Abstract
Background: To investigate impacts of sodium arsenic (NaAsO2) on embryonic cardiac development in rats and evaluate the protective role of folate in NaAsO2 exposure rats. Methods: We divided 90 female rats randomly into 9 groups. Group A was the control; group B-F were the animals fed with NaAsO2 in a series of increased doses, corresponding to 9.4 mg/L, 18.8 mg/L, 37.5 mg/L, 75 mg/L and 150 mg/L, respectively; group G-I were fed with 75 mg/L of NaAsO2, in addition of folate with doses of 0.53 mg/kg, 5.3 mg/kg, and 10.6 mg/kg, respectively. Their fetus’ general development and cardiovascular systems were examined. Nkx2.5, GATA4, TBX5 gene and protein expression were measured. Results: Relatively to group A, arsenic treated group C-F rats generated significantly lower weight of fetus and placenta (P<0.05), whereas the folate-treated groups H and I were significantly heavier than the arsenic-treated group E (P<0.05). We observed that incidences of cardiac malformations were significantly greater in arsenic-treated group E and F than group A (P<0.05). We found that the Nkx2.5 and GATA4 protein expression in the fetal hearts were downregulated in group B-F compared to group A. But the expression of them was significantly upregulated in group H-I relatively to group E (P<0.05). Moreover, the TBX5 gene expression was increased in both group D-F and G-I when they were compared to group A or group E, respectively (P<0.05). Conclusion: NaAsO2 induce embryonic cardiac defection and folate supplement alleviate this impairment through modulation of the Nkx2.5, GATA4 and TBX5 gene expression.
Keywords: Arsenic, folate, congenital heart disease, Nkx2.5, GATA-4, TBX5
Introduction
Congenital heart diseases (CHD) manifest cardiovascular malformations during embryonic development. They are commonly diagnosed in children, in a frequency of 4 to 10 children per 1000 live births [1]. The genetic and environmental factors are the key determinants contributing to the multifactorial etiology of CHD. Currently, Environmental pollution is generally identified to be a major factor significantly correlating with occurrences of high incidences of CHD. Therefore, investigation of some key risk factors contributing to high incidences of CHD and their underlying molecular mechanisms might provide of proof-of-principle for early protective intervention.
Arsenic is a highly toxic metal, existing widely in our nature; it is a component mainly compounding pesticides, herbicides, alloy material, and medicine. It is absorbed by the respiratory and intestinal tracts and even the skin by a direct contact, and leads to multiple organ dysfunctions. During pregnancy, accumulated arsenic in the body will penetrate the placental barrier [2,3] and directly cause embryonic dysplasia, manifesting a systemic fetal development malformations, with growth retardation, short limbs [4], low viability [5] and multiple organ malformations commonly including neural tube defects [6], urogenital abnormalities [7], limb bud and body hypoplasia [8]. Importantly, maternal arsenic exposure has been shown strongly correlating with the occurrence of CHD in their offspring. However, these retrospective studies are inevitable memory deviation [9-11] and the direct evidence of arsenic-induced CHD remains elusive.
The Nkx2.5, GATA-4 and TBX5 genes are the cardiac-specific transcription factors. They control many downstream genes, possibly involving cardiovascular development. Aberrant Nkx2.5, GATA-4 and TBX5 expression (by genetically modification of animals by gene knockout) are associated with mammal heart malformations and CHD [12]. We hypothesized that arsenic exposure during pregestation and pregnancy could cause the fetal CHD, potentially mediated by downregulation of the cardiac-specific transcription factors-Nkx2.5, GATA-4 and TBX5 genes.
Supplementation of folate during pregestation and pregnancy has been shown an effective decrease in incidence of the fetal CHD [13,14]. Folate breaks down the plasma homocystenine exerting a protective function [15-17]. However, whether folate supplementation during the pregnancy could protect cardiovascular development from arsenic toxicity remains largely unexplored; the associated protective activity involving changes of the expression of the cardiac-specific transcription factors-Nkx2.5, GATA-4 and TBX5 remains to be examined.
