Abstract
Objective: Common treatment methods have shown a lack of therapeutic effect in melanoma, a type of malignant tumor. The pathogenesis of melanoma is not yet fully clear, therefore, search for a new treatment strategy is urgent. Recent studies have demonstrated that miR-182 is remarkably over-expressed in human melanoma. Our study aimed to explore the underlying mechanism of miR-182 on melanoma. Methods: In this study, the expression level of miR-182 was detected using RT-PCR in melanoma and adjacent tissues as well as in HEM-m, A375, A2058, and WM35 cell lines. Functions of miR-182 were investigated by using CCK-8 on cell proliferation, apoptosis, invasion, and cell cycle on A375 cells. Moreover, expression levels of Frz, Dsh, β-catenin, APC, Axin, GSK-3β, and CK1 were detected by Western blotting after knockdown and overexpression of miR-182. Overexpression of miR-182 and knockdown of APC was used to demonstrate regulated functions in melanoma. Results: Expression levels of miR-182 were significantly upregulated in melanoma tissues and cell lines. Overexpression of miR-182 promoted cell proliferation, migration, and invasion while inhibiting cell apoptosis and cell cycle in S phase. Overexpression of miR-182 upregulated expression levels of β-catenin and APC. Overexpression of miR-182 and knockdown of APC inhibited proliferation of melanoma cells and tumors. Conclusion: Overexpression of miR-182 promotes cell proliferation and invasion by targeting to APC in melanoma. The pathogenesis of miR-182 and APC might provide therapeutic targets for treatment of melanoma in the molecular level.
Keywords: miR-182, APC, proliferation, apoptosis, melanoma
Introduction
Melanoma is a highly aggressive skin tumor with significantly increased incidence in recent years, gradually becoming a common malignant tumor. Its rapid progress, ease of transfer, and poor clinical outcome have caused serious harm to human health [1,2]. Early melanoma is mainly treated by surgical resection but for advanced metastatic melanoma, the current treatment has had little effect. Metastatic melanoma has shown little response to radiotherapy and chemotherapy treatments, with a high recurrence rate [3,4]. With development of tumor molecular biology, the novel drug vemurafenib was targeted to BRAF mutant melanoma. It brought new hope for the treatment of melanoma but resistance to targeted therapy remains a serious challenge [5,6]. Therefore, in order to improve the efficacy of melanoma treatment, its occurrence, development, and other molecular mechanisms of research have become necessary.
miRNAs are a class of single-stranded RNA, about 22 nucleotides in length, which are endogenous non-protein-encoded small RNAs in post-transcriptional regulation genes [7-9]. In 1993, Ambros discovered the first miRNA-Lin4, which has the powerful function of regulating expression of Lin-14 gene [10]. In 2000, expression of let-7 miRNA was confirmed in humans and in other species, to be playing an important regulatory role in the occurrence and development of many diseases [11,12]. In 2002, miRNAs were first confirmed to have correlation to tumors [13]. Mature miRNAs affect protein expression by specifically recognizing and binding to the 3’untranslated region (3’-UTR) of target mRNA, leading to mRNA degradation or inhibition of translation [14]. miRNAs play important roles in the biological processes of cell proliferation, differentiation, and apoptosis and changes of their expression levels are closely related to occurrence and development of many tumors, including melanoma [15].
Because of its high degree of malignancy, rapid development, and easy transfer, melanoma has been of great concern considering the relationship between metastasis and invasion. In this present study, we explored the effects of miR-182 on proliferation, apoptosis, metastasis, and invasion. Moreover, we show the possible connection to adenomatous polyposis coli (APC) in melanoma cells. This study could provide potential therapeutic targets for treatment of melanoma.
Materials and methods
Cells and surgical specimens
Melanoma cell lines were purchased from the Cell Culture Collection of the Chinese Academy of Sciences (Beijing, China) and cultured in DMEM medium containing 10% fetal bovine serum (HyClone, Logan, UT, USA), 100 mg/mL streptomycin, and 100 U/mL penicillin in a humidified incubator containing 5% CO2 at 37°C for 2-3 days. Nude mice were obtained from Chinese Academy of Medical Sciences (Beijing, China). Specimens of melanoma tissues and adjacent noncancerous tissues were collected from 60 patients with melanoma between October 2016 and May 2017, following surgical resection at China-Japan Union Hospital of Jilin University. All experiments were approved by the Medical Ethics Committee of China-Japan Union Hospital and written informed consent documents were signed by all of the patients.
