Abstract
The role of extracellular matrix proteins in glioma progression is largely unknown. In the current study, we screened different published GSE datasets and found that an extracellular matrix protein Lysyl Oxidase-Like 1 (LOXL1) is highly expressed in different cohorts of glioma patients. To confirm the results from datasets, we examined the level of LOXL1 in 30 matched glioma tissues and we found that LOXL1 is highly expressed in glioma malignant tissues. We further investigated the biological functions of LOXL1 in glioma cells with both loss- and gain-function assays. These show that highly expressed LOXL1 promotes glioma cell proliferation and growth through regulating Wnt/β-catenin signaling. In conclusion, we report that thesecretory protein LOXL1 is highly expressed in malignantglioma tissues and promotes glioma cell proliferation through Wnt/β-catenin signaling.
Keywords: Extracellular matrix, LOXL1, cell proliferation, Wnt/β-catenin, poor prognosis
Introduction
Gliomas are solid tumors in the central nervous system that account for approximately 70% of malignant primary brain tumors each year [1]. In spite of intense efforts to optimize the treatment of gliomas over the past decades, no significant advancement in patient survival has been achieved. Therefore, the underlying mechanisms about glioma tumorigenesis need to be elucidated.
The tumor microenvironment plays an essential role in tumorigenesis and progression [2] and consists of cancer cells, extracellular matrix proteins, immune cells, and tumor-associated fibroblasts [3]. There is much evidencesuggesting that interactions between cancer cells and extracellular matrix proteins mediate development of cancer [4]. In the current study, we found a poorly studied extracellular matrix protein LOXL1 which is highly expressed in glioma tissues compared with adjacent non-tumor tissues.
LOXL1 is a 63 KD extracellular matrix protein, which functions as an extracellular copper-dependent amine oxidase that catalyzes the first step in the formation of cross links in collagens and elastin [5]. Previous studies about LOXL1 mainly focused on its polymorphisms in eyes with exfoliation glaucoma [6]. However, the role of LOXL1 in tumorigenesis or cancer development remains largely unknown. In the current study, we found that LOXL1 is highly expressed in glioma and we revealed biological functions of LOXL1 in glioma.
Materials and methods
Ethic statement
30 fresh tumor tissues and matched adjacent tissues were collected from patients with pathologically and clinically confirmed glioma. All human tumor tissues were obtained with written informed consents from patients. The Institutional Review Board of Jiaxing First People’s Hospital approved the use of the tumor samples in this study.
Cell culture
U87MG and U251 cells were purchased from Cell Bank of the Chinese Academy of Sciences. Normal human astrocytes (NHA) were purchased from American Type Culture Collection (ATCC). All cells were maintained under standard culture conditions (37°C, 5% CO2) in culture medium recommended by Cell Bank of the Chinese Academy of Sciences or ATCC.
RNA isolation and quantitative real-time PCR
Total RNA was purified from fresh glioma and adjacent tissues or cells using TRIzol (Invitrogen) following the manufacturer’s protocol. RNA (1 μg) was reverse transcribed using SuperScript Reverse Transcriptase III (Invitrogen). Quantitative real time PCR was performed using SYBR green Supermix (ABI) in ABI 7500 PCR system. The housekeeping gene GAPDH was used as an internal standard. Primers used in this study were described in Table 1.
Table 1.
Sequence of primers and shRNAs
| Name | Sequence (5’-3’) |
|---|---|
| LOXL1 primer | F: GATGGCAAGTGGTATGGGGTC |
| R: CGAGGACAGGCTGATCTTCC | |
| GAPDH primer | F: AGCCTCAAGATCATCAGCAATGCC |
| R: TGTGGTCATGAGTCCTTCCACGAT | |
| Sh#1 | GTACCGGCACGTGAACCCAAAGTATATTCTCGAGAATATACTTTGGGTTCACGTGTTTTTTG |
| Sh#2 | CCGGAGGGAGCGAACGTGGATGAAACTCGAGTTTCATCCACGTTCGCTCCCTTTTTTG |
Western blots
Cells were lysed in WB/IP lysis buffer (P0013, Beyotime) and nuclear proteins were extracted using lysis buffer (P0028, Beyotime), all the procedures were performed following the manufacturer’s protocol. Subsequently the cell lysates were boiled in 5X SDS-PAGE loading buffer for 10 min and then resolved by 10% SDS-PAGE and transferred to nitrocellulose membrane. The following antibodies were used in this study: LOXL1 (Proteintech), GAPDH (Proteintech), β-catenin (CST), Lamin A/B (Proteintech). Bound antibodies were visualized with the ECL kit (Thermo Scientific).