Our present study now shows that NaAsO2 uptaken by SD rats increased high incidences of CHD. Conversely, folate supplementation significantly compromised the NaAsO2-induced CHD, whereby sustaining expression of several cardiac-specific transcription factors in rat embryonic hearts.
Materials and methods
Animals
Animals were 30-40 days old female SD rats purchased from the Animal Center of Fujian Medical University, Fuzhou, China. Animal care and experimental proceedings were according to guidelines established by Fujian Medical University Animal Care Commission and following approval of the ethical committee. Animals were kept at 22±2 degrees centigrade, relative humidity of 55%, in the light for 12±1 hours and in the dark for another 12±1 hours. Fed water was changed twice a week.
Groups and toxicity exposure
A total of 90 female rats were divided randomly into 9 groups after one week adaptively fed. Group A was the control; group B-F were the animals fed with NaAsO2 in a series of increased doses, corresponding to 9.4 mg/L, 18.8 mg/L, 37.5 mg/L, 75 mg/L and 150 mg/L, respectively, in drinking water; group G-I were fed with 75 mg/L of NaAsO2, in addition of folate with doses of 0.53 mg/kg, 5.3 mg/kg, and 10.6 mg/kg, respectively.
The female and adult SD rats were caged overnight at 2:1 ratio after 6 weeks feeding. On the next morning, the male rats were removed from cages; vaginal smears of the female rats were obtained. In the event that smears were observed, the rat was accepted as being at day 0 of the gestational period. There were 10 mice per group; they were sacrificed after 6 weeks treatment. Caesarean were proceed on the day 16 following previous feeding. It was recorded miscarriage if embryo could not be seen in the maternal uterus on that day.
General and pathological observation
Caesarean were proceeded on day 16 under general anesthesia using 10% chloral hydrate. Numbers of miscarriage rats were recorded, and the live fetus and placenta were weighted. We randomly pick 3 fetuses from each live birth and fixed in 10% formalin. After fixture for 24 h, heart and bilateral lung (set as control) were separated, dehydrated, paraffin embedded and continuously dissected at a 7 um thickness. The sections were dyed with HE staining. Heart histological examination was observed by light microscope.
RNA extraction and quantitative real time PCR (qRT-PCR)
Total RNA of fetal heart tissue was extracted according to Trizol reagent’s protocol (Invitrogen, USA). Amplification and detection of mRNA and synthesis of cDNA were accomplished by using iScript RT Supermix (Bio-Rad, USA) in accordance with the manufacturer’s protocol. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal standard. Primers of Nkx2.5: 5’-ACCCTCGGGCGGATAAGA-3’ and 5’-TCCTGCCGCTGTCGCTTAC-3’; GATA-4: 5’-AAACGGAAGCCCAAGAATC-3’ and 5’-CACTGGATGGATGGAGGAC-3’; TBX5: 5’-CTCCACCCAACCCATACCCACT-3’ and 5’-GCTGTGCCGACTCTGTCCTGTA-3’; GAPDH: 5’-TGATTCTACCCACGGCAAGT-3’ and 5’-AGCATCACCCCATTTGATGT-3’. The relative expression of Nkx2.5, GATA-4, TBX5 mRNA were measured by the comparative cycle threshold (Ct) method. All experiments were analyzed in triplicate.
Western blot (WB)
Approximately 50 mg of heart tissues were completely homogenated by using a plastic grinding rod. Total proteins were extracted and quantified by BCA assay. WB was performed using total proteins. Denatured SDS protein was loaded onto a 10% SDS-polyacrylamide gel and blotted onto polyvinylidene fluoride (PVDF) membrane. Then PVDF membranes were incubated with blocking buffer at room temperature for 90 min. Membrane incubated with polyclonal first antibody (Nkx2.5 was purchased from Biobyt, British; GATA-4 from Santa Cruz, USA; TBX5 from Thermo, USA and Lamin B1 was purchased from Boster, China) overnight at 4 degrees centigrade, washed and incubated with Anti-rabbit IgG (HRP-linked Antibody, Merck, German). Membranes were incubated in reacting lipid of chemiluminescence for 2 min and exposure to X-ray. Protein staining was recorded by film scanning and analyzed by using Quantity One to calculate gray scale ratio of Nkx2.5/Lamin B1, GATA-4/Lamin B1, TBX5/Lamin B1 in each group.
