Abstract
Cancer stem cells (CSCs) are responsible for cancer recurrence and metastasis and are related to poor prognosis in patients with hepatocellular carcinoma (HCC). CD133 is one of the most commonly used CSC markers. In this study, expression and the biological significance of CSC marker CD133 was evaluated in HCC, at mRNA and protein levels. We demonstrate that both mRNA and protein levels of CD133 are significantly elevated in HCC relative to that in adjacent non-cancerous tissue based on bioinformatics and immunohistochemical analysis, respectively (P < 0.01). Intriguingly, we detected nuclear distribution of CD133 and found that nuclear CD133 expression was indicative of poor patient prognosis (median survival 12 months versus 34.5 months) (Log-Rank, P = 0.0258). Meanwhile, our findings suggest that nuclear CD133 expression is positively correlated with tumor size and serves as an independent prognostic factor for HCC after surgical resection (HR = 0.564, 95% CI 0.313-1.018, P = 0.057). Nuclear CD133 expression can potentially serve as a biomarker for clinical diagnosis and prognosis of HCC.
Keywords: CD133, nuclear expression, hepatocellular carcinoma, prognosis
Introduction
Hepatocellular carcinoma (HCC) is currently the second most common cause of cancer-related deaths worldwide [1]. In China, HCC is one of five most commonly diagnosed cancers among men and also the third leading cause of cancer deaths among both men and women [2]. Although several approaches have been applied for treatment of HCC, unsatisfactory clinical outcomes highlight the need for novel indicators of survival as well as the need for reliable therapeutic targets.
Accumulating evidence suggests that cancer stem cells (CSCs) are responsible for recurrence, metastasis, and multidrug resistance of tumors. CSCs also participate in tumorigenesis and affect prognosis of patients. It is well documented that CSCs exist in several different cancers, including HCC. Among commonly used CSC markers, CD133 and CD44 were found to be significantly elevated in HCC compared to peritumoral tissues, indicating a potential prognostic value in HCC patients [3]. Therein, CD133 began gathering increasingly more attention from researchers due to its distinct distribution in cancers [4-6]. CD133 is a pentaspan transmembrane glycoprotein and originally identified in a subpopulation of CD34-positive haematopoietic stem cells [7]. The protein localizes to membrane protrusions and is known to be presented in adult stem cells. CD133 has been used to find CSCs in several cancers such as human colon cancer [8], acute myeloid leukemia [9], and liver cancer [10]. Moreover, many studies have suggested that CD133 contributes to the initiation and growth of HCC and is tightly linked to liver cancer [11].
In our present study, we investigated expression and the biological significance of CD133 in HCC, at mRNA and protein levels. Based on bioinformatics and immunohistochemical analysis, we showed that both mRNA and protein levels of CD133 were upregulated in HCC relative to that in adjacent non-cancerous tissues. Intriguingly, we found that CD133 was not only located on the membrane and in cytoplasm but also in the nucleus of HCC tissue. Nuclear CD133 expression was positively correlated with tumor size and poor patient prognosis in HCC. Additionally, our results demonstrate that nuclear expression is an independent prognostic factor for HCC patients.
Materials and methods
Bioinformatics analysis
RNA-Seq data of Liver Hepatocellular Carcinoma (LIHC) was downloaded from The Cancer Genome Atlas (TCGA) (http://cancergenome.nih.gov/). The data included 424 HCC patients, of which 50 patients were with paired non-cancerous tissues. Clinical information of only 377 patients was available on the website. For statistical analysis, patients with mRNA expression values greater than the median value were added to the high expression group while the rest were classified in low expression group.
Patients and tissues
Overall, 93 HCC and 87 non-tumor subjects with available clinical information and paraffin-embedded blocks were enrolled in this study. All of the patients had undergone surgery as initial treatment. Cancerous and adjacent non-cancerous tissues were collected from patients during surgery. Pathologic diagnosis was carried out by two pathologists, using existing hematoxylin-eosin (H&E) slides. Follow ups ranged from 4 to 6.7 years. Protocols were approved by the Ethical Committee and Institutional Review Board of Traditional Chinese Medicine-Integrated Hospital of Southern Medical University and written informed consent was obtained from each subject. All clinicopathological characteristics including age, gender, tumor stage, presence of hepatic cirrhosis, Edmondson-Steiner grade, tumor number, tumor size, and prognostic information were retrospectively collected from patient medical records. All procedures were performed in accordance with approved protocols.
