Abstract
Doxorubicin (DOX) is the most common chemotherapeutic drug for treatment of breast cancer but intrinsic and acquired resistance frequently occurs and severe side effects occur at high doses. DOX might induce activation of NF-κB causing this resistance, in which case proteasome inhibitors could inhibit activation of NF-κB by blocking inhibitory factor κB-alpha degradation. Triple-negative breast cancer (TNBC) is highly progressive and there are no established therapeutic targets against TNBC. Although some proteasome inhibitors have been shown to have antitumor effects in breast cancer, the effect of orally bioavailable proteasome inhibitor oprozomib on TNBC proliferation remains unclear. In the present study, we investigated the role of oprozomib in two TNBC lines, MDA-MB-231 and BT-549. Oprozomib had cytotoxic effects on TNBC cells and increased DOX-induced cytotoxic effects and apoptosis by enhancing DOX-induced JNK/p38 MAPK phosphorylation and inhibiting DOX-induced inhibitory factor êB alpha degradation. These results suggest that oprozomib has potent antitumor effects on TNBC in vitro and can sensitize TNBC cells to DOX treatment. The combination of DOX and oprozomib may be an effective and feasible therapeutic option for TNBC.
Keywords: Proteasome inhibitor, oprozomib, doxorubicin, drug resistance, breast cancer
Introduction
Breast cancer is the leading cancer type in women. For the year 2012, IARC estimated about 1.7 million new breast cancer cases and 522,000 deaths due to breast cancer worldwide [1]. Triple-negative breast cancer (TNBC), which accounts for approximately 15% of breast cancer in women, is defined by lack of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) [2]. TNBC is a heterogeneous group of tumors with more aggressive clinical features [3]. Because of the absence of receptor expression, only traditional chemotherapeutic agents are available for treatment of TNBC [2]. However, drug resistance remains a major impediment to TNBC chemotherapeutic treatment. Resistance to doxorubicin (DOX), the most common chemotherapeutic drug used to treat TNBC, necessitates use of increased therapeutic doses, resulting in adverse side effects and even discontinuation of therapy. There is, therefore, an urgent need for novel therapeutic strategies to overcome resistance to DOX.
Upregulated proteasomal activity has been shown to play a major role in cancer progression [4-6]. Therefore, proteasome inhibition has been extensively investigated as a selective anticancer strategy and has been validated in clinical trials using first- and second-generation proteasome inhibitors (PIs) [7]. Bortezomib, a first-generation PI, and carfilzomib, a second-generation PI, have been approved by the United States Food and Drug Administration for treatment of multiple myeloma [8-10]. Recently, there has been interest in proteasome inhibition as a therapeutic strategy in solid tumors as well, including breast cancer. Carfilzomib and MLN9708, an orally bioavailable next-generation PI, have antitumor effects in breast cancer and can sensitize breast cancer cells to DOX in vitro [11,12]. However, results from clinical trials indicate that first-generation PI and first-generation PI-containing therapies are not effective for treatment of solid tumors, including breast cancer, due to the inability of first-generation PIs to penetrate into tumors and achieve therapeutically relevant concentrations in tumors [13,14]. MLN9708, analogs of bortezomib, are boronate-based molecules that reversibly inhibit the β5 subunit. Oprozomib (OPZ), an orally bioavailable analog of carfilzomib, is an irreversible epoxyketone inhibitor with high specificity for the β5 subunit [15]. Although OPZ is only 20% as potent as carfilzomib, it demonstrates similar cytotoxicity with longer exposure as a result of its time-dependent proteasome inhibition [16]. Oprozomib has induced dose-dependent proteasome inhibition and increased pharmacokinetics and pharmacodynamics [17]. Combination of oprozomib with different unfolded protein response activators has enhanced antitumor efficacy in hepatocellular carcinoma [18]. However, to date, the antitumor effects of OPZ on breast cancer, especially TNBC, have not been fully investigated.
In our present study, we investigated the antitumor activity of OPZ, alone or in combination with DOX, in TNBC lines. We found that OPZ alone results in potent inhibition of cellular proliferation and induction of apoptosis and, when used in combination with DOX, sensitizes cells to DOX-induced toxicity and intensifies DOX-induced apoptosis in vitro.
Materials and methods
Antibodies and reagents
OPZ (7505) was purchased from Apex Biotech (Houston, TX, USA). DOX (D1515) was purchased from Sigma-Aldrich Corp (St. Louis, MO, USA). Antibodies against inhibitory factor κB alpha (IκBα; 9242), phospho-(p-)SAPK/JNK (Thr183/Tyr185; 9251), SAPK/JNK (9258), p-p38 MAPK (Thr180/Tyr182; 9211), p38 MAPK (8690), PARP (9532), Caspase 3 (9662), Caspase 7 (12827), and second antibodies against mouse (7076) and rabbit (7074) were purchased from Cell Signaling Technology (Danvers, MA, USA). α-tubulin (10D8; sc-53646) was purchased from Santa Cruz Biotechnology (Dallas, TX, USA).