Statistical analysis
Statistical analysis was performed by SPSS 19.0 version. The quantitative data (x̅±s) were analyzed using Students’ t-test, which in accordance with normal distribution and the variance was homogeneous. The Chi-square test or Fisher exact probability method was implied to identify count data. P<0.05 was accepted to be significant.
Results
General observation
In our study, the pregnancy rats were 8, 8, 10, 9, 8, 9, 8, 9, 9 in group A-I, respectively. Numbers of miscarriage rats in group A-I were 0, 0, 0, 0, 1, 2, 0, 0 and 0. Relatively to group A, arsenic treated group C-F rats generated significantly lower weight of fetus and placenta (P<0.05), whereas the folate-treated groups H and I were significantly heavier than the arsenic-treated group E (P<0.05). as showed in Table 1.
Table 1.
Toxicity of NaAsO2 on embryonic heart and preventive effect of folate
| Groups | Pregnancy female rats | Miscarriage female rats | Weight of fetus/g (x̅±s) | Weight of placenta/g (x̅±s) |
|---|---|---|---|---|
| A | 9 | 0 | 0.62±0.05 | 0.36±0.05 |
| B | 8 | 0 | 0.61±0.07▲ (P=0.270) | 0.36±0.05▲ (P=0.853) |
| C | 10 | 0 | 0.58±0.08* (P=0.000) | 0.33±0.04* (P=0.000) |
| D | 9 | 0 | 0.56±0.06* (P=0.000) | 0.33±0.05* (P=0.002) |
| E | 8 | 1 | 0.52±0.06* (P=0.000) | 0.30±0.03* (P=0.000) |
| F | 9 | 2 | 0.50±0.05* (P=0.000) | 0.27±0.04* (P=0.000) |
| G | 8 | 0 | 0.51±0.07# (P=0.240) | 0.30±0.07# (P=0.472) |
| H | 9 | 0 | 0.56±0.08Δ (P=0.001) | 0.33±0.06Δ (P=0.000) |
| I | 9 | 0 | 0.56±0.09Δ (P=0.000) | 0.32±0.09Δ (P=0.004) |
Compared with group A, P>0.05;
Compared with group A, P<0.05;
Compared with group E, P>0.05;
Compared with group E, P<0.05.
Arsenic-induced embryonic cardiac malformations in rats and protective role of folate
We eventually collected 27 fetal hearts in group A, none of them showed malformation by using light microscope. We observed normal four cavity structure; well-developed atrial wall, ventricular wall, interventricular septum and large vessels; and maturity of atrioventricular valve and large artery membrane (slim valve). We also found none disorders in 24 and 30 fetal hearts derived from group B and C, respectively. However, 2 were ventricular septal defect (VSD) in 27 fetal hearts derived from group D; 3 were atrial septal defect (ASD), 1 had pulmonary stenosis in 21 fetal hearts derived from group E. We gathered 24 fetal hearts from group F, among them 5 VSD, 1 ASD and 2 pulmonary stenosis were found. Totally 24, 27 and 27 fetal hearts were collected from folate-treated group G to I, respectively. We observed 2 VSD and 2 ASD in group G, 2 VSD and 1 ASD in group H, and 1 VSD and 2 ASD in group I. The malformation rate of fetal heart in group A-I were: 0%, 0%, 0%, 7.4%, 23.8%, 38.1%, 16.7%, 11.1% and 11.1%, respectively. We observed that incidences of cardiac malformations were significantly greater in arsenic-treated group E and F than group A (P<0.05), (Table 2). There was no significantly difference of cardiac malformations in group E in comparison with groups G-I (P>0.05) (Table 2). Additionally, retardation of atrioventricular valve and large artery of fetal heart were common manifestations in groups with high-dose arsenic (≥37.5 mg/L NaAsO2) and partly manifest thin ventricular wall, large atrial cavity and other disorders in fetal hearts (Figure 1).
Table 2.