Mimics and inhibitors designed for synthesis and transfection
Gemma Gene Company designed synthesis miR-182 mimics and inhibitors as well as si-APC. The miR-182 sequence was as follows: mimics: 5’-UUUGGCAAUGGUAGAACUCACACU-3’; mimics-nc: 5’-UUCUCCGAACGUGUCACGUTT-3’; inhibitors: 5’-AGUGUGAGUUCUACCAUUGCCAAA-3’; inhibitors-nc: 5’-CAGUACUUUUGUGUAGUACAA-3’. Centrifuge tubes containing mimics or inhibitors were added in sterile DEPCs and placed at a concentration of 20 μM. Two mL of serum-free medium without antibiotics was added to melanoma cells and transfection solution containing Opti-MEM and Lipofectamine 2000 was added. Replacement of serum containing antibiotics was added in the medium and continued to culture 24 hours after the collection of cells.
Cell viability assay
Cells were transfected with miR-182 mimics and inhibitors for 48 hours to detect cell viability, operating according to CCK-8 kit instructions (Beijing Li Weining Biological Technology Co., Ltd, Beijing). Transfected cells were incubated in a cell incubator for 0.5-4 hours and tested at 0.5, 1, 2, and 4 hours, respectively, using a microplate reader. Absorbance was measured at 450 nm.
Flow cytometry assay
Cells were digested with trypsin without EDTA and centrifuged at 1000 r/min for 5 minutes. Cells were harvested, washed twice with precooled PBS, and Annexin buffer was added. 5 μL of Annexin V and 1 μL of PI were added and incubated at room temperature for about 15 minutes. Cell apoptosis was measured on a flow cytometer. miR-182 overexpression and knockout of experimental groups were set up with two holes and the experiment was repeated 3 times.
Cell invasion assay
For the migration assay, 5 × 104 cells were suspended in 1% serum medium and upper chamber of the Transwell chamber was added to the pre-deposited matrix gel. The complete medium containing 10% fetal bovine serum was added to the lower chamber. After 48 hours of cell culturing, cells were migrated to the other side of the membrane and stained with 0.1% crystal violet. Cells were then stained with 100-fold optical microscopy (Olympus, Japan). All of the experiments were carried out three times.
Wound healing assay
Cultured plates were seeded on the back of the line before the label, cell digestion after access to 12-well plate, and perpendicular to the orifice to create cell scratches. We absorbed the cell culture medium, rinsed the orifice plate three times with PBS, and washed away scratches generated by cell debris. We added serum-free medium and culture plate into the incubator culture, every 4-6 hours pictures were taken. We analyzed the experimental results based on collected image data.
Effects on EMT process assay
Expression levels of tumor markers were analyzed in A375 cells by transfecting with miR-182 mimics and inhibitors, using mimics-nc and inhibitors-nc as positive control. Expression levels of Frz, Dsh, β-catenin, APC, Axin, GSK-3β, and CK1 were detected by Western blotting. β-actin was used as an internal standard.
In vivo tumorigenicity
Animal experiments were done according to Institutional Animal Care and Use Committee (IACUC) protocol and approved by the China-Japan Union Hospital of Jilin University for Use and Care of Animals. The establishment of the subcutaneous melanoma tumor model was treated by subcutaneous injection of A375 cells. A total of 16 4-week-old female BALB/C nude mice were required to establish melanoma model mice for 4 weeks. Mice were sacrificed 26 days after the injection and size of the tumor was measured by vernier caliper.
Statistical analysis
Results are presented as mean ± standard deviation (SD). Comparisons between more than two groups were performed by conducting an analysis of variance (one-way ANOVA) and P<0.05 was considered statistically significant.