Immunohistochemistry (IHC)
Tissue sections (4-μm thick) were de-paraffinized and rehydrated in a xylene and alcohol bath solution. Antigen retrieval was then conducted by incubating slides in 0.01 M citrate buffer (pH 6.0). The slides were blocked in 3% hydrogen peroxide for 10 minutes to eliminate endogenous peroxidase activity. Then, slides were incubated for 60 minutes at room temperature, with primary antibodies for CD133 (1:100, Proteintech, USA, 18470-1-AP). Finally, the slides were incubated with a horseradish peroxidase-labeled secondary antibody and counterstained using 3, 3’-diaminobenzidine and hematoxylin.
Evaluation and scoring
IHC sections were scored by two blinded pathologists and any disagreement was resolved by reaching a consensus. A third pathologist was then invited to review scores judged by the two pathologists. Staining was scored according to intensity and the percentage of positively-stained cells. Staining intensity was scored as 0 (no staining), 1 (weakly positive staining), 2 (moderately positive staining), and 3 (strong staining). Percentages were estimated on a scale of 0 to 4, as follows: 0 (0%), 1 (≤ 25%), 2 (26%-50%), 3 (51%-75%), and 4 (76%-100%). Final staining scores were calculated through multiplying the intensity score by the percentage score. For statistical analysis, a score ≤ 8 was regarded as low expression and > 8 as high expression. Tumor cells with more than 10% nuclear staining were judged as positive nuclear expression.
Statistical analysis
All data analyses were performed using SPSS software, version 21.0 (SPSS Inc., USA). For comparison, a nonparametric test was performed to investigate differential expression of each protein between the two groups of patients. Relationships between clinicopathological factors and gene expression were investigated using Pearson’s X2 test. Log-rank tests were performed on Kaplan-Meier survival curves to elucidate any significant relationship between gene expression and overall patient survival. Univariate and multivariate survival analysis was performed using Cox proportional hazards regression model. Univariate regression models were fitted to identify independent factors associated with overall survival and only variables with P < 0.1 were included in multivariate analysis. Hazard ratio (HR) and corresponding 95% confidence intervals (95% CI) were calculated for each factor. All tests were two-sided and P < 0.05 was considered statistically significant.
Results
CD133 mRNA levels are elevated in HCC
To explore the role of CD133 in HCC, we initially assessed its mRNA expression in HCC based on the TCGA LIHC dataset. RNA-seq data from HCC and adjacent non-cancerous tissues showed that CD133 expression was significantly elevated in HCC tissues compared with adjacent non-cancerous tissues (P < 0.001, Figure 1A). In addition, CD133 expression was positively correlated with pathological stage in HCC (P = 0.0458, Figure 1B). Furthermore, gene set enrichment analysis (GSEA), based on the TCGA LIHC dataset, indicated that low CD133 expression was positively correlated with upregulation of liver cancer survival (Figure 1C) and downregulation of liver cancer stem cell (Figure 1D).
Figure 1.
