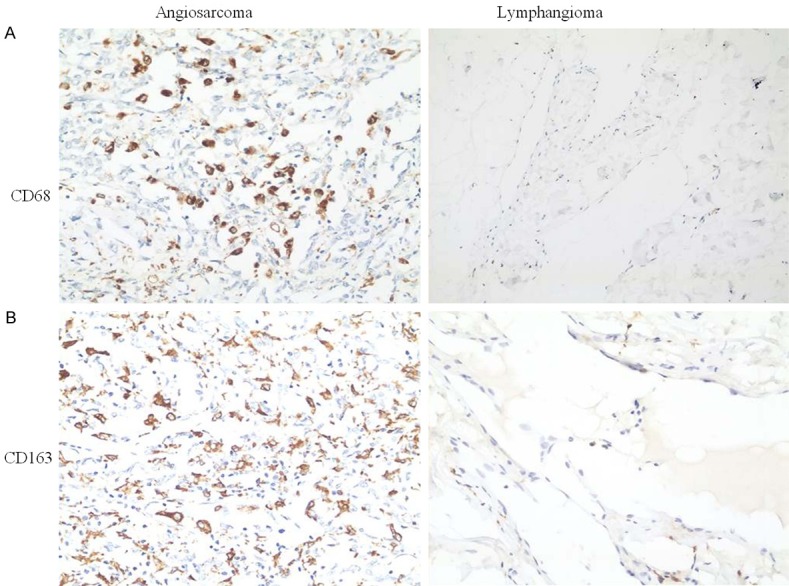
Immunohistochemical staining of CD68 and CD163 in patients with angiosarcoma and lymphangioma. A. Immunohistochemical staining with anti-CD68 for tissue specimens of angiosarcoma and lymphangioma. B. Expression levels of CD163 in angiosarcoma and lymphangioma.
Table 3.
Expression of CD68 and CD163 in patients with angiosarcoma and lymphangioma
| Disease | Patients (n) | CD68 score | P | CD163 score | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| 3 | 2 | 1 | 0 | 3 | 2 | 1 | 0 | ||||
| Angiosarcoma | 17 | 2 | 8 | 7 | 0 | 5 | 10 | 2 | 0 | ||
| Lymphangioma | 21 | 0 | 2 | 13 | 6 | 0.001 | 0 | 7 | 14 | 0 | 0.00065 |
Upregulation of CD163+ macrophages in angiosarcoma
CD163 immunoreactivity was characterized by a granular brownish pattern and membrane staining (Figure 1B). The expression level of CD163 was significantly higher in angiosarcoma than in lymphangioma (P<0.05, Table 3).
Expression of VEGFc or VEGFR3 in angiosarcoma
VEGFc immunoreactivity was localized within the cytoplasm of the macrophages and tumor cells in angiosarcoma (Figure 2A). VEGFR3 immunoreactivity was localized within the cytoplasm of the tumor cells in angiosarcoma (Figure 2B).
Figure 2.
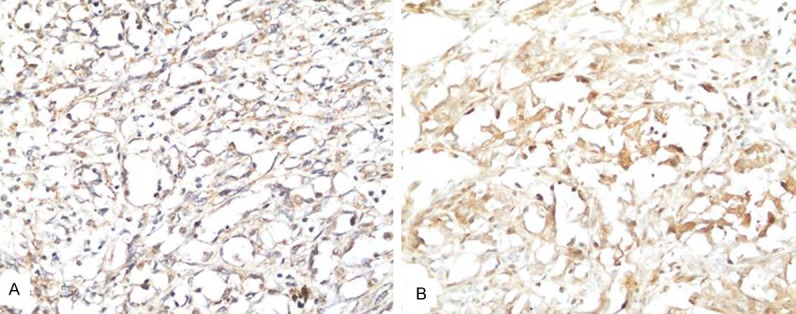
Immunohistochemical staining of VEGF-C and VEGFR3 in angiosarcoma. A. VEGF-C immunoreactivity was localized within the cytoplasm of the macrophages and tumor cells. B. VEGFR3 immunoreactivity was localized within the cytoplasm of the tumor cells.