Results
Expression of miR-182 was upregulated in melanoma
Quantitative reverse transcription PCR (qRT-PCR) was employed to detect miR-182 levels in melanoma tissues. Results showed that expression of miR-182 was significantly higher in melanoma tissue than in adjacent tissues (Figure 1A, 1B) and upregulated expression of miR-182 was also detected in melanoma cells (Figure 1C).
Figure 1.
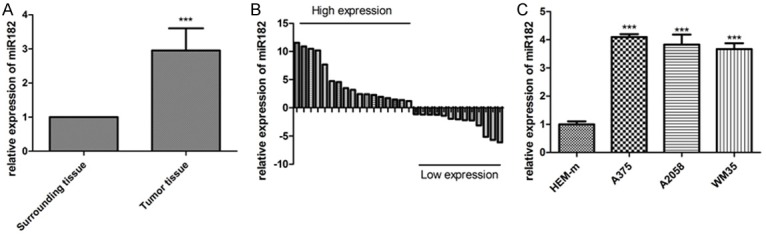
miR-182 was upregulated in melanoma. A, B. The expression level of miR-182 was upregulated in melanoma tissues, ***P<0.001. C. The expression level of miR-182 was upregulated in A375, A2058, and WM35 cell lines, ***P<0.001.
Expression level of miR-182 affected cell growth
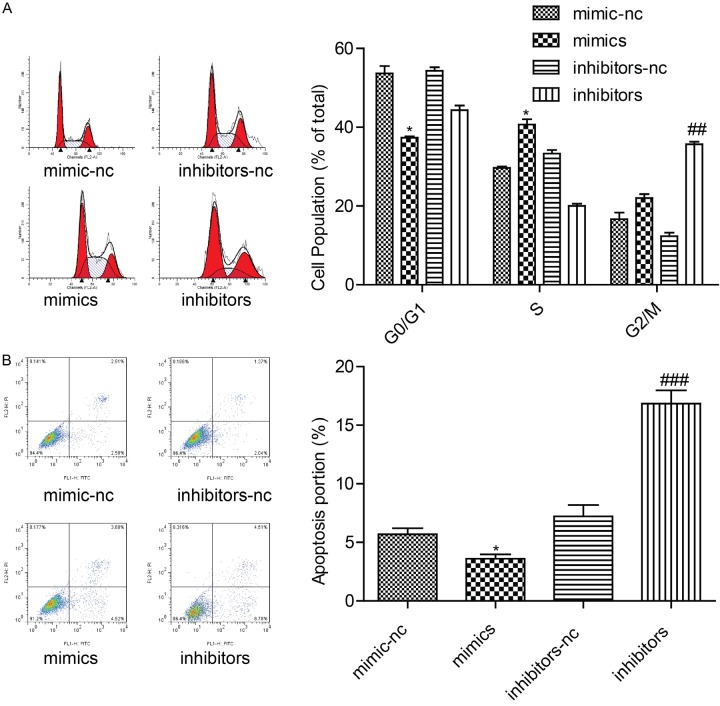
After transfecting with miR-182 mimics, inhibitors, mimics-nc, and inhibitor-nc in A375 cells, as shown in Figure 2A, expression of miR-182 was increased. Overexpression of miR-182 significantly promoted tumor cell proliferation while downregulated expression of miR-182 inhibited A375 cell proliferation (Figure 2B). Apoptosis of A375 cells was detected by flow cytometry. As shown in Figure 2C, overexpression of miR-182 enhanced invasion ability to tumor cells. Invasion was further weakened in the knockdown miR-182 tumor cell, in comparison to the control. The reduction in wound closures by miR-182 further confirmed its effect in promoting cell migration to A375 cells. Results show that it significantly reduced widths for residual gaps obtained in the transfection of miR-182 mimics (Figure 2D). As shown in Figure 3A, overexpression of miR-182 also blocked the cell cycle in S phase and inhibited A375 cell apoptosis (Figure 3B).
Figure 2.