Cell lines and cell culture
Two human TNBC lines were used in the study, MDA-MB-231 and BT-549. MDA-MB-231 were routinely cultured in high-glucose Dulbecco’s modified Eagle medium (Lonza, Walkersville, MD, USA) and BT-549 cell lines were maintained in RPMI-1640 medium (Lonza). All cells were supplemented with 10% fetal bovine serum (Sigma-Aldrich), 100 units/mL penicillin, and 100 mg/mL streptomycin. All cells were cultured at 37°C in a humidified atmosphere of 5% CO2.
Cytotoxicity assay
Cell cytotoxicity assays were performed with MTT (MKBH9792V; Sigma-Aldrich), following manufacturer instructions. Briefly, cells were seeded in 96-well plates at a density of 5 × 103 cells per well. After 24 hours of incubation at 37°C, cells were allowed to grow in medium either alone or with increasing concentrations of OPZ, DOX, or both and were incubated for 48 or 72 hours, depending on the experiment. Cells were then observed and photographed using an optical microscope. A mixture of 10 µL of MTT and 140 µL of medium with 10% fetal bovine serum was added to each well and incubated for another 2 hours. Then, 50 µL of dimethyl sulfoxide was added into each well and cells were incubated for another 10 minutes. Absorbance of each well was measured at 540 nm and plotted for cell viability curve. Each experiment was performed in triplicate.
Colony formation assay
Cells were seeded in 12-well plates at 2 × 103 cells per well. After 48 or 72 hours, cells were incubated with OPZ at 0 μM, 0.03 μM, or 0.1 μM for 72 hours and then cultured in drug-free medium for about 2 weeks. After that, cells were fixed and stained with methanol/crystal violet for 10 minutes and photographed. Each experiment was performed in triplicate.
Anchorage-independent growth assay
Cell anchorage-independent growth capabilities were evaluated by soft agar assays, performed as previously described [19]. Briefly, in six-well plates, the bottom layer was made by mixing 2 mL of 0.5% agar/RPMI/Dulbecco’s modified Eagle medium solution in each well and then allowing the layer to cool to semi-solid. For the upper layer, cells were mixed with 1.5 mL of 0.3% agar at 5 × 103 cells per well with the indicated concentrations of OPZ. Cells grew at 37°C for 2 to 3 weeks until colonies were visible to the naked eye. Cells were then stained with crystal violet dye (C3886; Sigma-Aldrich). The colonies were counted by QuantityOne software (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and plotted. Each experiment was performed in triplicate.
Protein immunoblotting
For protein immunoblotting, after the indicated treatments, cells were washed twice with ice-cold phosphate-buffered saline. Cell pallets were then dissolved in RIPA lysis buffer (50 mM Tris-HCl at pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.25% sodium deoxycholate) with protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 10 µg/mL leupeptin, 1 mM dithiothreitol, 50 mM sodium fluoride, 0.1 mM sodium orthovanadate) and phosphatase inhibitors (phosphatase inhibitor cocktail 2 and 3; p5726 and p0044, Sigma-Aldrich) for 30 minutes at 4°C. The solutions were then centrifuged at 13,000 rotations per minute for 15 minutes and supernatants were collected. Measurement of protein concentrations were performed using a Bradford reagent (Bio-Rad Laboratories). A 4X loading buffer was mixed with each sample and samples were heated at 100°C for 7 minutes. Cell lysates were then subjected to 10% or 15% SDS-PAGE electrophoresis and transferred to polyvinylidene fluoride membranes and blocked with 5% milk at room temperature (25°C) for 1 hour. The membranes were then incubated with primary antibodies at 4°C overnight. The following day, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies against rabbit or mouse immunoglobulin. The membranes were developed using ECL-Plus Western blot system (GE Health Care, Buckinghamshire, UK), according to the manufacturer’s instructions. Membranes were re-probed with α-Tubulin antibody as a loading control.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 software. All values are presented as mean ± standard deviation. P values < 0.05 were considered statistically significant. Student’s t-test (two-tailed) or analysis of variance (Dunnett’s multiple comparison post-test) were used to analyze differences between the drug treatment groups and control group.
Results
OPZ suppresses proliferation of TNBC
To assess the antitumor effect of OPZ on TNBC, we used two TNBC lines including MDA-MB-231 and BT-549, which together represent the major sources of TNBC (Table 1) [20-22]. These cells were treated with OPZ at the indicated concentrations of 0.001 μM to 10 μM for 72 hours, then subjected to an MTT assay. Results showed that OPZ reduced cell viability of tested TNBC in a dose-dependent manner (Figure 1A). The half-maximal inhibitory concentrations of OPZ were 0.079 μM (MDA-MB-231) and 0.05 μM (BT-549; Figure 1A).
