Abstract
Despite advances in the treatment of Hodgkin lymphoma (HL), there is a need for development of reliable prognostic biomarkers and improved stratification of patients for effective therapeutic intervention. The immune microenvironment is the key to HL pathophysiology. The aim of this study was to identify expression of microenvironment-related biomarkers (PD-1, FOXP3, and CSF-1R) immunohistochemically and determine their association with clinicopathological features and prognosis in HL. We found that a high number of non-HRS cells expressing CSF-1R confers inferior overall survival (OS), which is associated with the presence of Epstein-Barr virus in neoplastic cells (P=0.009). Increased FOXP3 expression confered superior OS and progression-free survival (PFS). PD-1 expression had no significant association with OS and PFS. The combination of FOXP3 and PD-1 or CSF-1R may yield better prognostic stratification.
Keywords: PD-1, FOXP3, CSF-1R, Hodgkin lymphoma, prognostic
Introduction
Hodgkin’s lymphoma (HL) has a good prognosis for most patients, with long-term remission greater than 80% following conventional chemotherapy or radiotherapy-based protocols [1-3]. However, a small proportion of patients still experience relapsing or refractory disease, which eventually leads to death, and many long-term patients suffer the late effects of excessively toxic treatments [4]. About 5% to 10% of patients are resistant to initial therapy, and 10% to 30% relapse after initial remission [5]. Several diagnostic scoring systems have been proposed, including the International Prognostic Score (IPS) [6]. The IPS is still considered as the “gold standard” for assessing prognosis and has performed consistently well in independent data sets. However, it was originally designed for patients with advanced disease, with limited relevance to early-stage disease [7]. There is no consensus on the routine management of HL patients [8]. Novel biomarkers are needed to improve prediction of the primary treatment outcome, and decrease the mortality and treatment-related late sequelae including secondary solid tumors and end-organ dysfunction [9].
HL is characterized by the presence of Hodgkin Reed-Sternberg (HRS) cells in classical HL (cHL). The HRS cells are embedded in a heterogeneous background of non-neoplastic bystanders that include lymphocytes, macrophages, eosinophils, mast cells, plasma cells, stromal cells, fibroblasts, micro-vessels and other cells [10]. They are recruited by the HRS cells via secretion of a variety of chemokines and cytokines, which play an essential role in HL pathogenesis. HRS cells supply growth factors, inhibit anti-tumor immune responses, and are in turn perpetuated by additional factors secreted by other reactive cells [11]. Increasing interest in the bystander cells has not only contributed to a better understanding of the underlying biological characteristics of the disease but also enabled the identification of new prognostic biomarkers.
The macrophage appears to play a major role in tumor support [12]. Previous studies demonstrated the adverse effect of increased macrophage infiltration [13,14]. Using gene expression and immunohistochemical (IHC) techniques, studies revealed a correlation between decreased tumor-associated macrophages (TAM) frequency and improved clinical outcome [15]. The CD4+T cells are also important in tumor pathophysiology, although functional data are lacking [10]. Several studies reported that increased numbers of cytotoxic T cells (Granzyme B+/TIA1+) were associated with worse outcome and shorter survival in HL patients. In the present study, expression of three HL microenvironment-related markers such as programmed death 1 (PD-1), a surface marker of follicular helper T-cells; regulatory T-cell marker FOXP3 [16-18] and colony-stimulating factor 1 receptor (CSF-1R), the receptor for colony-stimulating factor 1 was detected and their prognostic significance analyzed. Further, the role of Epstein-Barr virus (EBV) is poorly understood despite a clear association with this disease. Therefore, we used in situ hybridization (ISH) to further determine its role in tumor microenvironment.
Patients and methods
Between 2004 and 2013, 95 patients were recruited and diagnosed as HL from Tianjin Medical University Cancer Institute and Hospital, and Chinese PLA General Hospital, and 86 were subjected to immunohistochemical analysis. However, the final cohort showed characteristics similar to those of the original 95 patients. All primary diagnostic tumor biopsies were reviewed and reclassified according to the World Health Organization classification of tumors of the hematopoietic and lymphoid tissues [19]. The current study was performed in strict accordance with local ethical guidelines and recommendations of the Declaration of Helsinki (Seoul revision, 2008). In this retrospective study involving archived materials, no individual patient identification, or study-driven clinical interventions were performed.
Clinical and follow-up data were obtained from clinical records including histopathological subtype, age, gender, presence or absence of B symptoms, clinical stage according to the Cotswolds modification of the Ann Arbor staging system [20], lactate dehydrogenase level, EBV status, and primary treatment. Most patients were treated with four to eight cycles of ABVD (adriamycin, bleomycin, vinblastine, and dacarbazine) chemotherapy. Additional radiotherapy was administered in cases of pre-therapeutic bulky or localized residual masses. Relapsed or refractory patients received either salvage chemotherapy or high-dose chemotherapy with autologous stem-cell transplantation.
Immunohistochemistry and in situ hybridization
Tissue blocks of untreated patients were obtained from the Department of Pathology at two hospitals. Paraffin-embedded lymph node specimens of 86 patients were available. Immunohistochemical staining for CSF-1R, PD-1 and FOXP3 was performed as described below. Briefly, 5-μm-thick sections were transferred onto poly-L-lysine-coated adhesive slides and dried at 65°C for 90 min. After standard heat-induced epitope retrieval for 2 to 3 min in citrate buffer (pH 7.4), the samples were incubated with antibodies against PD-1 (dilution 1:50; R&D Systems, Germany), FOXP3 (dilution 1:100; Abcam, UK), and CSF-1R (dilution 1:100; Abcam, UK) overnight at 4°C. The sections were incubated with biotinylated anti-goat and anti-mouse/rabbit immunoglobulins, and DAB was used as a substrate.