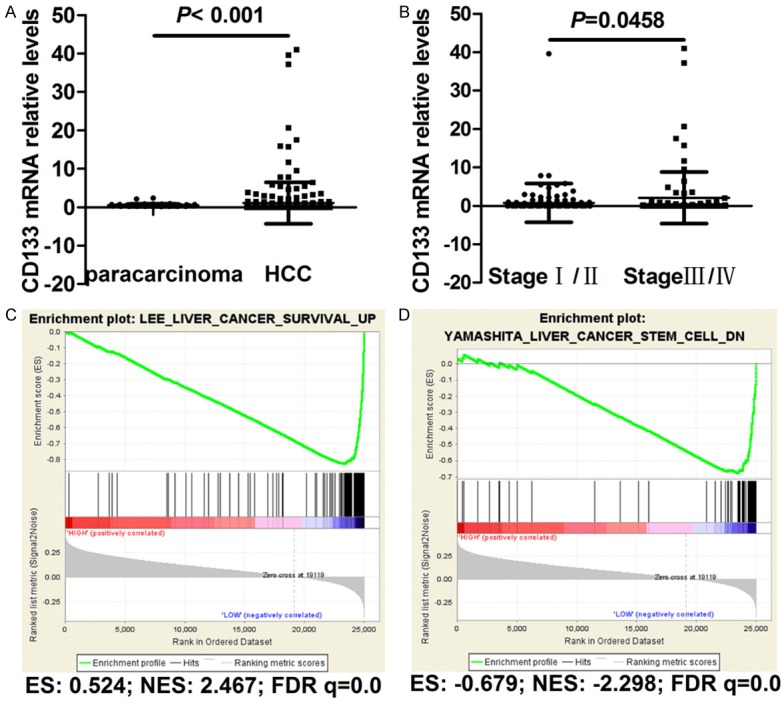
Bioinformatics analysis of CD133 in HCC tissues and adjacent non-cancerous tissue. A. Comparison of CD133 expression between HCC tissues and adjacent non-cancerous tissues. B. Comparison of CD133 expression between Stage I/II and Stage III/IV HCC. C. GSEA analysis of CD133 showing significant enrichment of the gene set involved in the regulation of liver cancer survival based on the TCGA database. D. GSEA analysis of CD133 showing significant enrichment of the gene set involved in the regulation of liver cancer stem cells based on the TCGA database.
Clinicopathological features
Clinicopathological features of the 93 HCC patients are shown in Table 1. Patient ages ranged from 25 to 73 years, with a mean of 54 years. Additionally, 43 out of 93 patients (46.7%) were older than 54 years and most patients (89.2%) were male. Of the total number of HCC patients, 12 were in stage I, 31 in stage II, 40 in stage III, and only 2 in stage IV based on criteria set by the AJCC 8th edition. According to available medical records, only 1 patient had lymph node metastasis and 1 case had distant metastasis.
Table 1.
Correlation between nuclear CD133 expression and clinicopathological features of hepatocellular carcinoma patients
| Characteristics | Tatal | Nuclear CD133 expression | P value | |
|---|---|---|---|---|
|
| ||||
| Low, n (%) | High, n (%) | |||
| Age (years) | ||||
| ≤ Median | 49 | 36 (73.5%) | 13 (26.5%) | 0.882 |
| > Median | 43 | 31 (72.1%) | 12 (27.9%) | |
| Gender | ||||
| Male | 83 | 59 (71.1%) | 24 (28.9%) | 0.188 |
| Female | 10 | 9 (90.0%) | 1 (10.0%) | |
| AJCC stage | ||||
| I-II | 43 | 34 (79.1%) | 9 (20.9%) | 0.130 |
| III-IV | 42 | 27 (33.3%) | 15 (35.7%) | |
| T classification | ||||
| T1-T2 | 43 | 34 (79.1%) | 9 (20.9%) | 0.130 |
| T3-T4 | 42 | 27 (33.3%) | 15 (35.7%) | |
| N classification | ||||
| N0 | 83 | 59 (71.1%) | 24 (28.9%) | 0.714 |
| N1 | 1 | 1 (100.0%) | 0 (0.0%) | |
| Distant metastasis | ||||
| No | 84 | 61 (72.6%) | 23 (27.4%) | 0.282 |
| Yes | 1 | 0 (0.0%) | 1 (100.0%) | |
| Hepatic cirrhosis | ||||
| No | 52 | 38 (73.1%) | 14 (26.9%) | 0.992 |
| Yes | 41 | 30 (73.2%) | 11 (26.8%) | |
| Edmondson-Steiner grade | ||||
| I-II | 61 | 45 (73.8%) | 16 (26.2%) | 0.845 |
| III-IV | 32 | 23 (71.9%) | 9 (28.1%) | |
| Tumor number | ||||
| Single | 24 | 15 (62.5%) | 9 (37.5%) | 0.255 |
| Multiple | 45 | 34 (75.6%) | 11 (24.4%) | |
| Tumor size (cm) | ||||
| ≤ 5 | 42 | 35 (83.3%) | 7 (16.7%) | 0.038 |
| > 5 | 50 | 32 (64.0%) | 18 (36.0%) | |
Correlations between nuclear CD133 expression and clinicopathological characteristics of HCC patients
To verify findings obtained via bioinformatics analysis, we then detected protein levels of CD133 by immunohistochemistry in 93 HCC cases. Analysis suggested that patients exhibited different CD133 levels, indicated by strength of staining (weak to strong; Figure 2A). There were significant differences in CD133 expression between HCC specimens and adjacent non-cancerous tissues (P = 0.002, Table 2; Figure 2B, 2C). Intriguingly, we found that CD133 was not only located on the membrane and in cytoplasm but also in the nucleus of HCC tissue (Figure 2D). Although there was no relationship between nuclear CD133 expression and most patient features such as age, gender, TNM classification, hepatic cirrhosis, Edmondson-Steiner grade, and number of tumors, we showed that high CD133 expression was positively correlated with tumor size (P = 0.038, Table 1).