Table 1.
| Cell line | Subtype | Hormone receptor status1 | Source | Tumor type | ||
|---|---|---|---|---|---|---|
|
| ||||||
| ER | PR | HER2 | ||||
| MDA-MB-231 | Basal B | - | [-] | - | Pleural effusion | Adenocarcinoma |
| BT-549 | Basal B | - | [-] | - | Primary BC2 | Invasive ductal carcinoma, papillary |
Estrogen receptor (ER) and progesterone receptor (PR) positivity and human epidermal receptor 2 (HER2) overexpression (obtained from the Sanger web site: www.sanger.ac.uk) are indicated. Square brackets indicate that levels are inferred from mRNA levels alone where protein data are not available.
BC, breast cancer.
Figure 1.
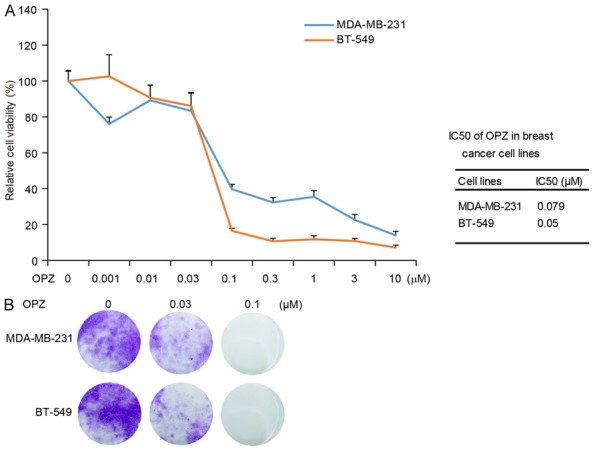
Oprozomib (OPZ) shows cytotoxic effects on TNBC cells. A. Two TNBC cell lines, MDA-MB-231 and BT-549, were treated with OPZ at concentrations of 0 μM, 0.001 μM, 0.01 μM, 0.03 μM, 0.1 μM, 0.3 μM, 1 μM, 3 μM, or 10 μM for 72 hours, then subjected to MTT assays. Absorbance of each well was measured at 540 nm and plotted for the cell viability curve. Data are represented as mean ± standard deviation. Half-maximal inhibitory concentration (IC50) values of OPZ in each cell line are listed on the right. B. Colony formation of breast cancer cells treated with OPZ. Two TNBC cell lines were seeded in 12-well plates at 2 × 103 per well, then incubated with oprozomib at 0 μM, 0.03 μM, or 0.1 μM for 72 hours and cultured in drug-free medium for about 2 weeks. Cell colonies were fixed, stained with crystal violet, and photographed.
To further validate the inhibitory effect of OPZ on cell proliferation, we performed a cellular colony formation assay. Cells were treated with OPZ at concentrations of 0 μM, 0.03 μM, and 0.1 μM for 72 hours and then cultured in drug-free medium for about 2 weeks. Treatment with OPZ remarkably inhibited cellular proliferation compared with control (Figure 1B). Taken together, these data indicate that OPZ has a potent inhibitory effect on proliferation of TNBC.
OPZ restrains anchorage-independent growth of TNBC
Cancer cells possess the ability to grow into ball-shaped colonies in a three-dimensional space when cultured in soft agar. To determine whether OPZ could impair anchorage-independent growth ability of TNBC cells, we performed soft agar assays. MDA-MB-231 and BT-549 cells were cultured with OPZ at 0 μM, 0.03 μM, or 0.3 μM for 3 weeks and then visible colonies were fixed and stained. The number of colonies decreased in OPZ-treated groups compared with control groups in each tested cell line (Figure 2A). Inhibitory effects of OPZ on colony formation were dose-dependent and statistically significant in OPZ-treated cells (Figure 2B).
Figure 2.
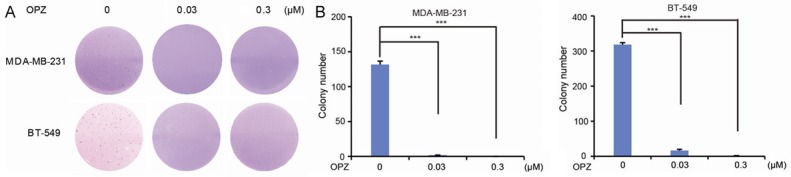
Oprozomib (OPZ) suppresses anchorage-independent growth ability of TNBC cells. Cell anchorage-independent growth ability was assessed using soft agar assays. (A) Two breast cancer cell lines, MDA-MB-231 and BT-549, were incubated with OPZ at concentrations of 0 μM, 0.03 μM, or 0.3 μM in soft agar plates for 3 weeks, stained with crystal violet, and photographed. (B) The colonies shown in (A) were counted and plotted. Data are represented as mean ± standard deviation. ***P < 0.001, by analysis of variance and Dunnett’s multiple comparison post-test.
OPZ induces apoptosis in TNBC
It has been reported that OPZ can induce apoptosis in a variety of tumor types including pediatric acute leukemia, hepatocellular carcinoma, and head and neck squamous cell carcinoma [18,23,24]. To examine whether OPZ could induce apoptosis in human TNBC, we treated the cells with OPZ at concentrations of 0 μM, 0.03 μM, 0.1 μM, 0.3 μM, and 1 μM and then harvested the cells and subjected them to immunoblotting. We found that OPZ could induce the cleavage of PARP and Caspase 3 in the tested cell lines in a dose-dependent manner (Figure 3). These results suggest that OPZ alone could trigger apoptosis in TNBC.