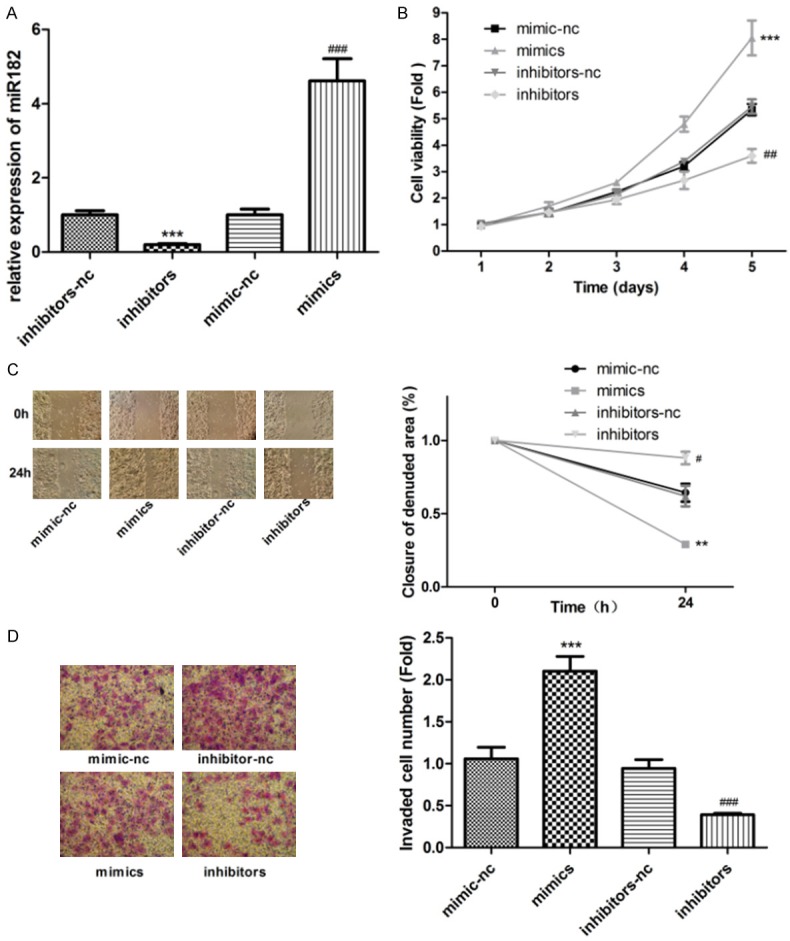
The expression level of miR-182 affected cell growth of A375 cells. A. The expression level of miR-182 was detected by qRT-PCR by transfection of miR-182 mimics and inhibitors, ***P<0.001, ###P<0.001. B. Wound Healing assay was used to detect the ability of cell migration, **P<0.01, #P<0.05. C. CCK-8 assay was used to detect cell proliferation by transfecting with mimics-nc, inhibitors-nc, miR-182 mimics, and inhibitors in A375 cells. ***P<0.001, ##P<0.01. D. Transwell assay was used to detect cell invasion, ***P<0.001, ###P<0.001.
Figure 3.
The expression level of miR-182 affected apoptosis and cell cycle. A. Flow cytometry assay was used to detect cell apoptosis by transfecting with mimics-nc, inhibitors-nc, miR-182 mimics, and inhibitors in A375 cells. *P<0.05, ##P<0.01. B. Cell cycle was detected by transfecting with mimics-nc, inhibitors-nc, miR-182 mimics, and inhibitors, *P<0.05, ###P<0.001.
Overexpression of miR-182 affected expression of related proteins in Wnt signaling pathway
After transfecting with miR-182 mimics in melanoma cells, expressions of Frz, Dsh, β-catenin, APC, Axin, GSK-3β, and CK1 were detected by Western blotting. As shown in Figure 4A, expression of β-catenin and APC significantly increased in the miR-182 mimics group while there was no significant difference in expression of other proteins between the two groups. The PCR assay found that expression of APC was upregulated in melanoma cells while the same tendency in expression of β-catenin did not exist (Figure 4B). Immunofluorescence assay indicated that expression of β-catenin was upregulated in the nucleus by transfection of miR-182 mimics (Figure 4C).
Figure 4.