The positive index was estimated by counting the number of positive cells in five randomly selected high power fields (HPF) at 40×10 magnification. For FOXP3+ quantification, the results were considered high when more than 25% of the cells were positive among the total cells. Among the percentage of non-HRS cells expressing CSF-1R, we selected more than 30% of CSF-1R-positive cells as the cut-off value for defining high-and low-CSF-1R groups. Cases stained with anti-PD-1 were scored according to the intensity of cytoplasmic and/or membranous positivity. It was considered positive when more than 20% of all cell population was stained.
In situ hybridization analysis for Epstein-Barr virus encoded RNA (EBER) was performed. EBER was considered as positive in case of dark-blue nuclear staining.
Statistical analysis
Overall survival (OS) was defined as the interval between the date of diagnosis and death from any cause. The follow-up of living patients (with or without events) was censored at their last follow-up date. Progression-free survival (PFS) was defined as the interval between the date of treatment and the date of disease progression, relapse, or death from any cause. Cumulative OS and PFS were analyzed by Kaplan-Meier method, and comparisons were made using the log-rank test. Multivariate prognostic analyses were performed for OS and PFS using the Cox proportional hazards regression model. All p values were two-sided, and a p value <0.05 was considered as significant. Statistical analyses were performed using the SPSS 17.0.
Results
Patient characteristics
The main clinical and histopathological characteristics are summarized in Table 1. The median age of HL patients was 31.5 years (ranging from 7 to 82 years) and 53 patients were male (61.6%). Most patients were diagnosed with advanced disease (stages III and IV) (n=39; 45.3%) and systemic B symptoms (n=22; 25.6%). Approximately 43% of patients had EBV infection (EBER-positive). The estimated 5-year OS and PFS were 75.6% and 54.2%, respectively.
Table 1.
Demographic and clinical Characteristics of the Patients (n=86)
| Variable | N (%) |
|---|---|
| Gender | |
| Male | 53 (62) |
| Female | 33 (38) |
| Age (years) | |
| ≥ 45 | 23 (27) |
| <45 | 63 (73) |
| Ann Arbor stage | |
| I-II | 46 (55) |
| III-IV | 39 (45) |
| B symptoms | |
| Yes | 22 (26) |
| No | 64 (74) |
| IPS score | |
| ≤2 | 74 (86) |
| >2 | 12 (14) |
| LDH | |
| Normal | 59 (69) |
| High | 27 (31) |
| β2-MG | |
| Normal | 64 (74) |
| High | 22 (26) |
| Histologic subtype | |
| Nodular lymphocyte-predominant | 13 (15) |
| Classical Hodgkin lymphoma | |
| Nodular sclerosis | 37 (43) |
| Mixed cellularity | 31 (36) |
| Lymphocyte-rich | 4 (5) |
| Lymphocyte-depleted | 1 (1) |
| Primary treatment | |
| Chemotherapy | 42 (21) |
| Chemoradiotherapy | 44 (79) |
| Response to primary therapy | |
| CR | 31 (36) |
| PR | 42 (49) |
| PD+SD | 13 (15) |
| EBER | |
| Positive | 37 (43) |
| Negative | 49 (57) |
CSF-1R, PD-1, and FOXP3 expression in HL tissue
Correlation between CSF-1R, PD-1, and FOXP3 and clinical variables are summarized in Table 2. Patients’ tumor samples were classified into a low-CSF-1R group expressing low number (<30%) of CSF-1R-positive non-HRS cells, and a high-CSF-1R group with 30% or more cells expressing CSF-1R. Compared with the low-CSF-1R group (n=49), the high-CSF-1R group (n=37) included more patients who were older ≥ 45 years) (40.5% vs. 16.3%, P=0.015), with advanced clinical stage (III/IV) (62.2% vs. 32.7%, P=0.010) and EBER positivity (40.5% vs. 30.6%, P=0.009). Patients were similarly divided into high and low groups according to their FOXP3 expression. The high-FOXP3 group (n=45) included more patients who had EBER positivity (56.1% vs. 31.1%, P=0.029) than the low-FOXP3 group (n=41). However, PD-1 expression was not associated with any of the clinical variables.
Table 2.
Correlation Between CSF-1R, PD-1 and FOXP3 and Clinical Variables
| Characteristic | CSF-1R NO. | Expression (%) | P value | FOXP3 NO. | Expression (%) | P value | PD-1 NO. | Expression (%) | P value |
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Low | High | Low | High | Positive | Negative | ||||
| (<30%) | (≥ 30%) | (<25%) | (≥ 25%) | (≥ 20%) | (<20%) | ||||
| (n=49) | (n=37) | (n=41) | (n=45) | (n=22) | (n=64) | ||||
| Gender | |||||||||
| Male | 32 | 21 | 0.503 | 23 | 30 | 0.377 | 11 | 42 | 0.213 |
| Female | 17 | 16 | 18 | 15 | 11 | 22 | |||
| Age (years) | |||||||||
| ≥ 45 | 8 | 15 | 0.015 | 13 | 10 | 0.341 | 8 | 15 | 0.270 |
| <45 | 41 | 22 | 28 | 35 | 14 | 49 | |||
| Ann Arbor stage | |||||||||
| I-II | 32 | 14 | 0.010 | 20 | 26 | 0.388 | 12 | 34 | 1.000 |
| III-IV | 16 | 23 | 21 | 18 | 10 | 29 | |||
| B symptoms | |||||||||
| Yes | 12 | 10 | 0.808 | 13 | 9 | 0.229 | 16 | 48 | 1.000 |
| No | 37 | 27 | 28 | 36 | 6 | 16 | |||
| IPS score | |||||||||
| ≤2 | 45 | 27 | 0.115 | 35 | 39 | 1.000 | 20 | 54 | 0.723 |
| >2 | 4 | 29 | 6 | 6 | 2 | 10 | |||
| LDH | |||||||||
| Normal | 38 | 21 | 0.060 | 30 | 29 | 0.487 | 13 | 46 | 0.295 |
| High | 11 | 16 | 11 | 16 | 9 | 18 | |||
| β2-MG | |||||||||
| Normal | 38 | 26 | 0.465 | 31 | 33 | 1.000 | 14 | 50 | 0.256 |
| High | 11 | 11 | 10 | 12 | 8 | 14 | |||
| EBER | |||||||||
| Positive | 15 | 15 | 0.009 | 23 | 14 | 0.029 | 12 | 25 | 0.223 |
| Negative | 34 | 22 | 18 | 31 | 10 | 39 | |||
We analyzed the relationship between CSF-1R, PD-1, and FOXP3, and found no correlation between CSF-1R expression in non-HRS cells and PD-1 expression (P=0.618). There was also no significant correlation between CSF-1R expression in non-HRS cells and FOXP3 expression (P=0.191) as shown in Table 3.