Figure 3.
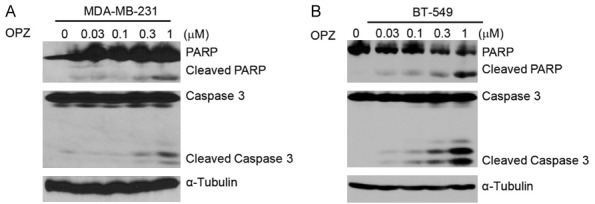
Oprozomib (OPZ) induces apoptosis in TNBC cells. TNBC cell lines MDA-MB-231 (A) and BT-549 (B) were treated with OPZ at concentrations of 0 μM, 0.03 μM, 0.1 μM, 0.3 μM, or 1 μM for 24 hours. Then, whole cell lysates were subjected to SDS-PAGE and immunoblotted with antibodies against PARP and Caspase 3 to detect apoptosis. α-Tubulin was used as loading control.
OPZ intensifies cytotoxic effect of DOX in TNBC
To determine whether OPZ sensitizes TNBC to treatment with DOX, we cultured cells with DOX at concentrations of 0 µM, 0.1 µM, 0.2 µM, 0.5 µM, or 1 µM, alone or in combination with 0.05 µM OPZ, for 48 hours. Cell proliferation was assessed using MTT assay. Results showed that cell viability was much lower with the combination treatment than with DOX alone, for TNBC lines (Figure 4). This suggests that OPZ could sensitize tested cell lines to the cytotoxicity of DOX.
Figure 4.
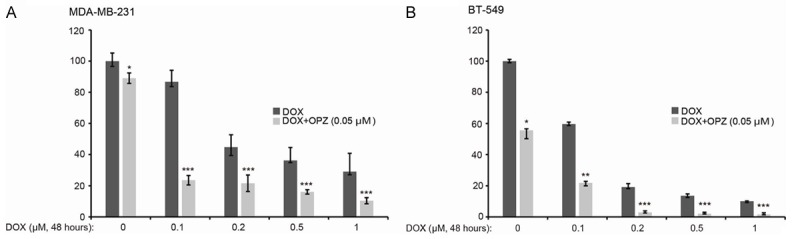
Oprozomib (OPZ) enhances cytotoxic effect of doxorubicin (DOX) in TNBC cells. TNBC cell lines MDA-MB-231 (A) and BT-549 (B) were treated with DOX at the indicated concentrations with or without OPZ (0.05 μM) for 72 hours. Cell viability was then measured by MTT assay. Data are represented as mean ± standard deviation. *P < 0.05, **P < 0.01, ***P < 0.001, by t-test.
OPZ enhances DOX-induced apoptosis in TNBC
To determine whether OPZ could strengthen DOX-induced apoptosis in breast cancer cells, we treated cells with DOX alone (1 μM) or combined with OPZ at concentrations of 0.2 μM (or 0.4 μM for MDA-MB-231 cells) for 0 or 24 hours (0 or 16 hours for BT-549 cells). MDA-MB-231 cells were not relatively sensitive to OPZ at concentration of 0.2 μM, so we checked the effect of OPZ at concentration of 0.4 μM on MDA-MB-231 cells. Nearly all BT-549 cells died after being exposed to the media with OPZ at concentration of 0.2 μM for 24 hours, therefore, we checked the effect of OPZ at concentration of 0.2 μM on BT-549 cells within 16 hours. Immunoblotting analyses demonstrated that OPZ could boost DOX-induced cleavage of PARP and Caspase 3 in TNBC lines tested (Figure 5). This indicates that OPZ could intensify DOX-induced apoptosis in TNBC.
Figure 5.
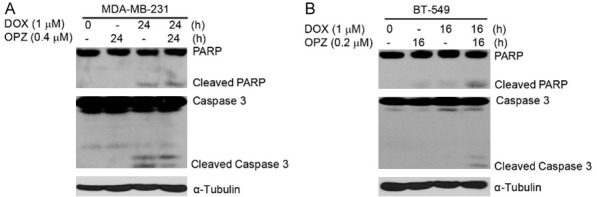
Oprozomib (OPZ) strengthens doxorubicin (DOX)-induced apoptosis in TNBC cells. TNBC cell lines MDA-MB-231 (A) and BT-549 (B) were treated with DOX (1-μM) alone or combined with OPZ (0.2 μM or 0.4 μM) for 0, 24, or 16 hours. Whole cell lysates were then subjected to SDS-PAGE and immunoblotted with antibodies against PARP and caspase 3 to detect apoptosis. α-tubulin was used as loading control.
