Abstract
Sclerostin is now being recognized as performing a multitude of physiological functions and is associated with coronary artery calcification. Peripheral arterial disease (PAD) is a common manifestation of atherosclerosis in elderly persons. This study aimed to determine whether high serum sclerostin is associated with PAD in elderly persons. Blood samples were obtained from 68 participants in the study older than 65 years. Ankle-brachial index (ABI) values were measured using the automated oscillometric method (VaSera VS-1000). PAD was considered present if the left or right ABI values were less than 0.9 and included in the low ABI group. Serum sclerostin levels were measured using a commercial enzyme-linked immunosorbent assay kit. Among elderly participants, 13 (19.1%) were in the low ABI group. Elderly participants had higher statin use (P = 0.015), serum intact parathyroid hormone (P < 0.001), C-reactive protein (P = 0.027), and sclerostin (P < 0.001) levels in the low ABI group than in the normal ABI group. Serum sclerostin (odds ratio: 1.050, 95% confidence interval: 1.013-1.089, P = 0.008) was the independent predictor of PAD in elderly participants in multivariate logistic regression analysis. A high serum sclerostin level is a risk factor for PAD in the elderly.
Keywords: Ankle-brachial index, aging, wingless pathway antagonist, peripheral arterial occlusive disease
Introduction
Peripheral arterial disease (PAD), an atherosclerotic-driven condition, influences more than 202 million people worldwide [1]. As an age-related disease, both the incidence and prevalence of PAD markedly increases among elderly persons, which is a threat to aging global societies [2]. The PAD overall prevalence ranges from 3% to 10%, which increases to 15%-20% in elderly persons beyond 70 years old [3]. In view of the upward trend in the prevalence of PAD, the socioeconomic burden would be considerable in the future [1,3]. Although PAD might be asymptomatic, it can progress to severe conditions, such as critical limb ischemia and the incidence of limb loss, which are associated with a reduction in the functional capacity and quality of life when symptomatic [4].
Sclerostin is a soluble glycoprotein secreted by osteocytes and has been identified as an important regulator of bone formation and an inhibitor of the Wnt/β-catenin signaling pathway [5]. The Wnt/β-catenin signaling pathway plays an important role in the regulation of endothelial inflammation, vascular calcification, and mesenchymal stem cell differentiation and therefore contributes to atherosclerosis disease [6]. As a result, it is suspected that sclerostin might play a role in patients with atherosclerosis [6-8].
There are few studies that focus on the pathophysiologic effects between sclerostin and PAD in elderly persons. Therefore, the aim of our study was to investigate the relationship between circulating serum sclerostin levels and PAD in elderly persons.
Materials and methods
Participants
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Protection of the Human Subjects Institutional Review Board of Tzu-Chi University and Hospital. All participants provided informed consent prior to participating in this study (IRB099-97). Participants who take medications related to warfarin or protease-activated receptor-1 antagonists were excluded. Participants were also excluded if they had acute infection e at the time of blood sampling, or if they refused to provide informed consent for the study. Finally, a total of 68 participants older than 65 years of age were enrolled in this cross-sectional study conducted between January and December 2012 in a medical center in Hualien, Taiwan. People who used oral hypoglycemic medications or insulin or with fasting plasma glucose level were ≥ 126 mg/dl were diagnosed with diabetes mellitus [9]. People who received any anti-hypertensive medication in the past 2 weeks or with a systolic BP ≥ 140 mmHg, diastolic BP ≥ 90 mmHg were regarded as having hypertension. Participants were measured blood pressure (BP) after sitting for at least 10 minutes in the morning.
Anthropometric analysis
Participant wearing light clothing and without shoes and participant’s height and weight was measured to the nearest 0.5 cm or 0.5 kg, respectively. Body mass index (BMI) was calculated by dividing weight in kilograms by body height in meters squared (kg/m2) [10-13].