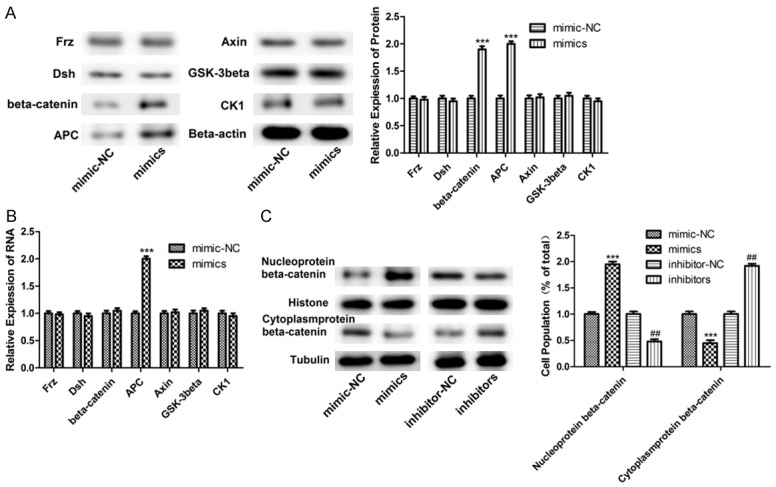
Overexpression of miR-182 affected expression of related proteins in Wnt signaling pathway. A. The protein levels of Frz, Dsh, β-catenin, APC, Axin, GSK-3β, and CK1 were detected by Western blot at overexpression of miR-182, ***P<0.001. B. The expression levels of Frz, Dsh, β-catenin, APC, Axin, GSK-3β, and CK1 were detected by PCR at overexpression of miR-182, ***P<0.001. C. Expression of β-catenin was detected by immunofluorescence assay, ***P<0.001, ##P<0.01.
Overexpression of miR-182 and knockdown of APC affect the growth of melanoma cells
After transfecting with si-APC in the melanoma cells, expression levels of APC were significantly downregulated (Figure 5A, 5B). Then, si-APC melanoma cells were transfecting with miR-182 mimics. Cell proliferation, migration, and invasion were downregulated compared with the miR-182 overexpression group (Figure 5C, 5D) and nuclear aggregation of β-catenin was also weakened in the melanoma cells (Figure 5E).
Figure 5.
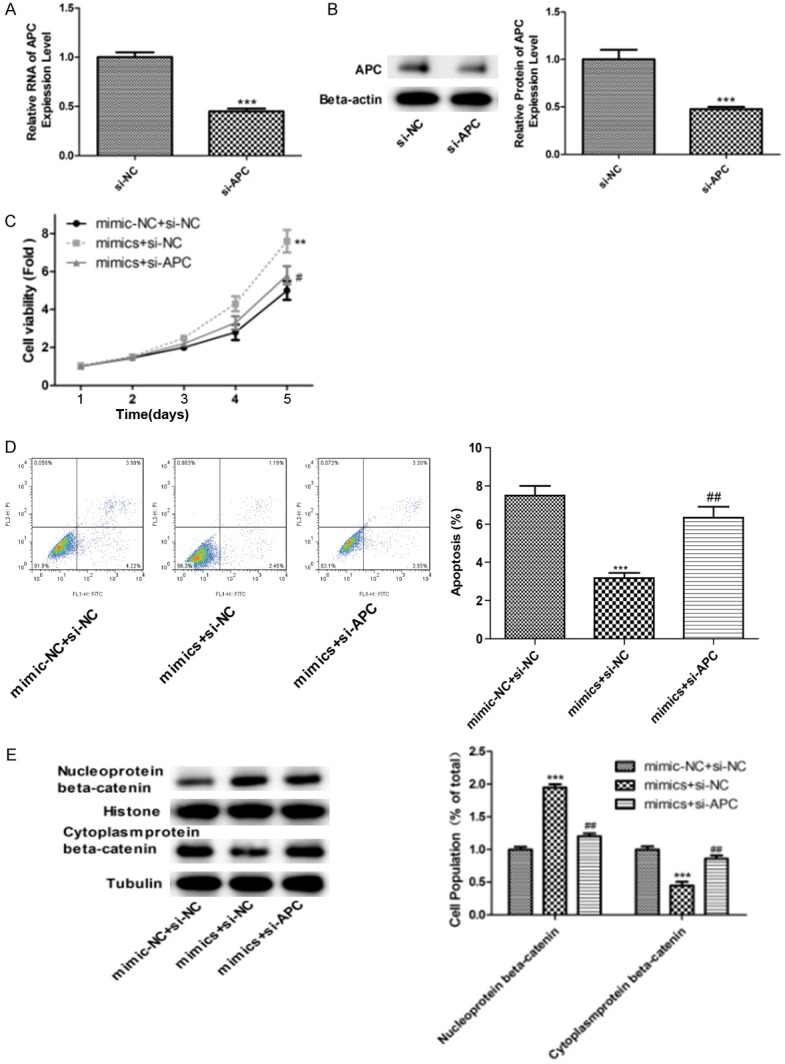
Overexpression of miR-182 and knockdown of APC affect growth of melanoma cells. A. mRNA expression of APC was detected by PCR, ***P<0.001. B. Protein expression of APC was detected by WB, ***P<0.001. C. Cell viability was detected at overexpression of miR-182 and knockdown of APC, **P<0.01, #P<0.05. D. Cell apoptosis was detected at overexpression of miR-182 and knockdown of APC, ***P<0.001, ##P<0.01. E. Protein expression of β-catenin was detected by immunofluorescence assay at overexpression of miR-182 and knockdown of APC, ***P<0.001, ##P<0.01.
Tumor experiment in vitro
A375 cells were transfected with miR-182 mimics and si-APC and then injected into the nude mice. After feeding for 2 weeks, the tumor tissue was tested for size. As shown in Figure 6, overexpression of miR-182 and knockdown of APC group had a small tumor size, indicating that downregulation of APC should inhibit growth of melanoma cells.
Figure 6.
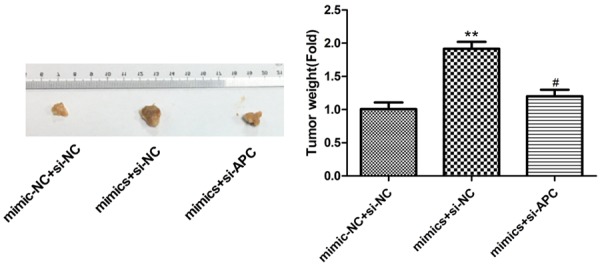
Tumor xenograft model in the nude mice was detected at overexpression of miR-182 and knockdown of APC, **P<0.01, #P<0.05.