Table 3.
Correlation between CSF-1R, PD-1, and FOXP3 expression
| Characteristic | CSF-1R | Expression | P |
|---|---|---|---|
|
| |||
| Low (n=49) | High (n=37) | ||
| PD-1 expression | |||
| Positive (n=22) | 35 (71.4) | 29 (78.4) | 0.618 |
| Negative (n=64) | 14 (28.6) | 8 (21.6) | |
| FOXP3 expression | |||
| Low (n=41) | 20 (40.8) | 21 (56.8) | 0.191 |
| High (n=45) | 29 (59.2) | 16 (43.2) | |
Univariate analysis revealed that age ≥ 45 years (P<0.01), advanced stage III/IV (P=0.032), IPS>2 score (P=0.001), high β2-MG levels (P=0.014), and EBER-positive status (P=0.005) were significant predictors of shorter OS, whereas age ≥ 45 years (P=0.017), B system-positive (P=0.015) and EBER-positive status (0.006) were significant factors associated with shorter PFS. Multivariate analysis revealed that older age (≥ 45 years) and elevated FOXP3 expression were independent prognostic markers for PFS and OS (P=0.027 and P=0.001) as shown in Table 4.
Table 4.
Analysis Overall Survival and Progression-Free-Survival
| Covariate | OS | P value | PFS | P value |
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | HR (95% CI) | |||
| Univariate analysis | ||||
| Age, ≥ 45 vs. <45 | 1.040 (1.019-1.062) | <0.001 | 0.419 (0.204-0.858) | 0.017 |
| Ann Arbor stage, I-II vs. III-IV | 2.131 (1.067-4.255) | 0.032 | 1.147 (0.577-2.279) | 0.695 |
| IPS score, ≤2 vs. >2 | 4.489 (1.847-10.909) | 0.001 | 1.343 (0.463-3.894) | 0.587 |
| β2-MG, Normal vs. High | 2.550 (1.213-5.361) | 0.014 | 1.245 (0.562-2.759) | 0.589 |
| EBER, Positive vs. Negative | 2.785 (1.367-5.672) | 0.005 | 2.605 (1.309-5.184) | 0.006 |
| B symptoms, Yes vs. No | 1.431 (0.663-3.090) | 0.361 | 2.381 (1.186-4.778) | 0.015 |
| CSF-1R, Low vs. High | 2.018 (1.015-4.013) | 0.045 | 1.336 (0.679-2.262) | 0.401 |
| PD-1, Positive vs. Negative | 1.826 (0.882-3.780) | 0.105 | 1.210 (0.564-2.597) | 0.624 |
| FOXP3, Low vs. High | 0.196 (0.084-0.453) | <0.001 | 0.209 (0.097-0.451) | <0.001 |
| Multivariate analysis | ||||
| Age, ≥ 45 vs. <45 | 5.615 (2.512-12.553) | 0.002 | 2.250 (1.073-4.715) | 0.027 |
| FOXP3, Low vs. High | 0.262 (0.108-0.634) | 0.002 | 0.253 (0.114-0.561) | 0.001 |
Prognostic significance of CSF-1R, PD-1, FOXP3, and EBER expression
OS was significantly different between low-CSF-1R and high-CSF-1R groups (P=0.045) with 5-year OS of 78.9% and 59.5%, respectively. No significant differences were found in PFS between low-and high-CSF-1R in non-HRS cell group(Figure 1). Patients in the low-FOXP3 group had lower 5-year PFS and OS rates than those in the high-FOXP3 group (34.1% vs. 75.3%, P<0.001; 62.9% vs. 83.6%, P<0.001, respectively) (Figure 2). There was no significant difference in OS and PFS between the positive-PD-1 and the negative-PD-1 group (Figure 3). Patients in the EBER-positive group had lower 5-year PFS rates (37.1% vs. 69.2%, P=0.006) and lower 5-year OS rates (55.6% vs. 83.1%, P=0.005) than those in the EBER-negative group (Figure 4). Based on PD-1 status and FOXP3 expression, all the patients were divided into three groups: high FOXP3 and PD-1-negative; FOXP3 and PD-1 discordant; and low FOXP3 and PD-1-positive, with significant differences in PFS and OS (P=0.002 and P<0.001, respectively). Furthermore, the FOXP3 and CSF-1R expression revealed a positive impact on PFS and OS (P=0.001 and P<0.001, respectively) (Figure 5).
Figure 1.