Construct stable cell lines
To generate stable silenced LOXL1 cell lines, vectors containing shRNAs were purchased from Sigma-Aldrich. To ectopic express LOXL1, vectors containing the ORF of LOXL1 were obtained from Genecopoeia. We then transfected NHA cells with these vectors by lipofectamine 2000 (Invitrogen) following the manufactur-er’s protocol. The supernatant media containing virus was collected by centrifugation to remove cellular contaminant. The resulting viruses were used to infect the indicated cells, and then integrated cells were selected by 2 μg/ml puromycin for 2 weeks. Alterations of LOXL1 in those cells were confirmed by Western blots before further analysis. The sequences of shRNAsaredescribed in Table 1.
CCK-8 cell viability assays
Cells were seeded into a 96-well plate at 3×103 cells per well with 100 μl cultured medium and cultured at 37°C, 5% CO2. Cell viability was quantified by addition 10 μl of cell counting kit (CCK-8, Dojindo). After 1.5 hours incubation, the plates were monitored by Power Wave XS microplate reader (BIO-TEK) at an absorbance 450 nm.
Colony formation assays
The indicated cells (1000/well) were seeded in the 6-well plates and incubated 14-21 days at 37°C, 5% CO2. Colonies were fixed and stained with 0.5% crystal violet and the number of colonies was counted.
Flow cytometry cell cycle assays
The indicated cells (20×104/well) were seeded in the 6-well plates. These cells were then collected at 12 h, 24 h, and 36 h. Cells were washed twice with 1×PBS, then re-suspended and fixed in 2 ml 70% ethanol at -20°C. Cells were then stained with PI (BD) and followed as the manufacturer’s protocol.
Luciferase reporter assays
The indicated cells were seeded in 96-well plates and transfected with TCF/β-catenin reporter plasmid (Wnt/β-catenin signaling) and 10 ng Renilla following the recommended protocol for the Lipofectamine 2000 transfection system. After 48 hours incubation, firefly and Renilla luciferase activities were measured using the dual-luciferase reporter assay system (Promega, Madison, WI) from the cell lysates.
Statistics analysis
Data are expressed as the mean ± standard deviation. Student’s t test was used for comparisons between groups and P<0.05 was considered statistically significant difference.
Results
LOXL1 is highly expressed in different cohort of Glioma malignant tissues
In the current study, we retrieved data from the Oncomine Database and surprisingly found that LOXL1 is significantly elevated in different cohorts of glioma patients. As shown in Figure 1A and 1B, LOXL1 is highly expressed in published GSE4290 [7] and GSE7696 [8] glioma patient tissues. Furthermore, we then determined the level of LOXL1 in fresh glioma tissues and the results were consistent with the data in the published datasets, as shown in Figure 1C, LOXL1 is significantly highly expressed in fresh glioma tissues when compared with adjacent tissues. In addition, we also examined the level of LOXL1 in NHA, U87MG and U251 cell lines, and found that the level of LOXL1 is higher in U87MG and U251 when compared with NHA. These data indicate that LOXL1 is highly expressed in glioma tumor tissues.
Figure 1.
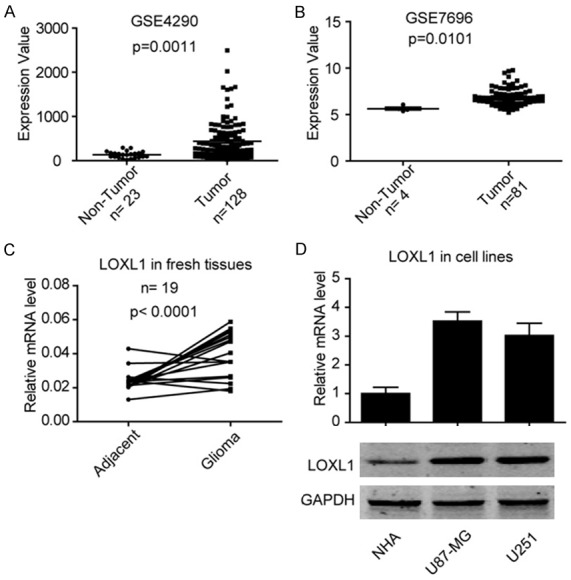
LncRNA LOXL1 is highly expressed in glioma malignant tissues and cell lines. A: LOXL1 is significantly up-regulated in GSE4290 patient’s cohort. B: LOXL1 is highly expressed in GSE7696 patient’s cohort. C: Quantitative real-time PCR reveals that LOXL1 is significantly elevated in fresh glioma tissues. D: Expression of LOXL1 in normal brain tissues cell line NHA and glioma cell lines U87MG and U251.