Discussion
Melanoma is a highly malignant tumor. The most common treatmens are surgical resection, chemotherapy, immunotherapy, and radiation therapy. Effectiveness of these treatments, however, has been unsatisfactory, especially in metastatic melanoma [16,17]. In the present study, our aim was to search for new treatment methods since the pathogenesis of melanoma is not yet completely clear and there are no clear effective ways to treat melanoma. We clarified that regulation of microRNA is involved in the development of melanoma. In this study, we focused on the function of miR-182 on proliferation of melanoma cells.
In the development of normal melanoma cells to malignant melanoma, miRNAs regulate expression of key proteins through the regulation of target genes, so as to promote or inhibit melanoma cell proliferation, differentiation, and apoptosis, affecting occurrence and development of melanoma. It has been discovered that Dicer enzyme, regulatory protein Argonaute2, and miRNA abnormally expressed in the primary cell culture of melanoma. At the same time, 157 different miRNAs were analyzed for expression differences between benign melanoma and primary melanoma by real-time quantitative PCR amplification, the results showing that there were 72 miRNA expression differences [18,19]. The present study found that abnormal expression of miRNA plays different roles in melanoma [20].
Microphthalmia-associated transcription factor (MITF) is a member of the transcription factor family, which regulates differentiation and migration of cells by various signaling pathway centers [21]. In melanoma, multiple miRNAs regulate cell invasion and metastasis of melanoma by MITF. Epithelial-mesenchymal transition (EMT) is a pathophysiological phenomenon in which epithelial cells lose polarity, cell connectivity, and adhesion decreases compared with cytoskeleton changing and cell motility increasing. Studies have also indicated that genes in the EMT control the invasion and metastasis process of melanoma [22].