Heart malformation effect of NaAsO2 exposure and folate protective function
| Group | Number of hearts | VSD | ASD | Pulmonary stenosis | Amount (abnormality rate) |
|---|---|---|---|---|---|
| A | 27 | 0 | 0 | 0 | 0 (0%) |
| B | 24 | 0 | 0 | 0 | 0 (0%) |
| C | 30 | 0 | 0 | 0 | 0 (0%) |
| D | 27 | 2 | 0 | 0 | 2 (7.4%)▲ (P=0.491) |
| E | 21 | 3 | 1 | 1 | 5 (23.8%)* (P=0.012) |
| F | 21 | 5 | 1 | 2 | 8 (38.1%)* (P=0.001) |
| G | 24 | 2 | 2 | 0 | 4 (16.7%)# (P=0.713) |
| H | 27 | 2 | 1 | 0 | 3 (11.1%)# (P=0.272) |
| I | 27 | 1 | 2 | 0 | 3 (11.1%)# (P=0.272) |
Compared with group A, P>0.05;
Compared with group A, P<0.05;
Compared with group E, P>0.05.
Figure 1.
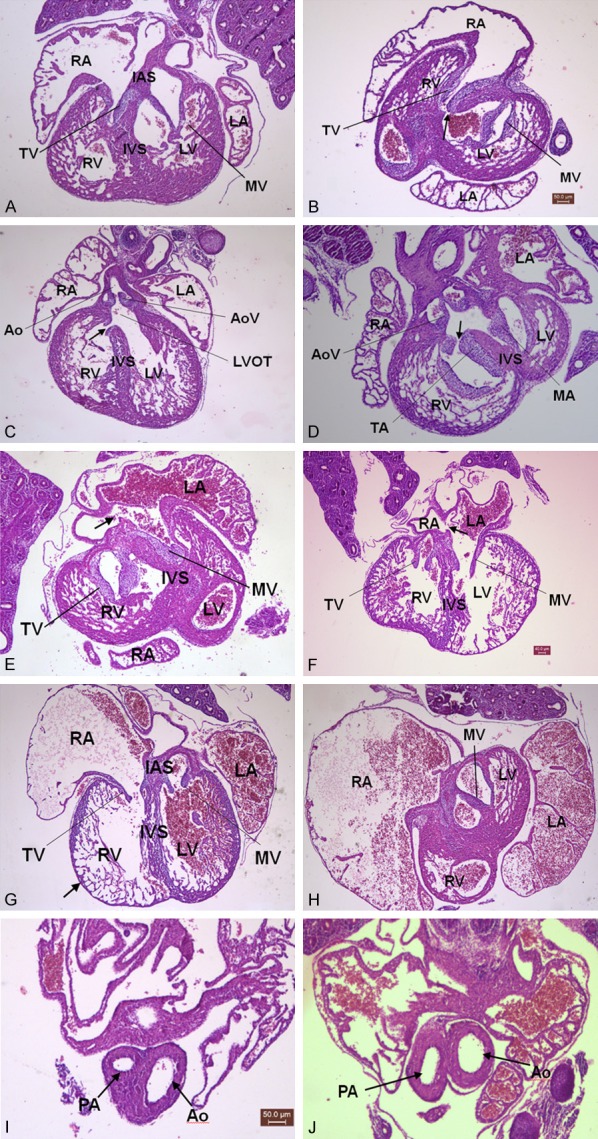
Effects of NaAsO2 exposure on embryonic heart development during Pregestation/gestation. ×40. A: Normal heart; B-D: ↑VSD; E: ↑ASD; F: ↑ASD; G: ↑Thin ventricular wall; H: Large atrial cavity; I, J: ↑Pulmonary stenosis. LV: left ventricular; RV: right ventricular; LA: left atrium; RA: right atrium; IVS: interventricular septum; IAS: interatrial septum; MV: mitral valve; TV: tricuspid valve; Ao: aorta; AoV: aortic valve; LVOT: left ventricular outflow tract; PA: pulmonary arterial; ↑: anomalous structure.