OPZ inhibits DOX-induced IκBα degradation in TNBC
Both NF-κB and JNK/p38 MAPK are key signal transduction pathways in regulating cell survival [25-28]. Studies have revealed that DOX could induce NF-κB and MAPK activation [29-31] but PIs could inhibit activation of NF-κB [32]. To confirm this, we assessed the effects of the combination of OPZ with DOX on activity of NF-κB and JNK/p38 MAPK in each of the TNBC lines. Cells were cultured with DOX alone or in combination with 5 μM OPZ for 0, 2, 3, or 4 hours. As shown in Figure 6, the addition of OPZ to DOX upregulated DOX-induced SAPK/JNK and p38 MAPK phosphorylation and DOX-induced IκBα degradation was suppressed by OPZ. These data demonstrate that OPZ reinforced DOX-induced SAPK/JNK and p38 MAPK phosphorylation and inhibited DOX-induced NF-κB activation.
Figure 6.
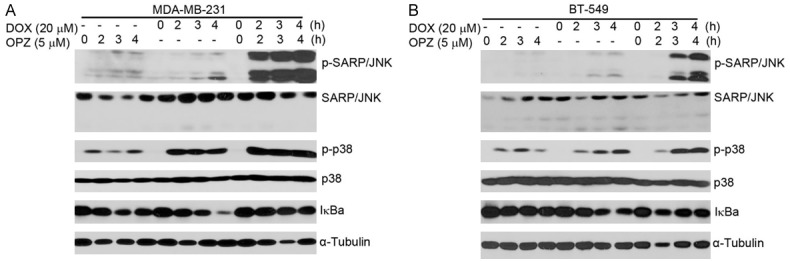
Oprozomib (OPZ) inhibits doxorubicin (DOX)-induced inhibitory factor κB alpha (IκBα) degradation in TNBC cells. TNBC cell lines MDA-MB-231 (A) and BT-549 (B) were treated with DOX (20 μM) alone or in combination with oprozomib (5 μM) for 0, 2, 3, or 4 hours. Whole cell lysates were then subjected to SDS-PAGE and immunoblotted with antibodies against phospho-(p-)SARP/JNK, SARP/JNK, p-p38 MAPK, p38 MAPK, and IκBα. α-Tubulin was used as loading control.
Discussion
Our results indicate that the PI OPZ inhibits proliferation of TNBC cells. We found that OPZ had high cytotoxic activity, reducing cellular proliferation, attenuating colony forming ability, and increasing apoptosis in tested TNBC lines. When combined with DOX, OPZ intensified DOX-induced apoptosis. Our findings suggest that OPZ has potent antitumor effects on TNBC cells and can sensitize TNBC cells to DOX in vitro.
Half-maximal inhibitory concentration (IC50) values for cell lines treated with OPZ were all in the low micromolar range, from 0.05 μM (BT-549) to 0.079 μM (MDA-MB-231). This is in the range of OPZ half-maximal inhibitory concentration values reported for other tumor cell lines (0.002-0.0994 μM) [24,33,34] and it implies that sensitivity to OPZ may be not obvious among different sources of TNBC. This also suggests that OPZ alone could effectively inhibit proteasome activity and has a significant antitumor effect on TNBC cells tested. Previous studies of OPZ have demonstrated that OPZ causes diminished cell proliferation and increased cell death across a variety of other cancer cell lines such as those from pediatric leukemia cells, hepatocellular carcinoma, and head and neck cancer [18,23,24].
Although DOX is a first-line treatment for cancer, including TNBC, intrinsic and acquired resistance presents a significant hindrance to the treatment efficacy of DOX [31,35,36]. Decreased sensitivity to DOX necessitates dose escalation, resulting in significant adverse effects including cardiotoxicity and suppression of bone marrow hematopoietic function. In the present study, we demonstrated that although TNBC showed high sensitivity to OPZ alone, cells showed further sensitivity to treatment with the combination of OPZ and DOX. It is notable that OPZ may sensitize TNBC to DOX chemotherapy and thus lessen DOX resistance and toxicity. Consistent with these findings, some reports have suggested that PIs exacerbate DOX-induced cytotoxicity in cardiomyocytes [37] and sensitize leukemia cells to DOX [38]. Collectively, these data suggest that OPZ could be beneficial as an addition to DOX for the treatment of TNBC, enabling the use of lower doses of DOX and reducing side effects.
Treatment with relatively low doses of OPZ prevented IκBα degradation induced by DOX in the NF-κB signaling pathway and enhanced p38 MAPK/ JNK apoptosis signaling activation induced by DOX, according to our assays. The NF-κB pathway is activated in many kinds of cancer, including breast cancer [39-41], and chemoresistance caused by NF-κB activation is a common off-target effect. Inhibition of NF-κB has been associated with increased effectiveness of DOX in breast cancer and lack of response to DOX has been linked to reduced NF-κB inhibition [11,42]. DOX also induces the activation of p38 MAPK/JNK apoptosis signaling in breast cancer cells [12].
One study has shown that when verapamil, a P-glycoprotein inhibitor, was combined with a PI (MG132, bortezomib, or carfilzomib), apoptosis and cytotoxic effects of verapamil in TNBC MDA-MB-231 cells were enhanced [43]. Similarly, in the present study, OPZ alone or in combination with DOX showed potent cytotoxic effects and induced apoptosis in MDA-MB-231 and BT-549 cells. OPZ may also be combined with other P-glycoprotein inhibitors to increase therapeutic efficacy in TNBC. In future studies, use of PIs and the development of novel strategies to combat cancer through inhibition of multiple targets may be indispensable in developing better treatment options for TNBC.