Figure 2.
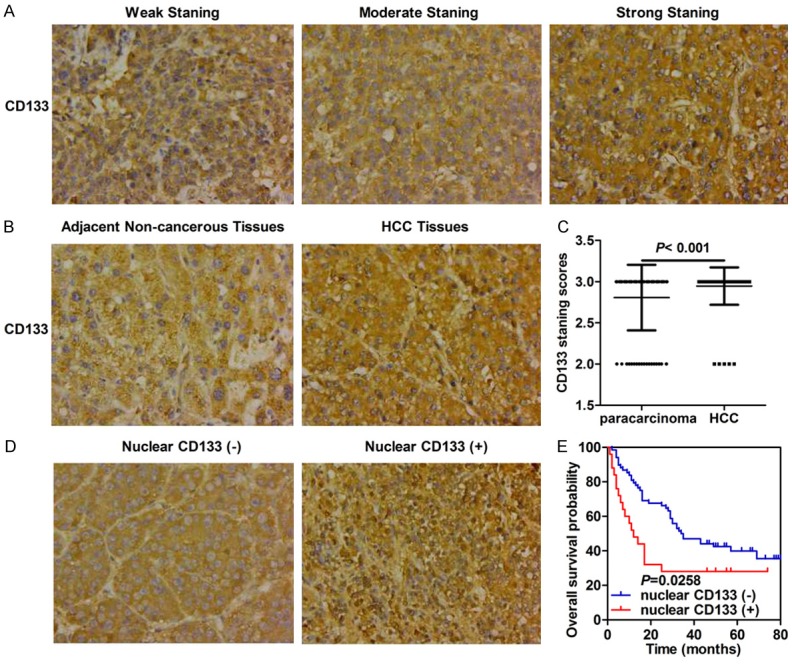
CD133 protein levels are elevated in HCC tissues and its nuclear expression confers poor prognosis for HCC patients. A. Representative images of CD133 staining in HCC (original magnification ×400). B. Representative images of CD133 staining in HCC tissues and adjacent non-cancerous tissues (original magnification ×400). C. Differential expression levels of CD133 in tumor tissues and adjacent non-cancerous tissues. D. Representative images of CD133 staining with and without nuclear distribution in HCC tissues (original magnification ×400). E. Kaplan-Meier survival analysis based on CD133 expression.
Table 2.
Nuclear CD133 expression in HCC tissues and adjacent non-cancerous tissues
| Group | Cases (n) | Nucler CD133 expression | P value | |
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| Hepatocellular carcinoma | 93 | 5 (5.4%) | 88 (94.6%) | 0.002 |
| Para-carcinoma tissue | 87 | 18 (20.7%) | 69 (79.3%) | |
Nuclear CD133 expression is indicative of poor prognosis in HCC
The relationship between CD133 protein levels and overall survival of HCC patients was further explored by survival analysis. However, there was no relationship between CD133 expression and HCC patient prognosis (data not shown). Subsequently, we investigated whether the nuclear location of CD133 was correlated with prognosis in HCC patients and found that nuclear translocation of CD133 was indicative of poor prognosis (median survival 12 months versus 34.5 months) (Log-Rank, P = 0.0258, Figure 2E).