Knockdown LOXL1 inhibits glioma cell proliferation and growth
To determine the role of LOXL1 in GLIOMA tumorigenesis, we silenced LOXL1 against the specific targets in U87MG and U251 cell lines using lentivirus-mediated short hairpins RNAs (shRNAs). As illustrated in Figure 2A, both shRNAs achieved effective knockdown efficiency. Notably, LOXL1 depletion significantly reduced U87MG and U251 cell viability compared with control (sh-NC) cells (Figure 2B, 2C). Aditionally, LOXL1 knockdown strongly impaired the ability of clone formation in U87MG and U251 cells (Figure 2D, 2E). As depletion of LOXL1 significantly inhibited glioma cell proliferation and growth, we then asked whether LOXL1 influences the cell cycle of glioma. In agreement with these results, we found that silencing LOXL1 induced cell cycle arrested in G0/G1 stage. Overall, these results indicate that LOXL1 silencing impairs the ability of glioma cell proliferation and growth.
Figure 2.
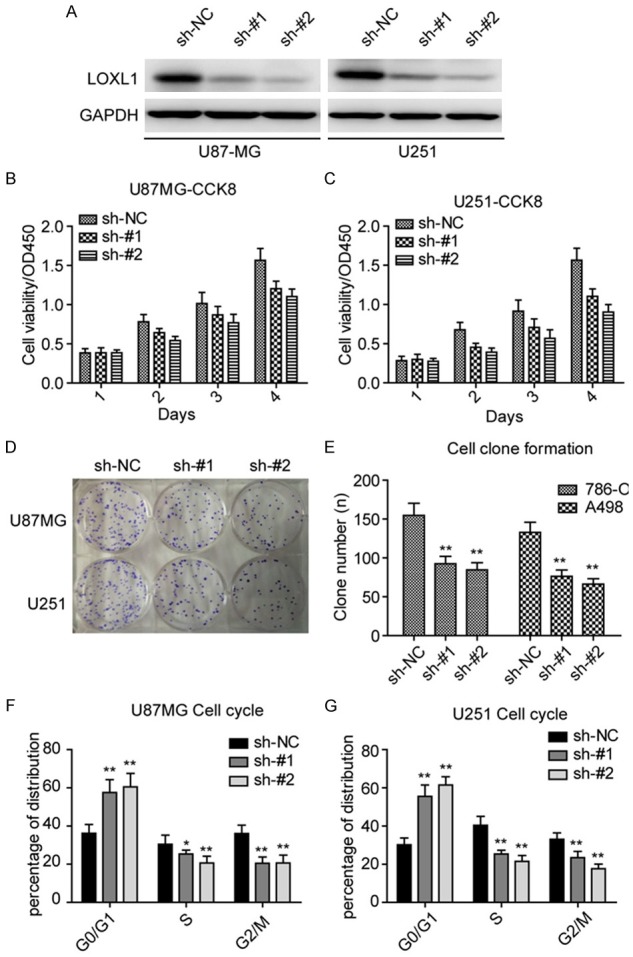
Knockdown LOXL1 inhibits GLIOMA cell proliferation and growth. (A) Expression of LOXL1 is significantly down-regulated in indicated cell lines after stably silencing LOXL1. Silencing LOXL1 inhibits U87MG (A) and U251 (B) cell viability. (B, C) Knockdown LOXL1 inhibits the cell viability of U87MG (B) and U251 (C). (D, E) Knockdown LOXL1 inhibits the ability of clone formation in U87MG and U251. (F, G) Depletion of LOXL1 leads to U87MG and U251 cell cycle arrest in G0/G1 stage. (G) Silencing LOXL1 inhibits subcutaneous tumor formation in nude mice.