Biochemical investigations
Serum levels of glucose, blood urea nitrogen, creatinine, total cholesterol (TCH), triglycerides (TG), high-density lipoprotein cholesterol, low-density lipoprotein cholesterol (LDL-C), total calcium, phosphorus, and C-reactive protein (CRP) were measured using an autoanalyzer (COBAS Integra 800; Roche Diagnostics, Basel, Switzerland) with fasting blood samples with participants that did not eat food for over 8 hours [10-13]. Serum sclerostin levels (Biomedica immunoassays, Vienna, Austria) and intact parathyroid hormone (iPTH; Diagnostic Systems Laboratories, Texas, USA) concentrations were quantified using commercially available enzyme-linked immunosorbent assays [13].
Ankle-brachial index measurements
Ankle-brachial index (ABI) values were using an oscillometric method to measure BP in both arms and ankles (VaSera VS-1000; Fukuda Denshi Co, Ltd, Tokyo, Japan) [14,15]. Under supine position, the occlusion and monitoring cuffs were appropriately applied to all four limbs of the participants, and the real-time electrocardiography was recorded for at fifteen minutes at least. ABI value was calculated as the ratio of the ankle SBP divided by the arm SBP. As previous study, PAD was diagnosed based on an ABI < 0.9 [16]. The low ABI group was defined if right or left ABI values < 0.9 as our previous study [15].
Statistical analysis
Data were analyzed using SPSS for Windows (version 19.0; SPSS Inc., Chicago, IL, USA). Data expressed as the number of patients were analyzed using the Chi-square test. Data were tested for normal distribution using the Kolmogorov-Smirnov test. Non-normally distributed data were expressed as medians and interquartile ranges, and comparisons were performed using the Mann-Whitney U test (fasting glucose, TG, and CRP) between participants. Normally distributed data were expressed as mean ± standard deviation, and comparisons were performed using the Student’s independent t-test (two-tailed) between participants. Variables that were significantly associated with PAD in elderly participants were tested for independence using multivariate logistic regression analysis (adopted factors: statin used, iPTH, CRP and sclerostin). Left or right ABI values that correlated with serum sclerostin levels in subjects were evaluated using univariate linear regression analysis. A P value < 0.05 was considered statistically significant.
Results
Demographic, clinical, and biochemical characteristics of the 68 elderly participants are presented in Table 1. In total, 33 (48.5%) elderly participants had diabetes mellitus and 55 (80.9%) had hypertension. In this study, thirteen (19.1%) elderly participants were defined in the low ABI group. Compared with elderly participants in the normal ABI group, those in the low ABI group had higher serum CRP (P = 0.027), iPTH (P < 0.001), and sclerostin (P < 0.001) levels. The use of drugs in these elderly participants included angiotensin receptor blockers (ARB; n = 27; 39.7%), angiotensin-converting enzyme inhibitors (ACEi; n = 18; 26.5%), β-blockers (n = 35; 51.5%), calcium channel blockers (CCB; n = 26; 38.2%), fibrate (n = 141; 20.6%), statins (n = 37; 54.4%), clopidogrel (n = 17; 25.0%), and aspirin (n = 40; 58.8%). In elderly participants, statin use (P = 0.015) was higher in the low ABI group than in the normal ABI group. However, compared with elderly participants in the normal ABI group, those in the low ABI group were not statistically significantly different based on gender, co-existing diabetes, hypertension, or the use of ARB, ACEi, β-blockers, CCB, fibrate, aspirin, or clopidogrel.
Table 1.