Following the above findings, univariate Cox proportional hazards analysis was performed to correlate nuclear CD133 expression with clinicopathological parameters and overall survival of the 93 HCC patients (as shown in Table 3). Non-nuclear CD133 expression, AJCC stages I-II, without distant metastasis, and small tumor size (≤ 5 cm in diameter) contributed to longer overall survival time of HCC patients. Meanwhile, multivariate Cox proportional hazards analysis was conducted to evaluate meaningful parameters identified by univariate Cox analysis. Since T classification was consistent with AJCC stage classification and only 1 patient had lymph node metastasis and 1 case had distant metastasis, we included nuclear CD133 expression, AJCC stage, and tumor size in multivariate Cox analysis for HCC patients. Nuclear CD133 expression (HR = 0.564, 95% CI 0.313-1.018, P = 0.057) and AJCC stage (HR = 0.319, 95% CI 0.152-0.673, P = 0.003) served as independent prognostic factors for HCC patients (Table 3).
Table 3.
Univariate and multivariate survival analysis of clinicpathological variables of hepatocellular carcinoma patients
| Clinical parameters | Overall survival | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Univariate analysis | Multivariate analysis | |||||
|
|
|
|||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Nuclear CD133 expression | 0.473 | (0.245-0.914) | 0.026 | 0.564 | (0.313-1.018) | 0.057 |
| Negative | ||||||
| Positive | ||||||
| Age (years) | 1.260 | (0.751-2.113) | 0.382 | |||
| ≤ Median | ||||||
| > Median | ||||||
| Gender | 1.393 | (0.626-3.097) | 0.416 | |||
| Male | ||||||
| Female | ||||||
| AJCC stage | 0.340 | (0.192-0.602) | < 0.001 | 0.319 | (0.152-0.673) | 0.003 |
| I-II | ||||||
| III-IV | ||||||
| T classification | 0.340 | (0.192-0.602) | < 0.001 | |||
| T1-T2 | ||||||
| T3-T4 | ||||||
| N classification | 0.121 | (0.004-3.930) | 0.235 | |||
| N0 | ||||||
| N1 | ||||||
| Distant metastasis | 0.000 | (0.000-0.000) | < 0.001 | |||
| No | ||||||
| Yes | ||||||
| Hepatic cirrhosis | 0.858 | (0.508-1.450) | 0.568 | |||
| No | ||||||
| Yes | ||||||
| Edmondson-Steiner grade | 0.784 | (0.451-1.363) | 0.388 | |||
| I-II | ||||||
| III-IV | ||||||
| Tumor number | 1.796 | (0.912-3.540) | 0.101 | |||
| Single | ||||||
| Multiple | ||||||
| Tumor size (cm) | 0.490 | (0.289-0.831) | 0.008 | 0.909 | (0.438-1.887) | 0.797 |
| ≤ 5 | ||||||
| > 5 | ||||||
Discussion
Cancer stem cells are resistant to chemotherapy and radiotherapy, contributing to cancer recurrence and metastasis. Therefore, CSC-associated biomarkers could be helpful in predicting prognosis of cancer patients and might also constitute potential targets for cancer treatment. In the present work, CD133 expr-ession and its biological significance in HCC was studied. Both mRNA and protein levels of CD133 were upregulated in HCC. Moreover, we showed that nuclear translocation of CD133 was presented in HCC tissues and that nuclear CD133 expression contributed to poor prognosis of HCC patients.