Possible mechanism of folate against arsenic toxicity on embryonic heart
Results of real-time PCR amplification curve and dissolution curve showed in Figure 2. We observed single sharp peak in dissolution curve in which confirmed results were specific products. Results of PCR showed that expression of Nkx2.5 and GATA4 mRNA in embryonic hearts were significantly downregulated in group B-F compared to group A (P<0.05). But the expression of them were significantly increased in group I, H relatively to group E (P<0.05). Moreover, expressions of TBX5 mRNA were significantly increased in both group D-F and G-I when they were compared to group A or group E, respectively (P<0.05), Figure 3.
Figure 2.
PCR amplification curve (left) and dissolution curve (right).
Figure 3.
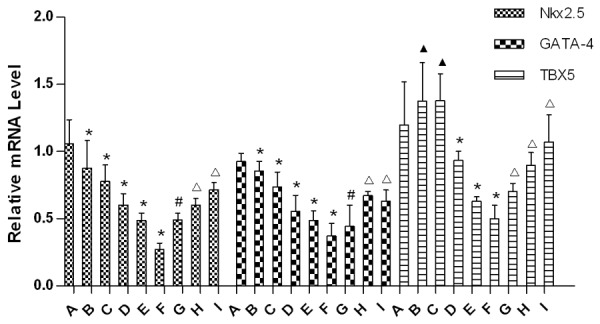
Relative mRNA levels of Nkx2.5, GATA4, TBX5 in each group’s embryonic heart. (▲Compared with group A, P>0.05; *Compared with group A, P<0.05; #Compared with group E, P>0.05; ΔCompared with group E, P<0.05).
WB assay revealed that Nkx2.5 and GATA4 protein expression in fetal heart were significantly decreased in group B-F compared to group A. However, the protein expression of them was significantly increased in group G-I relatively to group E (P<0.05), Figures 4, 5.
Figure 4.
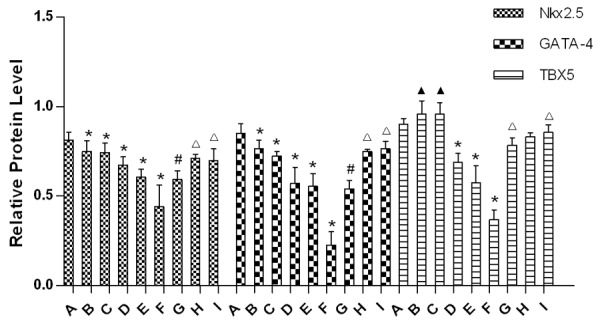
Relative protein levels of Nkx2.5, GATA4 and TBX5 in embryonic heart of each group. (▲Compared with group A, P>0.05; *Compared with group A, P<0.05; #Compared with group E, P>0.05; ΔCompared with group E, P<0.05).
Figure 5.
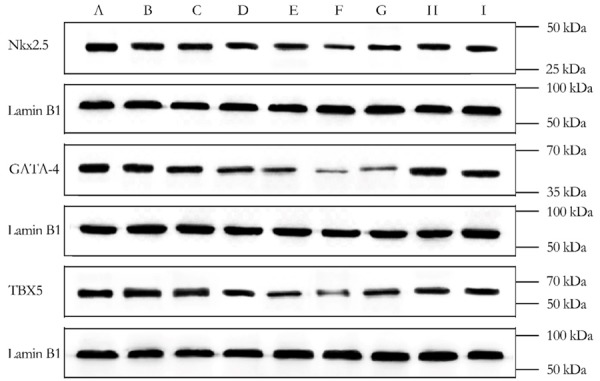
Results of Western Blot.
Discussion
Most environmental risk factors often pose threat to normal fetal development; they penetrate the placental blood barrier, and directly damage embryonic cells, in association with impaired cellular differentiation, proliferation and mobilization [18]. Arsenic and associated metabolites are such factors impair embryonic development at the teratogenic phase. Folate metabolism-essentially influences fetal development, regulating DNA synthesis, RNA transcription and tRNA methylation for protein expression and physiological organelle functions.