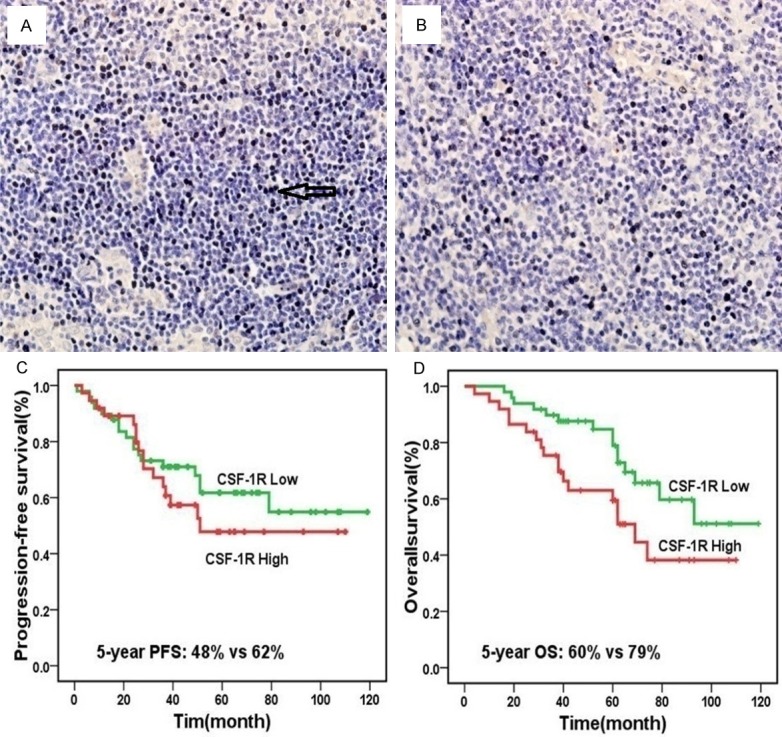
CSF-1 re-expression in non-HRS cells and prognosis, high (A) and low expression (B) of CSF-1R in Hodgkin Lymphoma (magnification ×40); Patients with a low number of CSF-1R-positive cells had better progression-free survival than those with high number (P=0.401) (C); a high number of CSF-1R-positive cells led to significantly worse overall survival compared with low numbers (P=0.045) (D).
Figure 2.
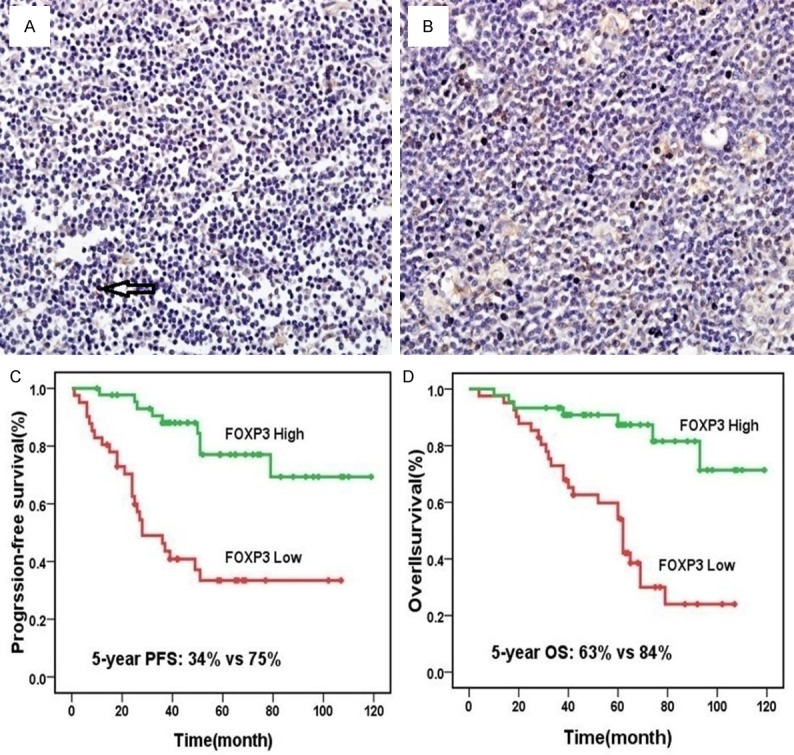
FOXP3 expression and prognosis, high (A) and low expression (B) of FOXP3 in Hodgkin Lymphoma (magnification ×40); Patients with a high number of FOXP3-positive cells had better progression-free survival than those with low number (P<0.001) (C); a low number of FOXP3-positive cells led to significantly worse overall survival compared with high numbers (P<0.001) (D).
Figure 3.
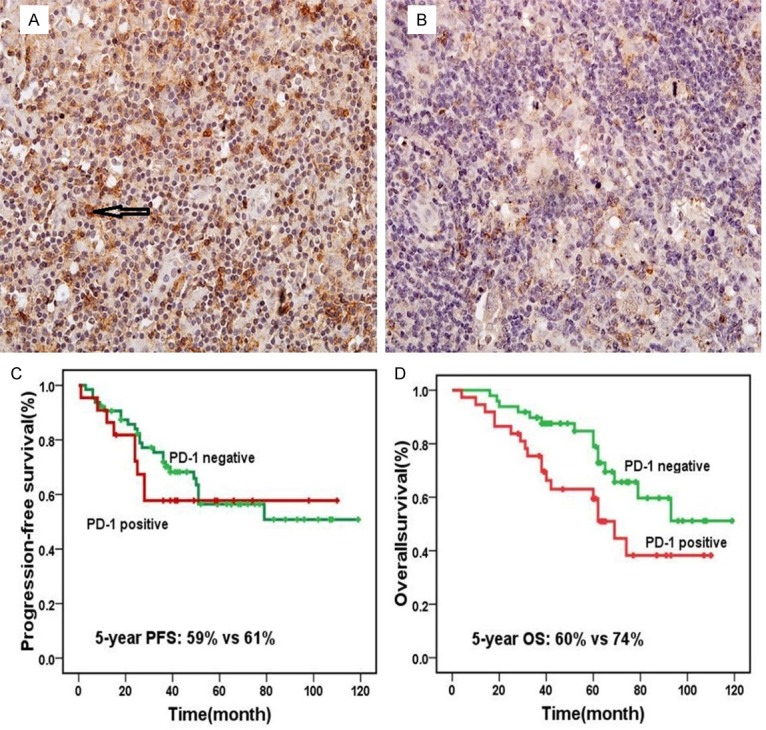
PD-1 expression and prognosis, positive (A) and negative (B) expression of PD-1 in Hodgkin Lymphoma (magnification ×40); Patients with PD-1-positive had no significant difference progression free survival than patients with PD-1-negative (P=0.624) (C); PD-1-positive led to significantly worse overall survival compared with PD-1-negative (P=0.105) (D).
Figure 4.