CD133, also named Prominin 1, is normally located on the cell membrane and is thought to function in maintaining stem cell properties by suppressing differentiation. As a putative marker of CSCs, CD133 also plays a vital role in several kinds of cancers. CD133 was shown to modulate cell differentiation and survival [12,13] and is closely related to the Wnt/β-catenin signaling pathway [14-16]. In our work, bioinformatics analysis confirmed that low CD133 expression was positively associated with downregulation of liver cancer stem cells. In recent years, many reports have shown that cytoplasmic and nuclear CD133 expression impacts patient survival in several types of cancer [17-20] and that CD133 could exert its pro-metastatic activity when released extracellularly [21]. In HCC, increased CD133 expression levels were correlated with increased tumor grade, advanced disease stage, elevated serum alpha-fetoprotein levels, higher recurrence rates, and poor patient prognosis. Moreover, increased CD133 expression was an independent prognostic factor for survival and tumor recurrence in patients with HCC [3,22]. In the present work, we found that low CD133 expression conferred to good prognosis in HCC based on the TCGA database. Although our present study detected no impact of CD133 protein levels on overall survival of HCC patients, we believe that significant results could be obtained by increasing the sample volume.
It has been reported that HCC patients with high cytoplasmic CD133 expression have poorer clinical outcomes while HCC patients with high nuclear CD133 expression have better clinical outcomes [23]. However, in most cases, cytoplasmic or nuclear CD133 expression was assessed as an indicator of poor prognosis in triple-negative breast cancer and non-small cell lung cancer [6,24] and was related to tumor diameter, tumor differentiation, and TNM stage in non-small cell lung cancer [24]. Herein, we demonstrated that nuclear localization of CD133 is positively correlated with poor prognosis in HCC patients. CD133 mRNA and protein levels were also elevated in HCC compared to non-cancerous tissues, based on bioinformatics and immunohistochemical analysis, respectively. Further analysis suggests that nuclear CD133 expression is correlated with tumor size and could serve as an independent prognostic factor for HCC patients.
Taken together, our results aid in unraveling the oncogenic roles of CD133 in HCC. We show that nuclear CD133 expression can potentially serve as a biomarker for clinical diagnosis and prognosis evaluation of HCC. Lastly, targeted inhibition of CD133 might be an alternative strategy for management of HCC.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (No. 81773151) and the Guangzhou Science and Technology Funding Projects (No. 201604020009).
Disclosure of conflict of interest
None.
References
- 1.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Zhao Q, Zhou H, Liu Q, Cao Y, Wang G, Hu A, Ruan L, Wang S, Bo Q, Chen W, Hu C, Xu D, Tao F, Cao J, Ge Y, Yu Z, Li L, Wang H. Prognostic value of the expression of cancer stem cell-related markers CD133 and CD44 in hepatocellular carcinoma: from patients to patient-derived tumor xenograft models. Oncotarget. 2016;7:47431–47443. doi: 10.18632/oncotarget.10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Immervoll H, Hoem D, Steffensen OJ, Miletic H, Molven A. Visualization of CD44 and CD133 in normal pancreas and pancreatic ductal adenocarcinomas: non-overlapping membrane expression in cell populations positive for both markers. J Histochem Cytochem. 2011;59:441–455. doi: 10.1369/0022155411398275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hashimoto K, Aoyagi K, Isobe T, Kouhuji K, Shirouzu K. Expression of CD133 in the cytoplasm is associated with cancer progression and poor prognosis in gastric cancer. Gastric Cancer. 2014;17:97–106. doi: 10.1007/s10120-013-0255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantile M, Collina F, D’Aiuto M, Rinaldo M, Pirozzi G, Borsellino C, Franco R, Botti G, Di Bonito M. Nuclear localization of cancer stem cell marker CD133 in triple-negative breast cancer: a case report. Tumori. 2013;99:e245–250. doi: 10.1177/030089161309900523. [DOI] [PubMed] [Google Scholar]