The loss of folate is essentially associated with dysmaturity of cellular DNA synthesis and cell proliferation. Folate supplementation at tetratogenesis for female rat decreased incidence of CHD in by 16.5% [19]. In line with these findings, our present study demonstrated the embryonic protective effects by folate; it decreased the incidences of heart malformation in rat embryo when they were exposed to arsenic.
Our present study showed that exposure of pregnant rat to arsenic led to a decrease in fetal and placental weights corresponding to a series increased arsenic-fed. We speculate that the embryonic cytotoxic NaAsO2 might restrain embryonic development and cell differentiation and proliferation [20]; consequently leading to hypoplasia and inhibit placental angiogenesis [21]. Attenuation of fetal angiogenesis in turn induces chronic intrauterine hypoxia and hence fetal growth retardation.
In this report, we showed that the beneficial effects of folate supplementation; it compromises toxicity of NaAsO2 and significantly increased the weights of rat fetus and placenta. Interestingly, protective effect of folate at 5.3 mg/kg and 10.6 mg/kg remained just the same. Based on that, intake proper dose of folate but not as much as possible is suitable to against toxicity of NaAsO2. Our present study showed that the higher doses of arsenic were uptaken by pregnant rat the more incidences of CHD observed in the embryos. The most CHD happened to be VSD, ASD, pulmonary stenosis and other cardiac malformation The exposure to high doses of arsenic often occurred in parallel with retardation of atrioventricular valve and large vessels, thin ventricular wall and large atrial cavity. The developmental stages of heart were highly sensitive to external factors. These essential multiple stages including formation of atrioventricular, valve, large vessel of fetal heart and atrioventricular separation [22]. As an external toxicity, NaAsO2 could interfere in any developmental stages of heart and lead to multi-structure abnormalities.
Conversely, we found that supplementation of folate during pregestation and gestation counteracted NaAsO2-induced CHD, with a decrease in incidences of cardiovascular malformations.
Several previous studies have shown that mammal heart development is regulated by cardiac-specific transcription factors. The Nkx2.5 gene is a subtype of the NK type of homologous nuclear gene family of Nkx2, controlling the differentiation of heart cells, formation and looping of cardiovascular, separation of atrioventricular, formation of atrioventricular valve and conductive in atrioventricular. The Nkx2.5 mutations caused abnormal downstream expression of genes: ANF, BMP, MLC2V, N-myc, MEF2C, dHAND, Msx2. They are involved in heart malformations [23].
The GATA family of zinc-finger transcription factors, GATA4 is proposed to regulate genes involved in myocardial differentiation and function. Since GATA4 contributes to 30%-40% of normal threshold values for regular development of heart. Homozygous GATA4 deletion is embryonic lethal due to defects of cardiac formations. Additionally, early alterations in GATA4 gene expression may result in cardiac dysplasia, endocardial cushion defects [24], ASD, pulmonary valve thickening, cardiac valve agenesia and other several cardiac malformation types [25].
The TBX5 gene is a member of a phylogenetically conserved T-box family of genes, which has recently been found to play a unique role in heart development. TXB5 combines downstream target genes with specific T-box domain, and regulates heart development throughout. It is necessary for initiation of atrioventricular cavity, separation of ventricular and cardiac [26]. The TBX5 deficiency is thus responsible for severe agenesia of atria and ventriculus sinister. Nkx2.5, GATA-4, TBX5 are the upstream transcriptional factors; they target several key genes and regulate cardiac development. They act in a coordinated fashion and decide each process of cardiac formation. Our present study now displays for the first time that the arsenic cardiac cytotoxic effect is associated with downregulation them at particularly in the phases of pregestation and gestation.
Conclusion
In conclusion, addition of folate had involved in multiple pathways to decrease the incidence of CHD before or during gestation. We present that folate could prevent NaAsO2-induced CHD through sustaining expression of several cardiac-specific transcription factors. Moreover, this article highlighted a new thought to explore etiology and preventative therapy of CHD.
Acknowledgements
The authors would like to thank financial support from the Science and Technology Major Projects of Fujian Province, China (No. 2013YZ0002-1) , Fujian Provincial Key Laboratory of Prenatal Diagnosis and Birth Defect and Key Clinical Specialty Discipline Construction Program of Fujian, China.