LOXL1 overexpression enhances NHA cell proliferation and growth
We next overexpressed LOXL1 in NHA cells and established a LOXL1 stably overexpressing cell line. As shown in Figure 3A, LOXL1 overexpression dramatically increased the level of LOXL1 in NHA cell. Furthermore, ectopic expression of LOXL1 significantly heightened cell viability and the ability of cell clone formation (Figure 3B, 3C). We also explored the cell cycle in overexpressed LOXL1 NHA cells. Notably, LOXL1 overexpression significantly enlarged the distribution of S stage cells (Figure 3D). All together, these data indicate that LOXL1 plays a role in NHA cell proliferation and growth.
Figure 3.
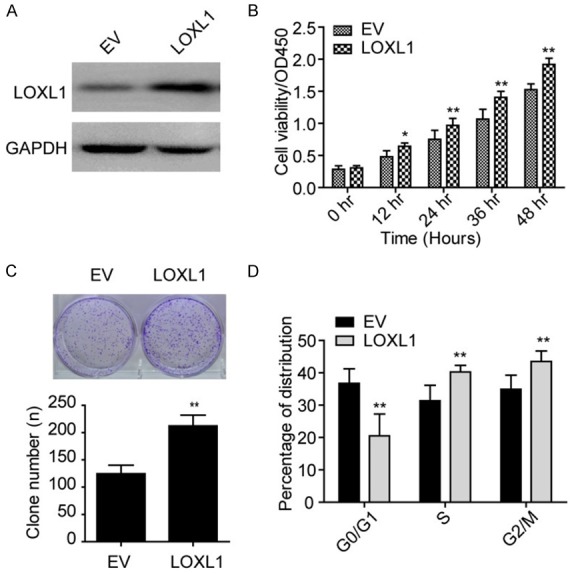
Ectopic expression of LOXL1 promotes NHA cell proliferation and growth. A: Expression of LOXL1 is significantly elevated in NHA cell when ectopic expressed LOXL1 in NHA cell lines. B: CCK-8 cell viability assays show that overexpression LOXL1 elevates NHA cell viability. C: Clone formation assays reveal that LOXL1 promotes NHA cell growth. D: LOXL1 promotes NHA cell transition from G0/G1 to S stage.
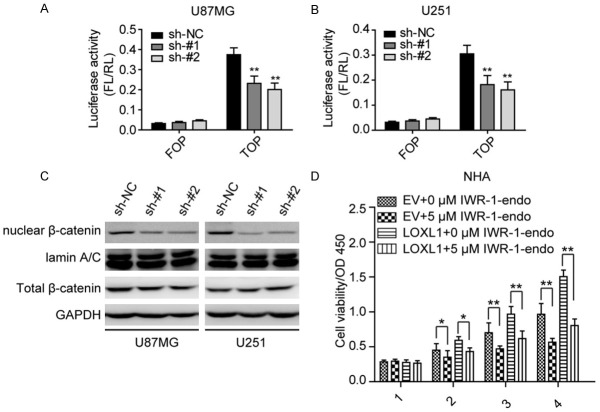
LOXL1 regulates the activation of Wnt/β-catenin signaling in glioma cells
As Wnt/β-catenin signaling could regulate these cyclins, we then asked whether LOXL1 regulates Wnt/β-catenin signaling to subsequently modulate cell proliferation. As shown in Figure 4A, luciferase reporter assays revealed that silencing LOXL1 impairs the activation of Wnt/β-catenin signaling in U87MG (Figure 4A) and U251 (Figure 4B) cell lines. We then examined the level of nuclear β-catenin, as illustrated in Figure 4C, we found that depletion of LOXL1 significantly reduced the level of nuclear β-catenin signaling. Moreover, we then used IWR1-endo, an inhibitor of β-catenin, treating NHA stable cell lines, and found that IWR-1-endo could rescue the LOXL1 induced cell proliferation. These data suggest that LOXL1 regulates the activity of Wnt/β-catenin signaling.
Figure 4.
LOXL1 regulates Wnt/β-catenin signaling in glioma cell lines. (A, B) Dual luciferase reporter assays revealed that depletion of LOXL1 significantly inhibits Wnt/β-catenin signaling in U87MG (A) and U251 (B) cells. (C) Silencing LOXL1 significantly inhibits the nuclear β-catenin level in U87MG and U251 cells. (D) β-catenin inhibitor IWR-1-endo rescues the promotive effects in NHA cell overexpression cell lines.