- 7.Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, Bray RA, Waller EK, Buck DW. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90:5013–5021. [PubMed] [Google Scholar]
- 8.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 9.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 10.Piao LS, Hur W, Kim TK, Hong SW, Kim SW, Choi JE, Sung PS, Song MJ, Lee BC, Hwang D, Yoon SK. CD133+ liver cancer stem cells modulate radioresistance in human hepatocellular carcinoma. Cancer Lett. 2012;315:129–137. doi: 10.1016/j.canlet.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, Guan XY. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 12.Mak AB, Nixon AM, Moffat J. The mixed lineage leukemia (MLL) fusion-associated gene AF4 promotes CD133 transcription. Cancer Res. 2012;72:1929–1934. doi: 10.1158/0008-5472.CAN-11-3589. [DOI] [PubMed] [Google Scholar]
- 13.Mak AB, Pehar M, Nixon AM, Williams RA, Uetrecht AC, Puglielli L, Moffat J. Post-translational regulation of CD133 by ATase1/ATase2-mediated lysine acetylation. J Mol Biol. 2014;426:2175–2182. doi: 10.1016/j.jmb.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng Y, Su Q, Mo J, Fu X, Zhang Y, Lin EH. Celecoxib downregulates CD133 expression through inhibition of the Wnt signaling pathway in colon cancer cells. Cancer Invest. 2013;31:97–102. doi: 10.3109/07357907.2012.754458. [DOI] [PubMed] [Google Scholar]
- 15.Mak AB, Nixon AM, Kittanakom S, Stewart JM, Chen GI, Curak J, Gingras AC, Mazitschek R, Neel BG, Stagljar I, Moffat J. Regulation of CD133 by HDAC6 promotes beta-catenin signaling to suppress cancer cell differentiation. Cell Rep. 2012;2:951–963. doi: 10.1016/j.celrep.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su YJ, Lin WH, Chang YW, Wei KC, Liang CL, Chen SC, Lee JL. Polarized cell migration induces cancer type-specific CD133/integrin/Src/Akt/GSK3beta/beta-catenin signaling required for maintenance of cancer stem cell properties. Oncotarget. 2015;6:38029–38045. doi: 10.18632/oncotarget.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yiming L, Yunshan G, Bo M, Yu Z, Tao W, Gengfang L, Dexian F, Shiqian C, Jianli J, Juan T, Zhinan C. CD133 overexpression correlates with clinicopathological features of gastric cancer patients and its impact on survival: a systematic review and meta-analysis. Oncotarget. 2015;6:42019–42027. doi: 10.18632/oncotarget.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jao SW, Chen SF, Lin YS, Chang YC, Lee TY, Wu CC, Jin JS, Nieh S. Cytoplasmic CD133 expression is a reliable prognostic indicator of tumor regression after neoadjuvant concurrent chemoradiotherapy in patients with rectal cancer. Ann Surg Oncol. 2012;19:3432–3440. doi: 10.1245/s10434-012-2394-3. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Zhao H, Gu J, Zheng L. Prognostic value of cancer stem cell marker CD133 expression in pancreatic ductal adenocarcinoma (PDAC): a systematic review and meta-analysis. Int J Clin Exp Pathol. 2015;8:12084–12092. [PMC free article] [PubMed] [Google Scholar]
- 20.Nunukova A, Neradil J, Skoda J, Jaros J, Hampl A, Sterba J, Veselska R. Atypical nuclear localization of CD133 plasma membrane glycoprotein in rhabdomyosarcoma cell lines. Int J Mol Med. 2015;36:65–72. doi: 10.3892/ijmm.2015.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rappa G, Mercapide J, Anzanello F, Le TT, Johlfs MG, Fiscus RR, Wilsch-Brauninger M, Corbeil D, Lorico A. Wnt interaction and extracellular release of prominin-1/CD133 in human malignant melanoma cells. Exp Cell Res. 2013;319:810–819. doi: 10.1016/j.yexcr.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song W, Li H, Tao K, Li R, Song Z, Zhao Q, Zhang F, Dou K. Expression and clinical significance of the stem cell marker CD133 in hepatocellular carcinoma. Int J Clin Pract. 2008;62:1212–1218. doi: 10.1111/j.1742-1241.2008.01777.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen YL, Lin PY, Ming YZ, Huang WC, Chen RF, Chen PM, Chu PY. The effects of the location of cancer stem cell marker CD133 on the prognosis of hepatocellular carcinoma patients. BMC Cancer. 2017;17:474. doi: 10.1186/s12885-017-3460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang M, Zhu H, Feng J, Ni S, Huang J. High CD133 expression in the nucleus and cytoplasm predicts poor prognosis in non-small cell lung cancer. Dis Markers. 2015;2015:986095. doi: 10.1155/2015/986095. [DOI] [PMC free article] [PubMed] [Google Scholar]