Disclosure of conflict of interest
None.
References
- 1.Miranovic V. The incidence of congenital heart disease: previous findings and perspectives. Srp Arh Celok Lek. 2014;142:243–8. doi: 10.2298/sarh1404243m. [DOI] [PubMed] [Google Scholar]
- 2.Xi S, Jin Y, Lv X, Sun G. Distribution and speciation of arsenic by transplacental and early life exposure to inorganic arsenic in offspring rats. Biol Trace Elem Res. 2010;134:84–97. doi: 10.1007/s12011-009-8455-1. [DOI] [PubMed] [Google Scholar]
- 3.Hall M, Gamble M, Slavkovich V, Liu X, Levy D, Cheng Z, van Geen A, Yunus M, Rahman M, Pilsner JR, Graziano J. Determinants of arsenic metabolism: blood arsenic metabolites, plasma folate, cobalamin, and homocysteine concentrations in maternal-newborn pairs. Environ Health Perspect. 2007;115:1503–9. doi: 10.1289/ehp.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hood RD, Bishop SL. Teratogenic effects of sodium arsenate in mice. Arch Environ Health. 1972;24:62–5. doi: 10.1080/00039896.1972.10666051. [DOI] [PubMed] [Google Scholar]
- 5.Tabocova S, Hunter ER, Gladen BC. Developmental toxicity of inorganic arsenic in whole embryo: culture oxidation state, dose, time, and gestational age dependence. Toxicol Appl Pharmacol. 1996;138:298–307. doi: 10.1006/taap.1996.0128. [DOI] [PubMed] [Google Scholar]
- 6.Hill DS, Wlodarczyk BJ, Finnell RH. Reproductive consequences of oral arsenate exposure during pregnancy in a mouse model. Birth Defects Res B Dev Reprod Toxicol. 2008;83:40–7. doi: 10.1002/bdrb.20142. [DOI] [PubMed] [Google Scholar]
- 7.Burk D, Beaudoin AR. Arsenate-induced renal agenesis in rats. Teratology. 1977;16:247–59. doi: 10.1002/tera.1420160303. [DOI] [PubMed] [Google Scholar]
- 8.Mirkes PE, Cornel L. A comparison of sodium arsenite- and hyperthermia-induced stress responses and abnormal development in cultured postimplantation rat embryos. Teratology. 1992;46:251–9. doi: 10.1002/tera.1420460308. [DOI] [PubMed] [Google Scholar]
- 9.Rudnai T, Sándor J, Kádár M, Borsányi M, Béres J, Métneki J, Maráczi G, Rudnai P. Arsenic in drinking water and congenital heart anomalies in Hungary. Int J Hyg Environ Health. 2014;217:813–8. doi: 10.1016/j.ijheh.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Zierler S, Theodore M, Cohen A, Rothman KJ. Chemical quality of maternal drinking water and congenital heart disease. Int J Epidemiol. 1988;17:589–94. doi: 10.1093/ije/17.3.589. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Wang ZH, Zhang XD, Wang SX, Jia QZ, Han LL, Qiao XY, Wu ZM, Jing YL, Wu M. [Investigation and analysis of neonate deformity in water arsenic exposure areas] . Zhonghua Yu Fang Yi Xue Za Zhi. 2008;42:93–5. [PubMed] [Google Scholar]
- 12.Pang S, Shan J, Qiao Y, Ma L, Qin X, Wanyan H, Xing Q, Wu G, Yan B. Genetic and functional analysis of the NKX2-5 gene promoter in patients with ventricular septal defects. Pediatr Cardiol. 2012;33:1355–61. doi: 10.1007/s00246-012-0346-0. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Li S, Mu D, Liu Z, Li Y, Lin Y, Chen X, You F, Li N, Deng K, Deng Y, Wang Y, Zhu J. The association between periconceptional folic acid supplementation and congenital heart defects: a case-control study in China. Prev Med. 2013;56:385–9. doi: 10.1016/j.ypmed.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Botto LD, Mulinare J, Erickson JD. Occurrence of congenital heart defects in relation to maternal mulitivitamin use. Am J Epidemiol. 2000;151:878–84. doi: 10.1093/oxfordjournals.aje.a010291. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Li Z, Chen X, Qi P, Li S. [Effects of homocysteine on cardiovascular development in early chicken embryo] . Zhonghua Yu Fang Yi Xue Za Zhi. 1999;33:137–9. [PubMed] [Google Scholar]