miRNAs encoded by the let-7 family were the first inhibitory miRNAs found to negatively regulate expression of oncogene Ras [23]. When compared to primary melanocytes and melanoma cells, let-7a was found to be absent in melanoma cells. In contrast, when melanocytes were transfected with let-7a antagonist, expression of both of them was induced and invasion ability was enhanced [24,25]. In 2012, Giles demonstrated for the first time that miR-7-5p inhibits melanoma cell migration in melanoma by directly regulating IRS-2 expression [26]. Compared with primary melanoma cells, expression of miR-7-5p is downregulated in metastatic melanoma cell lines. Abnormal expression of miR-7-5p could significantly inhibit migration and invasion of melanoma cells. It has been indicated that upregulation of miR-7-5p is beneficial in the treatment of melanoma and might be a novel therapeutic target.
miR-182 is known as a new research hotspot and it had been found that miR-182 is closely related to occurrence and development of lung cancer, gastric cancer, colorectal cancer, pancreatic cancer, and so forth. Researchers have found that the role of miR-182 is complex. It could inhibit occurrence of tumors and in some cases could also promote tumorigenesis. Studies have found that miR-182 could inhibit proliferation of gastric cancer by inhibiting the CREB1 gene [27].
In breast cancer, colorectal cancer, lung cancer, and prostate cancer research, miR-182 may promote occurrence and development. miR-182 has a complex relationship with tumors and its specific mechanism for regulating tumors remains unclear. It is either by inhibiting DNA repair or by hypermethylation of its promoters [28]. Some studies have found that miR-182 was downregulated by playing roles of tumor suppressor gene in some gastric cancers and kidney cancers. The study also demonstrated that miRNA could increase sensitivity of melanoma in clinical treatment. Through the present experimental results, it was found that miR-182 plays an important regulatory role in development of melanoma.
In the present study, miR-182 is found to play important roles in the pathogenesis and progression of melanoma, promoting migration and invasion of melanoma cells by the potential target of APC. In conclusion, miR-182 has a broad application of prospects in early diagnosis, prognosis, and treatment of melanoma. miR-182 may be a significant factor in the possible curing of melanoma.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (81671220, 81502516) and the Jilin Scientific and Technological Development Program (20150311069YY, 20160101077JC).
Disclosure of conflict of interest
None.
References
- 1.Chamberlain MC, Baik CS, Gadi VK, Bhatia S, Chow LQ. Systemic therapy of brain metastases: non-small cell lung cancer, breast cancer, and melanoma. Neuro Oncol. 2017;19:1–24. doi: 10.1093/neuonc/now197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jagtap DB, Rosenberg CA, Martin L, Pettinger M, Greenland P, Khandekar JD, Levy R, Lane D, Simon MS. Prospective analysis of association between use of statins or other lipid-lowering agents andmelanoma risk in the women’s health initiative. J. Clin. Oncol. 2011;29:1586. [Google Scholar]
- 3.Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma. Lancet. 2005;365:687–701. doi: 10.1016/S0140-6736(05)17951-3. [DOI] [PubMed] [Google Scholar]
- 4.Puig Sardá S. New dermatological insights in melanoma diagnosis and treatment. Actas Dermosifiliogr. 2017;108:1–2. doi: 10.1016/j.ad.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, McArthur GA, Hutson TE, Moschos SJ, Flaherty KT, Hersey P, Kefford R, Lawrence D, Puzanov I, Lewis KD, Amaravadi RK, Chmielowski B, Lawrence HJ, Shyr Y, Ye F, Li J, Nolop KB, Lee RJ, Joe AK, Ribas A. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, Kehoe SM, Johannessen CM, Macconaill LE, Hahn WC, Meyerson M, Garraway LA. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J. Clin. Oncol. 2011;29:3085–3096. doi: 10.1200/JCO.2010.33.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohammadi A, Mansoori B, Baradaran B. Regulation of miRNAs by herbal medicine: an emerging field in cancer therapies. Biomed Pharmacother. 2016;86:262–270. doi: 10.1016/j.biopha.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 8.Barwari T, Joshi A, Mayr M. MicroRNAs in cardiovascular disease. J Am Coll Cardiol. 2016;68:2577–2584. doi: 10.1016/j.jacc.2016.09.945. [DOI] [PubMed] [Google Scholar]