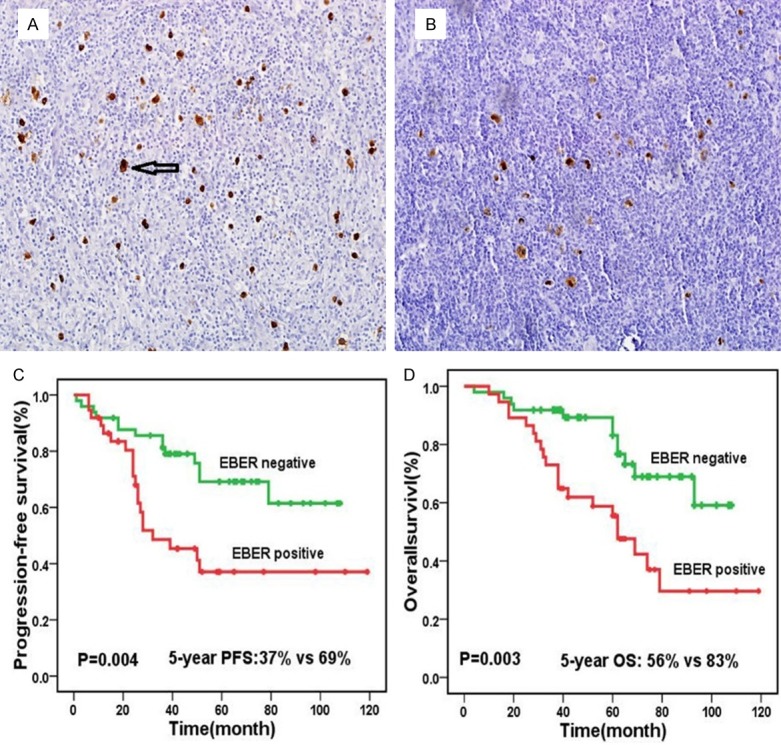
EBER expression and prognosis, positive (A) and negative (B) expression of EBER in Hodgkin Lymphoma (magnification ×40); Patients with EBER-positive had worse progression free survival than those with EBER-negative (P=0.006) (C); EBER-positive led to significantly worse overall survival compared with EBER-negative (P=0.005) (D).
Figure 5.
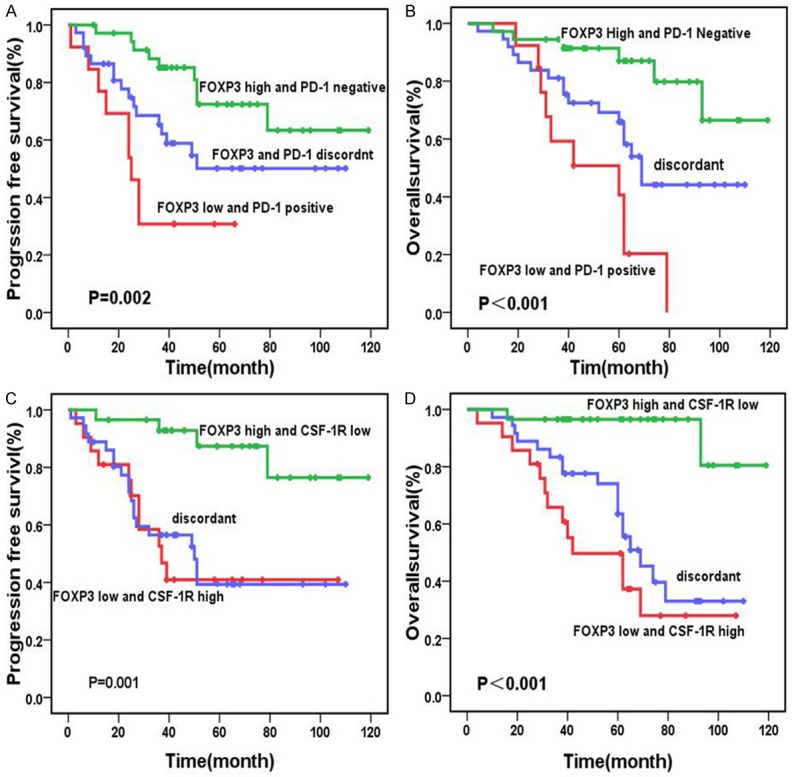
Comparison of survival rates according to FOXP3/PD-1 and FOXP3/CSF-1R expression: Patients with a low number of FOXP3-positive cells and PD-1 positive cells showed significantly worse overall survival than patients with other expression patterns (P<0.001) (A). Patients with a high number of FOXP3-positive and PD-1 negative cells manifested significantly better progression-free survival than those with other expression patterns (P=0.002) (B). Patients with a low number of FOXP3-positive cells and high number of CSF-1R showed significantly worse overall survival than patients with the other expression patterns (P<0.001) (C). Patients with a high number of FOXP3-positive and low number of CSF-1R manifested significantly better progression-free survival than those with the other expression patterns (P=0.001) (D).
Discussion
Hodgkin lymphoma (HL) is characterized by massive reactive infiltrates surrounding HRS cells. Several studies reported that tumor microenvironment was positively related to HL prognosis [10]. Lymphoma-associated macrophages may have a prognostic role in several lymphoproliferative disorders, including cHL. High expression of the macrophage/monocyte-related antigens CD68 and CD163 correlates with adverse outcomes and the presence of Epstein-Barr virus in the tumor cell population in classical HL [13]. CSF-1 is the most pleiotropic of the macrophage growth factors, stimulating the survival, proliferation and differentiation of mononuclear phagocytes and promoting the spread and motility of macrophages [21]. Furthermore, CSF-1R expression in the peri-tumoral area is associated with poor prognosis in leiomyosarcoma, prostate cancer [22,23] and cHL [24,25]. In cHL groups, CSF1/CSF1R signaling plays an essential role in the reciprocal crosstalk between tumor HRS cells and microenvironment. Previous studies revealed anti-tumor activity following disruption of CSF1/CSF1R signaling, probably due to inhibition of the recruitment and activation of TAMs [26]. In this study, we validated that high CSF-1R expression in non-HRS cells led to an inferior prognosis in HL. In addition, we also found that expression of CSF-1R was associated with EBER status. Although no positive relationship was observed between CSF-1R expression and FOXP3 expression, the combination of CSF-1R and FOXP3 expression was predictive of survival.