In summary, in studying two TNBC cell lines, we provide compelling evidence that OPZ alone could suppress proliferation and induce apoptosis in TNBC cells and, when combined with DOX, enhance the cytotoxic effect of DOX and DOX-induced apoptosis by preventing IκBα degradation in the NF-κB signaling pathway and activating p38 MAPK/ JNK apoptotic signaling. These findings suggest that OPZ might serve as an effective drug in potential combination therapies for chemoresistant TNBC.
Acknowledgements
This work was supported by the Scientific Research Foundation Post- doctoral Program at Xinjiang Medical University, China (to Yonghua Shi). We would like to thank Erica for scientific editing of the manuscript.
Disclosure of conflict of interest
None.
Abbreviations
- PI
proteasome inhibitor
- OPZ
oprozomib
- DOX
doxorubicin
References
- 1.Oberaigner W, Geiger-Gritsch S, Edlinger M, Daniaux M, Knapp R, Hubalek M, Siebert U, Marth C, Buchberger W. Reduction in advanced breast cancer after introduction of a mammography screening program in Tyrol/Austria. Breast. 2017;33:178–82. doi: 10.1016/j.breast.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Collignon J, Lousberg L, Schroeder H, Jerusalem G. Triple-negative breast cancer: treatment challenges and solutions. Breast Cancer. 2016;8:93–107. doi: 10.2147/BCTT.S69488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abramson VG, Lehmann BD, Ballinger TJ, Pietenpol JA. Subtyping of triple-negative breast cancer: implications for therapy. Cancer. 2015;121:8–16. doi: 10.1002/cncr.28914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedford L, Lowe J, Dick LR, Mayer RJ, Brownell JE. Ubiquitin-like protein conjugation and the ubiquitin-proteasome system as drug targets. Nat Rev Drug Discov. 2011;10:29–46. doi: 10.1038/nrd3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huber EM, Groll M. Inhibitors for the immuno- and constitutive proteasome: current and future trends in drug development. Angew Chem Int Ed Engl. 2012;51:8708–20. doi: 10.1002/anie.201201616. [DOI] [PubMed] [Google Scholar]
- 6.Shen M, Schmitt S, Buac D, Dou QP. Targeting the ubiquitin-proteasome system for cancer therapy. Expert Opin Ther Targets. 2013;17:1091–108. doi: 10.1517/14728222.2013.815728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Wu P, Hu Y. Clinical and marketed proteasome inhibitors for cancer treatment. Curr Med Chem. 2013;20:2537–51. doi: 10.2174/09298673113209990122. [DOI] [PubMed] [Google Scholar]
- 8.Bross PF, Kane R, Farrell AT, Abraham S, Benson K, Brower ME, Bradley S, Gobburu JV, Goheer A, Lee SL, Leighton J, Liang CY, Lostritto RT, McGuinn WD, Morse DE, Rahman A, Rosario LA, Verbois SL, Williams G, Wang YC, Pazdur R. Approval summary for bortezomib for injection in the treatment of multiple myeloma. Clin Cancer Res. 2004;10:3954–64. doi: 10.1158/1078-0432.CCR-03-0781. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg AL. Development of proteasome inhibitors as research tools and cancer drugs. J Cell Biol. 2012;199:583–8. doi: 10.1083/jcb.201210077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajkumar SV, Richardson PG, Hideshima T, Anderson KC. Proteasome inhibition as a novel therapeutic target in human cancer. J. Clin. Oncol. 2005;23:630–9. doi: 10.1200/JCO.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 11.Shi Y, Yu Y, Wang Z, Wang H, Bieerkehazhi S, Zhao Y, Suzuk L, Zhang H. Second-generation proteasome inhibitor carfilzomib enhances doxorubicin-induced cytotoxicity and apoptosis in breast cancer cells. Oncotarget. 2016;7:73697–710. doi: 10.18632/oncotarget.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Yu Y, Jiang Z, Cao WM, Wang Z, Dou J, Zhao Y, Cui Y, Zhang H. Next-generation proteasome inhibitor MLN9708 sensitizes breast cancer cells to doxorubicin-induced apoptosis. Sci Rep. 2016;6:26456. doi: 10.1038/srep26456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmid P, Kuhnhardt D, Kiewe P, Lehenbauer-Dehm S, Schippinger W, Greil R, Lange W, Preiss J, Niederle N, Brossart P, Freier W, Kümmel S, Van de Velde H, Regierer A, Possinger K. A phase I/II study of bortezomib and capecitabine in patients with metastatic breast cancer previously treated with taxanes and/or anthracyclines. Ann Oncol. 2008;19:871–6. doi: 10.1093/annonc/mdm569. [DOI] [PubMed] [Google Scholar]