- 9.Garofalo M, Croce CM. MicroRNAs: master regulators as potential therapeutics in cancer. Annu Rev Pharmacol Toxicol. 2011;51:25–43. doi: 10.1146/annurev-pharmtox-010510-100517. [DOI] [PubMed] [Google Scholar]
- 10.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 11.Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 12.Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B, Müller P, Spring J, Srinivasan A, Fishman M, Finnerty J, Corbo J, Levine M, Leahy P, Davidson E, Ruvkun G. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408:86–89. doi: 10.1038/35040556. [DOI] [PubMed] [Google Scholar]
- 13.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 15.Bonazzi VF, Stark MS, Hayward NK. Micro RNA regulation of melanoma progression. Melanoma Res. 2012;22:101–113. doi: 10.1097/CMR.0b013e32834f6fbb. [DOI] [PubMed] [Google Scholar]
- 16.Gangi A, Zager JS. The safety of talimogene laherparepvec for the treatment of advanced melanoma. Expert Opin Drug Saf. 2016;23:1–5. doi: 10.1080/14740338.2017.1274729. [DOI] [PubMed] [Google Scholar]
- 17.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O’Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Huang J, Yang N, Greshock J, Megraw MS, Giannakakis A, Liang S, Naylor TL, Barchetti A, Ward MR, Yao G, Medina A, O’brien-Jenkins A, Katsaros D, Hatzigeorgiou A, Gimotty PA, Weber BL, Coukos G. microRNAs exhibit high frequency genomic alterations in human cancer. Proc Natl Acad Sci U S A. 2006;103:9136–9141. doi: 10.1073/pnas.0508889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schultz J, Lorenz P, Gross G, Ibrahim S, Kunz M. MicroRNA let-7b targets important cell cycle molecules in malignant melanoma cells and interferes with anchorage-independent growth. Cell Res. 2008;18:549–557. doi: 10.1038/cr.2008.45. [DOI] [PubMed] [Google Scholar]
- 20.Howell PM Jr, Li X, Riker AI, Xi Y. MicroRNA in Melanoma. Ochsner J. 2010;10:83–92. [PMC free article] [PubMed] [Google Scholar]
- 21.Denat L, Larue L. Malignant melanoma and the role of the paradoxal protein microphthalmia transcription factor. Bull Cancer. 2007;94:81–92. [PubMed] [Google Scholar]
- 22.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelialmesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Müller DW, Bosserhoff AK. Integrin beta 3 expression is regulated by let-7a miRNA in malignant melanoma. Oncogene. 2008;27:6698–6706. doi: 10.1038/onc.2008.282. [DOI] [PubMed] [Google Scholar]
- 25.Fu TY, Chang CC, Lin CT, Lai CH, Peng SY, Ko YJ, Tang PC. Let-7b-mediated suppression of basigin expression and metastasis in mouse melanoma cells. Exp Cell Res. 2011;317:445–451. doi: 10.1016/j.yexcr.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Giles KM, Brown RA, Epis MR, Kalinowski FC, Leedman PJ. miRNA-7-5pinhibits melanoma cell migration and invasion. Biochem Biophys Res Commun. 2013;430:706–710. doi: 10.1016/j.bbrc.2012.11.086. [DOI] [PubMed] [Google Scholar]
- 27.Sachdeva M, Mito JK, Lee CL, Zhang M, Li Z, Dodd RD, Cason D, Luo L, Ma Y, Van Mater D, Gladdy R, Lev DC, Cardona DM, Kirsch DG. MicroRNA-182drives metastasis of primary sarcomas by targeting multiplegenes. J Clin Invest. 2014;124:4305–4319. doi: 10.1172/JCI77116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moskwa P, Buffa FM, Pan YF, Panchakshari R, Gottipati P, Muschel RJ, Beech J, Kulshrestha R, Abdelmohsen K, Weinstock DM, Gorospe M, Harris AL, Helleday T, Chowdhury D. miR-182-mediateddown-regulation of BRCA1 impacts DNA repair and sensitivityto PARP inhibitors. Mol Cell. 2011;41:210–220. doi: 10.1016/j.molcel.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]