T cells play a vital role in the immune system, especially in the tumor microenvironment. Therefore, in this study, we selected PD-1 and FOXP3 as two important CD4+T cells in the microenvironment-related biomarkers. PD-1, expressed on T cells after activation, is one of the most important immune checkpoints, which mediate immune suppression [27]. The PD-1/PD-L axis is very important in tumor pathogenesis. Prognostic significance of PD-1 or PD-L1 expression has been evaluated in many solid tumors, including lymphoma [28]. PD-1 signaling results in “T-cell exhaustion”, which is essential to the pathogenesis of HL [29]. Increased number of PD-1 (+) tumor infiltrating lymphocytes have been found to be an independent negative prognostic factor for survival in HL patients [30]. Another study involving cHL found that macrophages constitute the major source of PD-L1. Macrophage-mediated upregulation of PD-1/PD-L1 signaling axis and suppression of anti-tumor immunity are critical factors inpatient prognosis. In our study, although OS was found to be shorter in PD-1-positive subgroups, there was no significant difference. Paydas S et al. [31] concluded that PD-1 and PD-L1 co-expression is associated with significantly shorter disease-free survival (DFS) and OS when compared with those without PD expression. Furthermore, we found that EBV was involved in nearly 43% of HLs. EBER-positive status was related to CSF-1R and FOXP3 expression. Studies evaluating the association between EBV and PD-1/PD-L1 yielded complex results. EBV infection has been implicated in PD ligand expression. EBV-encoded LMP1 promoted PD-L1 expression in tumor cells [32]. However, in another HL study, they found that EBV status does not predict the presence or absence of PD-L1 expression [33]. In this study, we did not find an important relationship between EBV and PD-1.
FOXP3, representing the regulatory T cells (Tregs), plays a critical role in the modulation of immune response, particularly suppression of tumor-associated antigen-reactive lymphocytes [34]. Increased number of Tregs was correlated with unfavorable prognosis in non-small cell lung cancer [35] and ovarian carcinoma [36]. However, data regarding the importance of Tregs in the prognosis of cHL remain controversial. A few studies have shown that Tregs are a positive prognostic marker in cHL [16,18]. However, Schreck et al. [37] showed that Tregs negatively influenced the prognosis with a high risk of relapse. In the present study, we found that high FOXP3 expression confers superior PFS and OS. We also found that the presence of EBV in HRS cells correlated with increased FOXP3 expression, which is consistent with other studies [38]. EBV-specific cytotoxic T cells (CTLs) are unable to eliminate EBV-infected tumor cells in vivo [39] due to an increase in the recruitment of Tregs, which play an essential role in controlling autoimmunity and inhibit the development of successful anti-tumoral immune response. Further, the expression of EBNA-1 in HL cells mediates upregulation of CCL20, facilitating migration of natural Tregs into the tumor [40]. To the best of our knowledge, our study is the first of its kind to report that the prognostic role played by the combination of FOXP3 and CSF-1R or PD-1 in the survival of HL patients, indicating a reciprocal relationship between microenvironment biomarkers [41]. Expression of PD-1, FOXP3 and CSF-1R requires further evaluation in a larger group of patients with HL. These biomarkers could facilitate diagnosis and predict treatment response in patients with HL.
In conclusion, our data show that high levels of FOXP3 expression are associated with superior survival. Increased CSF-1R level in non-HRS confers worse survival in HL. Further, the combination of FOXP3 and PD-1 or CSF-1R expression may improve prognostic stratification. The three biomarkers in tumor microenvironment represent new strategies for the diagnosis, prognosis and treatment of HL.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81600163, 81570201).
Disclosure of conflict of interest
None.
References
- 1.Marcheselli R, Bari A, Tadmor T, Marcheselli L, Cox MC, Pozzi S, Ferrari A, Baldini L, Gobbi P, Aviv A, Pugliese G, Federico M, Polliack A, Sacchi S. Neutrophil-lymphocyte ratio at diagnosis is an independent prognostic factor in patients with nodular sclerosis Hodgkin lymphoma: results of a large multicenter study involving 990 patients. Hematol Oncol. 2017;35:561–566. doi: 10.1002/hon.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viviani S, Zinzani PL, Rambaldi A, Brusamolino E, Levis A, Bonfante V, Vitolo U, Pulsoni A, Liberati AM, Specchia G, Valagussa P, Rossi A, Zaja F, Pogliani EM, Pregno P, Gotti M, Gallamini A, Rota Scalabrini D, Bonadonna G, Gianni AM Michelangelo Foundation; Gruppo Italiano di Terapie Innovative nei Linfomi; Intergruppo Italiano Linfomi. ABVD versus BEACOPP for Hodgkin’s lymphoma when high-dose salvage is planned. N Engl J Med. 2011;365:203–212. doi: 10.1056/NEJMoa1100340. [DOI] [PubMed] [Google Scholar]
- 3.Engert A, Plütschow A, Eich HT, Lohri A, Dörken B, Borchmann P, Berger B, Greil R, Willborn KC, Wilhelm M, Debus J, Eble MJ, Sökler M, Ho A, Rank A, Ganser A, Trümper L, Bokemeyer C, Kirchner H, Schubert J, Král Z, Fuchs M, Müller-Hermelink HK, Müller RP, Diehl V. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363:640–652. doi: 10.1056/NEJMoa1000067. [DOI] [PubMed] [Google Scholar]