- 14.Trinh XB, Sas L, Van Laere SJ, Prove A, Deleu I, Rasschaert M, Van de Velde H, Vinken P, Vermeulen PB, Van Dam PA, Wojtasik A, De Mesmaeker P, Tjalma WA, Dirix LY. A phase II study of the combination of endocrine treatment and bortezomib in patients with endocrine-resistant metastatic breast cancer. Oncol Rep. 2012;27:657–63. doi: 10.3892/or.2011.1562. [DOI] [PubMed] [Google Scholar]
- 15.Johnson DE. The ubiquitin-proteasome system: opportunities for therapeutic intervention in solid tumors. Endocr Relat Cancer. 2015;22:T1–17. doi: 10.1530/ERC-14-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naymagon L, Abdul-Hay M. Novel agents in the treatment of multiple myeloma: a review about the future. J Hematol Oncol. 2016;9:52. doi: 10.1186/s13045-016-0282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Infante JR, Mendelson DS, Burris HA 3rd, Bendell JC, Tolcher AW, Gordon MS, Gillenwater HH, Arastu-Kapur S, Wong HL, Papadopoulos KP. A first-in-human dose-escalation study of the oral proteasome inhibitor oprozomib in patients with advanced solid tumors. Invest New Drugs. 2016;34:216–24. doi: 10.1007/s10637-016-0327-x. [DOI] [PubMed] [Google Scholar]
- 18.Vandewynckel YP, Coucke C, Laukens D, Devisscher L, Paridaens A, Bogaerts E, Vandierendonck A, Raevens S, Verhelst X, Van Steenkiste C, Libbrecht L, Geerts A, Van Vlierberghe H. Next-generation proteasome inhibitor oprozomib synergizes with modulators of the unfolded protein response to suppress hepatocellular carcinoma. Oncotarget. 2016;7:34988–5000. doi: 10.18632/oncotarget.9222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bieerkehazhi S, Chen Z, Zhao Y, Yu Y, Zhang H, Vasudevan SA, Woodfield SE, Tao L, Yi JS, Muscal JA, Pang JC, Guan S, Zhang H, Nuchtern JG, Li H, Li H, Yang J. Novel Src/Abl tyrosine kinase inhibitor bosutinib suppresses neuroblastoma growth via inhibiting Src/Abl signaling. Oncotarget. 2017;8:1469–80. doi: 10.18632/oncotarget.13643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011;13:215. doi: 10.1186/bcr2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, Speed T, Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo WL, Stilwell JL, Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB, Johnson MD, Lippman M, Ethier S, Gazdar A, Gray JW. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–27. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 23.Niewerth D, Franke NE, Jansen G, Assaraf YG, van Meerloo J, Kirk CJ, Degenhardt J, Anderl J, Schimmer AD, Zweegman S, de Haas V, Horton TM, Kaspers GJ, Cloos J. Higher ratio immune versus constitutive proteasome level as novel indicator of sensitivity of pediatric acute leukemia cells to proteasome inhibitors. Haematologica. 2013;98:1896–904. doi: 10.3324/haematol.2013.092411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zang Y, Thomas SM, Chan ET, Kirk CJ, Freilino ML, DeLancey HM, Grandis JR, Li C, Johnson DE. Carfilzomib and ONX 0912 inhibit cell survival and tumor growth of head and neck cancer and their activities are enhanced by suppression of Mcl-1 or autophagy. Clin Cancer Res. 2012;18:5639–49. doi: 10.1158/1078-0432.CCR-12-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo Y, Lin D, Zhang M, Zhang X, Li Y, Yang R, Lu Y, Jin X, Yang M, Wang M, Zhao S, Quan C. CLDN6-induced apoptosis via regulating ASK1-p38/JNK signaling in breast cancer MCF-7 cells. Int J Oncol. 2016;48:2435–44. doi: 10.3892/ijo.2016.3469. [DOI] [PubMed] [Google Scholar]
- 26.Saba NS, Liu D, Herman SE, Underbayev C, Tian X, Behrend D, Weniger MA, Skarzynski M, Gyamfi J, Fontan L, Melnick A, Grant C, Roschewski M, Navarro A, Beà S, Pittaluga S, Dunleavy K, Wilson WH, Wiestner A. Pathogenic role of B-cell receptor signaling and canonical NF-kappaB activation in mantle cell lymphoma. Blood. 2016;128:82–92. doi: 10.1182/blood-2015-11-681460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Koschembahr AM, Swope VB, Starner RJ, Abdel-Malek ZA. Endothelin-1 protects human melanocytes from UV-induced DNA damage by activating JNK and p38 signalling pathways. Exp Dermatol. 2015;24:269–74. doi: 10.1111/exd.12638. [DOI] [PubMed] [Google Scholar]
- 28.Zhen Y, Ding C, Sun J, Wang Y, Li S, Dong L. Activation of the calcium-sensing receptor promotes apoptosis by modulating the JNK/p38 MAPK pathway in focal cerebral ischemia-reperfusion in mice. Am J Transl Res. 2016;8:911–21. [PMC free article] [PubMed] [Google Scholar]