Discussion
Angiosarcoma represents less than 1% of all sarcomas. This disease can be either primary or secondary to chronic lymphoedema with cytogenetic differences between these two forms [18].
Lymphedema-associated cutaneous angiosarcoma was first described in 1948 by Stewart and Treves, also known as Stewart-Treves syndrome. This type of tumor develops on the lymphedematous limb or chest wall after mastectomy and axillary lymph node dissection [19]. Previous reports have described angiosarcoma development in patients with lymphedema secondary to congenital lymphedema, lymph node dissection, filarial infection, and chronic idiopathic lymphedema [20]. In the presence of lymphoedema, angiosarcoma can grow as plaques or cutaneous and subcutaneous nodules, single or multiple, which may coalesce, with an unknown etiology. The infrequent occurrence of this disease and the innocuous appearance of the tumor lead to delays in diagnosis and treatment. In addition, precise mechanisms for the development of angiosarcoma on the basis of lymphedema are unknown.
Persuasive evidence from clinical and preclinical studies demonstrated that macrophages could promote cancer initiation, progression, and metastasis. Tumor associated macrophages (TAMs) influence tumor progression to different extents depending on tumor types [21]. Macrophages invade massively osteosarcoma tissues [22-24] and establish an immune-tolerant environment during tumor growth [23,25].
Our study identified a large number of CD68+/CD163+ macrophages in angiosarcoma with lymphedema. While in lymphangioma, there were no or very few CD68+/CD163+ macrophages. These results suggested a critical role for CD68+/CD163+ macrophages in development of angiosarcoma with lymphedema.
Lymphatic injury may contribute to excessive production of proangiogenic cytokines through vascular endothelial growth factor (VEGF) signaling pathway. Indeed, VEGF is overexpressed in most angiosarcomas [26]. VEGF-C-expressing TAMs are involved in peritumoral lymphangiogenesis and subsequent dissemination in human cancer [27].
Our study found that both CD68+/CD163+ macrophages and tumor cells highly expressed VEGF-C in patients with angiosarcoma. Tumor cells also highly expressed VEGFR3 in angiosarcoma. These results indicated that VEGF-C/VEGFR3 signal pathway might promote the development and progression of angiosarcoma.
In conclusion, our study demonstrates a positive association between expression of CD68+/CD163+ macrophages and carcinogenesis of angiosarcomas.
Acknowledgements
We thank Mr. Yongqi Chen (Department of Pathology in Beijing Aerospace General Hospital) for English editing for this paper.
Disclosure of conflict of interest
None.
References
- 1.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Itakura E, Yamamoto H, Oda Y, Tsuneyoshi M. Detection and characterization of vascular endothelial growth factors and their receptors in angiosarcomas. J Surg Oncol. 2008;97:74–81. doi: 10.1002/jso.20766. [DOI] [PubMed] [Google Scholar]
- 3.Banerji S, Ni V, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson DG, Prevo R, Clasper S, Banerji S. LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends Immunol. 2001;22:317–321. doi: 10.1016/s1471-4906(01)01936-6. [DOI] [PubMed] [Google Scholar]
- 5.Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol. 1998;22:683–697. doi: 10.1097/00000478-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Condeelis J, Pollard JW. Macrophages obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Wang YC, He F, Feng F, Liu XW, Dong GY, Qin HY, Hu XB, Zheng MH, Liang L, Feng L, Liang YM, Han H. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res. 2010;70:4840–4849. doi: 10.1158/0008-5472.CAN-10-0269. [DOI] [PubMed] [Google Scholar]