- 4.Castellino SM, Geiger AM, Mertens AC, Leisenring WM, Tooze JA, Goodman P, Stovall M, Robison LL, Hudson MM. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the Childhood cancer survivor study. Blood. 2011;117:1806–1816. doi: 10.1182/blood-2010-04-278796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerrie AS, Power MM, Shepherd JD, Savage KJ, Sehn LH, Connors JM. Chemoresistance can be overcome with high-dose chemotherapy and autologous stem-cell transplantation for relapsed and refractory Hodgkin lymphoma. Ann Oncol. 2014;25:2218–23. doi: 10.1093/annonc/mdu387. [DOI] [PubMed] [Google Scholar]
- 6.Gisselbrecht C, Mounier N, André M, Casanovas O, Reman O, Sebban C, Divine M, Brice P, Briere J, Hennequin C, Fermé C. How to define intermediate stage in Hodgkin’s lymphoma? Eur J Haematol Suppl. 2005;66:111–4. doi: 10.1111/j.1600-0609.2005.00463.x. [DOI] [PubMed] [Google Scholar]
- 7.Gobbi PG, Zinzani PL, Broglia C, Comelli M, Magagnoli M, Federico M, Merli F, Iannitto E, Tura S, Ascari E. Comparison of prognostic models in patients with advanced Hodgkin disease. Promising results from integration of the best three systems. Cancer. 2001;91:1467–1478. doi: 10.1002/1097-0142(20010415)91:8<1467::aid-cncr1154>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 8.Harel S, Fermé C, Poirot C. Management of fertility in patients treated for Hodgkin’s lymphoma. Haematologica. 2011;96:1692–9. doi: 10.3324/haematol.2011.045856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Küppers R, Engert A, Hansmann ML. Hodgkin lymphoma. J Clin Invest. 2012;122:3439–47. doi: 10.1172/JCI61245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steidl C, Connors JM, Gascoyne RD. Molecular pathogenesis of Hodgkin’s lymphoma: increasing evidence of the importance of the microenvironment. J. Clin. Oncol. 2011;29:1812–1826. doi: 10.1200/JCO.2010.32.8401. [DOI] [PubMed] [Google Scholar]
- 11.Martin-Moreno AM, Roncador G, Maestre L, Mata E, Jiménez S, Martínez-Torrecuadrada JL, Reyes-García AI, Rubio C, Tomás JF, Estévez M, Pulford K, Piris MA, García JF. CSF1R Protein expression in reactive lymphoid tissues and lymphoma: its relevance in classical Hodgkin lymphoma. PLoS One. 2015;10:e0125203. doi: 10.1371/journal.pone.0125203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Kamper P, Bendix K, Hamilton-Dutoit S, Honore B, Nyengaard JR, d’Amore F. Tumor-infiltrating macrophages correlate with adverse prognosis and Epstein-Barr virus status in classical Hodgkin’s lymphoma. Haematologica. 2011;96:269–276. doi: 10.3324/haematol.2010.031542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tzankov A, Matter MS, Dirnhofer S. Refined prognostic role of CD68-positive tumor macrophages in the context of the cellular micromilieu of classical Hodgkin lymphoma. Pathobiology. 2010;77:301–308. doi: 10.1159/000321567. [DOI] [PubMed] [Google Scholar]
- 15.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, Bast MA, Rosenwald A, Muller-Hermelink HK, Rimsza LM, Campo E, Delabie J, Braziel RM, Cook JR, Tubbs RR, Jaffe ES, Lenz G, Connors JM, Staudt LM, Chan WC, Gascoyne RD. Tumor-Associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362:875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dada R. Program death inhibitors in classical Hodgkin’s lymphoma: a comprehensive review. Ann Hematol. 2018;97:555–561. doi: 10.1007/s00277-017-3226-0. [DOI] [PubMed] [Google Scholar]
- 17.Chetaille B, Bertucci F, Finetti P, Esterni B, Stamatoullas A, Picquenot JM, Copin MC, Morschhauser F, Casasnovas O, Petrella T, Molina T, Vekhoff A, Feugier P, Bouabdallah R, Birnbaum D, Olive D, Xerri L. Molecular profiling of classical Hodgkin lymphoma tissues uncovers variations in the tumor microenvironment and correlations with EBV infection and outcome. Blood. 2009;113:2765–3775. doi: 10.1182/blood-2008-07-168096. [DOI] [PubMed] [Google Scholar]
- 18.Tzankov A, Meier C, Hirschmann P, Went P, Pileri SA, Dirnhofer S. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin’s lymphoma. Haematologica. 2008;93:193–200. doi: 10.3324/haematol.11702. [DOI] [PubMed] [Google Scholar]
- 19.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117:5019–5032. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, Rosenberg SA, Coltman CA, Tubiana M. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: cotswolds meeting. J. Clin. Oncol. 1989;7:1630–1636. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Zhang H, Shen Z, Lin C, Wang X, Qin J, Qin X, Xu J, Sun Y. Increased expression of CSF-1 associates with poor prognosis of patients with gastric cancer undergoing gastrectomy. Medicine (Baltimore) 2016;95:e2675. doi: 10.1097/MD.0000000000002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Espinosa I, Beck AH, Lee CH, Zhu S, Montgomery KD, Marinelli RJ, Ganjoo KN, Nielsen TO, Gilks CB, West RB, van de Rijn M. Coordinate expression of colony-stimulating factor-1 and colony-stimulating factor-1-related proteins is associated with poor prognosis in gynecological and nongynecological leiomyosarcoma. Am J Pathol. 2009;174:2347–2356. doi: 10.2353/ajpath.2009.081037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardsen E, Uglehus RD, Due J, Busch C, Busund LT. The prognostic impact of M-CSF, CSF-1 receptor, CD68 and CD3 in prostatic carcinoma. Histopathology. 2008;53:30–38. doi: 10.1111/j.1365-2559.2008.03058.x. [DOI] [PubMed] [Google Scholar]