- 16.Jiang Y, Mei J, Zhang W, Qian X, Zhang S, Liu C, Yang H. [Correlation between offspring congenital heart disease and MTHFR 677C/T polymorphism and general status of pregnant women] . Zhonghua Liu Xing Bing Xue Za Zhi. 2015;36:1072–6. [PubMed] [Google Scholar]
- 17.van Beynum IM, Kapusta L, den Heijer M, Vermeulen SH, Kouwenberg M, Daniëls O, Blom HJ. Maternal MTHFR 677C>T is a risk factor for congenital heart defects: effect modification by periconceptional folate supplementation. Eur Heart J. 2006;27:981–7. doi: 10.1093/eurheartj/ehi815. [DOI] [PubMed] [Google Scholar]
- 18.Faustman EM, Sweeney C. Effects of ethylnitrosourea on expression of proto-oncogene pp60c-src and high-molecular-weight neurofilament protein in rodent embryo central nervous system cells in vitro. Toxicol Appl Pharmacol. 1994;128:182–8. doi: 10.1006/taap.1994.1196. [DOI] [PubMed] [Google Scholar]
- 19.Cipollone D, Carsetti R, Tagliani A, Rosado MM, Borgiani P, Novelli G, D’Amati G, Fumagalli L, Marino B, Businaro R. Folic acid and methionine in the prevention of teratogen-induced congenital defects in mice. Cardiovasc Pathol. 2009;18:100–9. doi: 10.1016/j.carpath.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Zhu H, Kong P. [Influence of arsenic on proliferation and differentiation of rat bud cells in vitro] . Wei Sheng Yan Jiu. 1998;27:161–3. [PubMed] [Google Scholar]
- 21.He W, Greenwell RJ, Brooks DM, Calderón-Garcidueñas L, Beall HD, Coffin JD. Arsenic exposure in pregnant mice disrupts placental vasculogenesis and causes spontaneous abortion. Toxicol Sci. 2007;99:244–53. doi: 10.1093/toxsci/kfm162. [DOI] [PubMed] [Google Scholar]
- 22.Marcela SG, Cristina RM, Angel PG, Manuel AM, Sofía DC, Patricia de LR, Bladimir RR, Concepción SG. Chronological and morphological study of heart development in the rat. Anat Rec (Hoboken) 2012;295:1267–90. doi: 10.1002/ar.22508. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka M, Chen Z, Bartunkova S, Yamasaki N, Izumo S. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development. 1999;126:1269–80. doi: 10.1242/dev.126.6.1269. [DOI] [PubMed] [Google Scholar]
- 24.Zeisberg EM, Ma Q, Juraszek AL, Moses K, Schwartz RJ, Izumo S, Pu WT. Morphogenesis of the right ventricle requires myocardial expression of Gata4. J Clin Invest. 2005;115:1522–31. doi: 10.1172/JCI23769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moskowitz IP, Wang J, Peterson MA, Pu WT, Mackinnon AC, Oxburgh L, Chu GC, Sarkar M, Berul C, Smoot L, Robertson EJ, Schwartz R, Seidman JG, Seidman CE. Transcription factor genes Smad4 and Gata4 cooperatively regulate cardiac valve development. [corrected] . Proc Natl Acad Sci U S A. 2011;108:4006–11. doi: 10.1073/pnas.1019025108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moskowitz IP, Pizard A, Patel VV, Bruneau BG, Kim JB, Kupershmidt S, Roden D, Berul CI, Seidman CE, Seidman JG. The T-Box transcription factor Tbx5 is required for the patterning and maturation of the murine cardiac conduction system. Development. 2004;131:4107–16. doi: 10.1242/dev.01265. [DOI] [PubMed] [Google Scholar]