Discussion
In this study we found that extracellular matrix protein LOXL1 is highly expressed in glioma malignant tissues compared with normal tissue. Our study shows that LOXL1 promotes glioma cell proliferation and growth through affecting Wnt/β-catenin signaling. Extracellular matrix proteins play pivotal roles in tumor development through interaction with cancer cells. A previous study reveals that extracellular matrix proteins protect small cell lung cancer cells against apoptosis. Extracellular matrix protein TGFB1 promotes metastasis of colon cancer by enhancing cell extravasation [9]. POSTN enhances the malignant phenotypes of the pancreatic cancer cell [10]. However, study about the role of extracellular matrix protein in glioma is rare. In current study, we found that LOXL1, which is rarely reported in the field of tumor biology, is highly expressed in glioma. We further foundthat silencing LOXL1 significantly inhibited cell proliferation, and ectopic expression of LOXL1 promoted NHA cell proliferation and growth. These findings indicate that highly expressed LOXL1 promotes glioma cell proliferation and growth in the tumor microenvironment.
However, the role of LOXL1 in cell proliferation and growth hasn’t been reported to date. To our knowledge, Wnt/β-catenin signaling is an activated pathway in many kinds of tumors and it plays a pivotal role in regulating cancer cell proliferation, growth, and survival [11-13]. Our findings shed light on the mechanism that LOXL1 regulates Wnt/β-catenin signaling to promote glioma cell proliferation. However, de-tailed mechanisms how LOXL1 regulates Wnt/β-catenin signaling remain to be fully elucidated in future studies.
Taken together, we reveal the extracellular matrix protein LOXL1 is commonly up-regulated in glioma tissues, and highly expressed LOXO1 plays an oncogenic role in glioma cell proliferation. Patients with a high level of LOXL1 might suffer a poor prognosis. We further report that LOXL1 regulates Wnt/β-catenin signaling to regulate glioma cell proliferation. As LOXL1 is an extracellular secretory protein, it is potentially a novel serological biomarker for glioma.
Acknowledgements
This work was supported by Scientific Research fund of Wenling Science and Technology Bureau (2016C31BA0036).
Disclosure of conflict of interest
None.
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Whiteside T. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Cell. 2005;7:513–520. doi: 10.1016/j.ccr.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Li H, Fan X, Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem. 2007;101:805–815. doi: 10.1002/jcb.21159. [DOI] [PubMed] [Google Scholar]
- 5.Csiszar K. Lysyl oxidases: a novel multifunctional amine oxidase family. Prog Nucleic Acid Res Mol Biol. 2001;70:1–32. doi: 10.1016/s0079-6603(01)70012-8. [DOI] [PubMed] [Google Scholar]
- 6.Schlötzer-Schrehardt U. Molecular pathology of pseudoexfoliation syndrome/glaucoma-new insights from LOXL1 gene associations. Exp Eye Res. 2009;88:776–785. doi: 10.1016/j.exer.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Sun L, Hui AM, Su Q, Vortmeyer A, Kotliarov Y, Pastorino S, Passaniti A, Menon J, Walling J, Bailey R, Rosenblum M, Mikkelsen T, Fine HA. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell. 2006;9:287–300. doi: 10.1016/j.ccr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Murat A, Migliavacca E, Gorlia T, Lambiv WL, Shay T, Hamou MF, de Tribolet N, Regli L, Wick W, Kouwenhoven MC, Hainfellner JA, Heppner FL, Dietrich PY, Zimmer Y, Cairncross JG, Janzer RC, Domany E, Delorenzi M, Stupp R, Hegi ME. Stem cell-related “self-renewal” signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J. Clin. Oncol. 2008;26:3015–3024. doi: 10.1200/JCO.2007.15.7164. [DOI] [PubMed] [Google Scholar]
- 9.Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Céspedes MV, Sevillano M, Nadal C, Jung P, Zhang XH, Byrom D, Riera A, Rossell D, Mangues R, Massagué J, Sancho E, Batlle E. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22:571–584. doi: 10.1016/j.ccr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erkan M, Kleeff J, Gorbachevski A, Reiser C, Mitkus T, Esposito I, Giese T, Büchler MW, Giese NA, Friess H. Periostin creates a tumor-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology. 2007;132:1447–1464. doi: 10.1053/j.gastro.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 11.Cadigan KM. Wnt-β-catenin signaling. Curr Biol. 2008;18:R943–R947. doi: 10.1016/j.cub.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]