- 29.Esparza-Lopez J, Medina-Franco H, Escobar-Arriaga E, Leon-Rodriguez E, Zentella-Dehesa A, Ibarra-Sanchez MJ. Doxorubicin induces atypical NF-kappaB activation through c-Abl kinase activity in breast cancer cells. J Cancer Res Clin Oncol. 2013;139:1625–35. doi: 10.1007/s00432-013-1476-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finn NA, Kemp ML. Pro-oxidant and antioxidant effects of N-acetylcysteine regulate doxorubicin-induced NF-kappa B activity in leukemic cells. Mol Biosyst. 2012;8:650–62. doi: 10.1039/c1mb05315a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez de Vergara O, Pablos R, Ruiz-Sanz MB, Ruiz-Larrea JI, Navarro R. Characterization of the NF-?B signaling triggered by doxorubicin. Free Radic Biol Med. 2014;75(Suppl 1):S38. doi: 10.1016/j.freeradbiomed.2014.10.784. [DOI] [PubMed] [Google Scholar]
- 32.Yan Y, Furumura M, Gouya T, Iwanaga A, Teye K, Numata S, Karashima T, Li XG, Hashimoto T. Shikonin promotes skin cell proliferation and inhibits nuclear factor-kappaB translocation via proteasome inhibition in vitro. Chin Med J (Engl) 2015;128:2228–33. doi: 10.4103/0366-6999.162512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teicher BA, Tomaszewski JE. Proteasome inhibitors. Biochem Pharmacol. 2015;96:1–9. doi: 10.1016/j.bcp.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Zang Y, Kirk CJ, Johnson DE. Carfilzomib and oprozomib synergize with histone deacetylase inhibitors in head and neck squamous cell carcinoma models of acquired resistance to proteasome inhibitors. Cancer Biol Ther. 2014;15:1142–52. doi: 10.4161/cbt.29452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang L, Fan Y, Cheng J, Cheng D, Zhao Y, Cao B, Ma L, An L, Jia W, Su X, Yang J, Zhang H. TAK1 ubiquitination regulates doxorubicin-induced NF-kappaB activation. Cell Signal. 2013;25:247–54. doi: 10.1016/j.cellsig.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan C, Wang X, Shi K, Zheng Y, Li J, Chen Y, Jin L, Pan Z. MiR-122 reverses the doxorubicin-resistance in hepatocellular carcinoma cells through regulating the tumor metabolism. PLoS One. 2016;11:e0152090. doi: 10.1371/journal.pone.0152090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spur EM, Althof N, Respondek D, Klingel K, Heuser A, Overkleeft HS, Voigt A. Inhibition of chymotryptic-like standard proteasome activity exacerbates doxorubicin-induced cytotoxicity in primary cardiomyocytes. Toxicology. 2016;353-354:34–47. doi: 10.1016/j.tox.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 38.Ortiz-Lazareno PC, Bravo-Cuellar A, Lerma-Diaz JM, Jave-Suarez LF, Aguilar-Lemarroy A, Dominguez-Rodriguez JR, Jave-Suarez LF, Aguilar-Lemarroy A, del Toro-Arreola S, de Celis-Carrillo R, Sahagun-Flores JE, de Alba-Garcia JE, Hernandez-Flores G. Sensitization of U937 leukemia cells to doxorubicin by the MG132 proteasome inhibitor induces an increase in apoptosis by suppressing NF-kappa B and mitochondrial membrane potential loss. Cancer Cell Int. 2014;14:13. doi: 10.1186/1475-2867-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennett L, Quinn J, McCall P, Mallon EA, Horgan PG, McMillan DC, Paul A, Edwards J. High IKKalpha expression is associated with reduced time to recurrence and cancer specific survival in oestrogen receptor (ER)-positive breast cancer. Int J Cancer. 2017;140:1633–44. doi: 10.1002/ijc.30578. [DOI] [PubMed] [Google Scholar]
- 40.Bian D, Gao C, Bao K, Song G. The long non-coding RNA NKILA inhibits the invasion-metastasis cascade of malignant melanoma via the regulation of NF-κB. Am J Cancer Res. 2017;7:28–40. [PMC free article] [PubMed] [Google Scholar]
- 41.Martin M, Hua L, Wang B, Wei H, Prabhu L, Hartley AV, Jiang G, Liu Y, Lu T. Novel serine 176 phosphorylation of YBX1 activates NF-kappaB in colon cancer. J Biol Chem. 2017;292:3433–44. doi: 10.1074/jbc.M116.740258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z, Zhang H, Shi M, Yu Y, Wang H, Cao WM, Zhao Y, Zhang H. TAK1 inhibitor NG25 enhances doxorubicin-mediated apoptosis in breast cancer cells. Sci Rep. 2016;6:32737. doi: 10.1038/srep32737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deshmukh RR, Kim S, Elghoul Y, Dou QP. P-glycoprotein inhibition sensitizes human breast cancer cells to proteasome inhibitors. J Cell Biochem. 2017;118:1239–48. doi: 10.1002/jcb.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]