- 8.Medrek C, Ponten F, Jirstrom K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi: 10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, Bast MA, Rosenwald A, Muller-Hermelink HK, Rimsza LM, Campo E, Delabie J, Braziel RM, Cook JR, Tubbs RR, Jaffe ES, Lenz G, Connors JM, Staudt LM, Chan WC, Gascoyne RD. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362:875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27:462–472. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen TT, Schwartz EJ, West RB, Warnke RA, Arber DA, Natkunam Y. Expression of CD163 (hemoglobin scavenger receptor) in normal tissues, lymphomas, carcinomas, and sarcomas is largely restricted to the monocyte/macrophage lineage. Am J Surg Pathol. 2005;29:617–624. doi: 10.1097/01.pas.0000157940.80538.ec. [DOI] [PubMed] [Google Scholar]
- 12.Jensen TO, Schmidt H, Moller HJ, Høyer M, Maniecki MB, Sjoegren P, Christensen IJ, Steiniche T. Macrophage markers in serum and tumor have prognostic impact in American joint committee on cancer stage I/II melanoma. J. Clin. Oncol. 2009;27:3330–3337. doi: 10.1200/JCO.2008.19.9919. [DOI] [PubMed] [Google Scholar]
- 13.Komohara Y, Hasita H, Ohnishi K, Fujiwara Y, Suzu S, Eto M, Takeya M. Macrophage infiltration and its prognostic relevance in clear cell renal cell carcinoma. Cancer Sci. 2011;102:1424–1431. doi: 10.1111/j.1349-7006.2011.01945.x. [DOI] [PubMed] [Google Scholar]
- 14.Lima L, Oliveira D, Tavares A, Amaro T, Cruz R, Oliveira MJ, Ferreira JA, Santos L. The predominance of M2-polarized macrophages in the stroma of low-hypoxic bladder tumors is associated with BCG immunotherapy failure. Urol Oncol. 2014;32:449–457. doi: 10.1016/j.urolonc.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Falini B, Flenghi L, Pileri S, Gambacorta M, Bigerna B, Durkop H, Eitelbach F, Thiele J, Pacini R, Cavaliere A, Martelli M, Cardarelli N, Sabattini E, Poggi S, Stein H. PG-M1: a new monoclonal antibody directed against a fixative-resistant epitope on the macrophage-restricted form of the CD68 molecule. Am J Pathol. 1993;142:1359–1372. [PMC free article] [PubMed] [Google Scholar]
- 16.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu X, Bai Q, Lu Y, Lu Y, Zhu L, Zhou X, Wu L. Expression and function of CXCL12/CXCR4/CXCR7 in thyroid cancer. Int J Oncol. 2016;48:2321–2329. doi: 10.3892/ijo.2016.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison WD, Chandrasekar CR. Stewart-Treves syndrome following idiopathic leg lymphoedema: remember sarcoma. J Wound Care. 2015;24(Suppl):S5–S7. doi: 10.12968/jowc.2015.24.Sup6.S5. [DOI] [PubMed] [Google Scholar]
- 19.Cui L, Zhang J, Zhang X, Chang H, Qu C, Zhang J, Zhong D. Angiosarcoma (Stewart-Treves syndrome) in postmastectomy patients: report of 10 cases and review of literature. Int J Clin Exp Pathol. 2015;8:11108–11115. [PMC free article] [PubMed] [Google Scholar]
- 20.Requena L, Sangueza OP. Cutaneous vascular proliferations. Part III. Malignant neoplasms, other cutaneous neoplasms with significant vascular component, and disorders erroneously considered as vascular neoplasms. J Am Acad Dermatol. 1998;38:143–175. doi: 10.1016/s0190-9622(98)70237-3. [DOI] [PubMed] [Google Scholar]
- 21.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 22.Liu T, Fang XC, Ding Z, Sun ZG, Sun LM, Wang YL. Pre-operative lymphocyte-to-monocyte ratio as a predictor of overall survival in patients suffering from osteosarcoma. FEBS Open Biol. 2015;5:682–687. doi: 10.1016/j.fob.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inagaki Y, Hookway E, Williams KA, Hassan AB, Oppermann U, Tanaka Y, Soilleux E, Athanasou NA. Dendritic and mast cell involvement in the inflammatory response to primary malignant bone tumours. Clin Sarcoma Res. 2016;6:13. doi: 10.1186/s13569-016-0053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;14:139–151. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Itakura E, Yamamoto H, Oda Y, Tsuneyoshi M. Detection and characterization of vascular endothelial growth factors and their receptors in a series of angiosarcomas. J Surg Oncol. 2008;97:74–81. doi: 10.1002/jso.20766. [DOI] [PubMed] [Google Scholar]
- 27.Schoppmann SF, Birner P, Stöckl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-Associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