- 24.Koh YW, Park C, Yoon DH, Suh C, Huh J. CSF-1R expression in tumor-associated macrophages is associated with worse prognosis in classical Hodgkin lymphoma. Am J Clin Pathol. 2014;141:573–583. doi: 10.1309/AJCPR92TDDFARISU. [DOI] [PubMed] [Google Scholar]
- 25.Steidl C, Diepstra A, Lee T, Chan FC, Farinha P, Tan K, Telenius A, Barclay L, Shah SP, Connors JM, van den Berg A, Gascoyne RD. Gene expression profiling of microdissected Hodgkin Reed-Sternberg cells correlates with treatment outcome in classical Hodgkin lymphoma. Blood. 2012;120:3530–3540. doi: 10.1182/blood-2012-06-439570. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald KP, Palmer JS, Cronau S, Seppanen E, Olver S, Raffelt NC, Kuns R, Pettit AR, Clouston A, Wainwright B, Branstetter D, Smith J, Paxton RJ, Cerretti DP, Bonham L, Hill GR, Hume DA. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood. 2010;116:3955–3963. doi: 10.1182/blood-2010-02-266296. [DOI] [PubMed] [Google Scholar]
- 27.Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, Mulder GE, Toebes M, Vesely MD, Lam SS, Korman AJ, Allison JP, Freeman GJ, Sharpe AH, Pearce EL, Schumacher TN, Aebersold R, Rammensee HG, Melief CJ, Mardis ER, Gillanders WE, Artyomov MN, Schreiber RD. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–81. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myklebust JH, Irish JM, Brody J, Czerwinski DK, Houot R, Kohrt HE, Timmerman J, Said J, Green MR, Delabie J, Kolstad A, Alizadeh AA, Levy R. High PD-1 expression and suppressed cytokine signaling distinguish T cells infiltrating follicular lymphoma tumors from peripheral T cells. Blood. 2013;121:1367–1376. doi: 10.1182/blood-2012-04-421826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto R, Nishikori M, Kitawaki T, Sakai T, Hishizawa M, Tashima M, Kondo T, Ohmori K, Kurata M, Hayashi T, Uchiyama T. PD-1-PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood. 2008;111:3220–3224. doi: 10.1182/blood-2007-05-085159. [DOI] [PubMed] [Google Scholar]
- 30.Ansell SM. Hodgkin lymphoma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:434–442. doi: 10.1002/ajh.24272. [DOI] [PubMed] [Google Scholar]
- 31.Paydas S, Bagir E, Seydaoglu G, Ercolak V, Ergin M. Programmed death-1 (PD-1), programmed death-ligand 1 (PD-L1), and EBV-encoded RNA (EBER) expression in Hodgkin lymphoma. Ann Hematol. 2015;94:1545–1552. doi: 10.1007/s00277-015-2403-2. [DOI] [PubMed] [Google Scholar]
- 32.Green MR, Rodig S, Juszczynski P, Ouyang J, Sinha P, O’Donnell E, Neuberg D, Shipp MA. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res. 2012;18:1611–1618. doi: 10.1158/1078-0432.CCR-11-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MG, Xu ML, Yu H, Fletcher CD, Freeman GJ, Shipp MA, Rodig SJ. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19:3462–3473. doi: 10.1158/1078-0432.CCR-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Assis MC, Campos AH, Oliveira JS, Soares FA, Silva JM, Silva PB, Penna AD, Souza EM, Baiocchi OC. Increased expression of CD4+ CD25+FOXP3+ regulatory T cells correlates with Epstein-Barr virus and has no impact on survival in patients with classical Hodgkin lymphoma in Brazil. Med Oncol. 2012;29:3614–9. doi: 10.1007/s12032-012-0299-4. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu K, Nakata M, Hirami Y, Yukawa T, Maeda A, Tanemoto K. Tumor-infiltrating Foxp3+ regulatory T cells are correlated with cyclooxygenase-2 expression and are associated with recurrence in resected non-small cell lung cancer. J Thorac Oncol. 2010;5:585–590. doi: 10.1097/JTO.0b013e3181d60fd7. [DOI] [PubMed] [Google Scholar]
- 36.Preston CC, Maurer MJ, Oberg AL, Visscher DW, Kalli KR, Hartmann LC, Goode EL, Knutson KL. The ratios of CD8+ T cells to CD4+CD25+ FOXP3+ and FOXP3- T cells correlate with poor clinical outcome in human serous ovarian cancer. PLoS One. 2013;8:e80063. doi: 10.1371/journal.pone.0080063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schreck S, Friebel D, Buettner M, Distel L, Grabenbauer G, Young LS, Niedobitek G. Prognostic impact of tumour-infiltrating Th2 and regulatory T cells in classical Hodgkin lymphoma. Hematol Oncol. 2009;27:31–39. doi: 10.1002/hon.878. [DOI] [PubMed] [Google Scholar]
- 38.Kiyasu J, Aoki R, Tanaka PY, Pracchia LF, Calore EE, Perez NM, Kimura Y, Niino D, Sugita Y, Takayanagi R, Abe Y, Matsuoka M, Ohshima K. FOXP3(+) regulatory and TIA-1(+) cytotoxic T lymphocytes in HIV-associated Hodgkin lymphoma. Pathol Int. 2012;62:77–83. doi: 10.1111/j.1440-1827.2011.02754.x. [DOI] [PubMed] [Google Scholar]
- 39.Cohen JI, Bollard CM, Khanna R, Pittaluga S. Current understanding of the role of Epstein-Barr virus in lymphomagenesis and therapeutic approaches to EBV-associated lymphomasb. Leuk Lymphoma. 2008;49:27–34. doi: 10.1080/10428190802311417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baumforth KR, Birgersdotter A, Reynolds GM, Wei W, Kapatai G, Flavell JR, Kalk E, Piper K, Lee S, Machado L, Hadley K, Sundblad A, Sjoberg J, Bjorkholm M, Porwit AA, Yap LF, Teo S, Grundy RG, Young LS, Ernberg I, Woodman CB, Murray PG. Expression of the Epstein-Barr virus-encoded Epstein-Barr virus nuclear antigen 1 in Hodgkin’s lymphoma cells mediates up-regulation of CCL20 and the migration of regulatory T cells. Am J Pathol. 2008;173:195–204. doi: 10.2353/ajpath.2008.070845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiraz Y, Baran Y, Nalbant A. T cells in tumor microenvironment. Tumour Biol. 2016;37:39–45. doi: 10.1007/s13277-015-4241-1. [DOI] [PubMed] [Google Scholar]