Clinical variable of the 68 elderly participants in the normal or low ankle brachial index group
| Characteristic | All participants (n = 68) | Normal ABI Group (n = 55) | Low ABI Group (n = 13) | P value |
|---|---|---|---|---|
| Age (years) | 72.69 ± 5.34 | 72.35 ± 4.94 | 74.15 ± 6.83 | 0.275 |
| Height (cm) | 159.41 ± 8.37 | 159.80 ± 7.67 | 157.77 ± 11.08 | 0.436 |
| Body weight (kg) | 65.19 ± 10.66 | 65.02 ± 10.73 | 65.92 ± 10.72 | 0.785 |
| Body mass index (kg/m2) | 25.67 ± 3.78 | 25.44 ± 3.64 | 26.61 ± 4.34 | 0.321 |
| Left ankle-brachial index | 1.05 ± 0.12 | 1.09 ± 0.08 | 0.88 ± 0.12 | < 0.001* |
| Right ankle-brachial index | 1.02 ± 0.13 | 1.07 ± 0.07 | 0.82 ± 0.10 | < 0.001* |
| Systolic blood pressure (mmHg) | 128.90 ± 15.59 | 128.00 ± 14.60 | 132.69 ± 19.46 | 0.333 |
| Diastolic blood pressure (mmHg) | 70.29 ± 8.74 | 70.56 ± 8.92 | 69.15 ± 8.19 | 0.605 |
| Total cholesterol (mg/dL) | 167.51 ± 36.41 | 169.18 ± 36.75 | 160.46 ± 35.47 | 0.442 |
| Triglyceride (mg/dL) | 115.50 (84.00-159.75) | 113.00 (83.00-169.00) | 121.00 (86.50-153.00) | 0.925 |
| HDL-C (mg/dL) | 47.18 ± 13.11 | 47.22 ± 12.48 | 47.00 ± 16.11 | 0.957 |
| LDL-C (mg/dL) | 97.37 ± 30.24 | 98.55 ± 31.77 | 92.38 ± 23.04 | 0.513 |
| Fasting glucose (mg/dL) | 108.00 (95.25-137.75) | 108.00 (95.00-153.00) | 111.00 (92.50-132.50) | 0.815 |
| Blood urea nitrogen (mg/dL) | 17.82 ± 5.99 | 17.56 ± 5.45 | 18.92 ± 8.07 | 0.466 |
| Creatinine (mg/dL) | 1.17 ± 0.34 | 1.14 ± 0.31 | 1.29 ± 0.44 | 0.138 |
| Total calcium (mg/dL) | 9.11 ± 0.34 | 9.12 ± 0.34 | 9.06 ± 0.32 | 0.541 |
| Phosphorus (mg/dL) | 3.43 ± 0.47 | 3.45 ± 0.50 | 3.35 ± 0.35 | 0.504 |
| Intact parathyroid hormone (pg/mL) | 55.78 ± 30.30 | 49.44 ± 23.26 | 82.61 ± 41.56 | < 0.001* |
| CRP (mg/dL) | 0.21 (0.16-0.27) | 0.20 (0.15-0.24) | 0.25 (0.19-0.59) | 0.027* |
| Sclerostin (pmoL/L) | 63.78 ± 31.32 | 56.46 ± 26.38 | 94.76 ± 32.56 | < 0.001* |
| Female, n (%) | 23 (33.8) | 18 (32.7) | 5 (38.5) | 0.694 |
| Diabetes, n (%) | 33 (48.5) | 28 (50.9) | 5 (38.5) | 0.419 |
| Hypertension, n (%) | 55 (80.9) | 44 (80.0) | 11 (84.6) | 0.704 |
| ACE inhibitor use, n (%) | 18 (26.5) | 16 (29.1) | 2 (15.4) | 0.314 |
| ARB use, n (%) | 27 (39.7) | 20 (36.4) | 7 (53.8) | 0.247 |
| β-blocker use, n (%) | 35 (51.5) | 28 (50.90) | 7 (53.8) | 0.849 |
| CCB use, n (%) | 26 (38.2) | 22 (40.0) | 4 (30.8) | 0.538 |
| Statin use, n (%) | 37 (54.4) | 26 (47.3) | 11 (84.6) | 0.015* |
| Fibrate use, n (%) | 14 (20.6) | 9 (16.4) | 5 (38.5) | 0.076 |
| Aspirin, n (%) | 40 (58.8) | 32 (58.2 | 8 (61.5) | 0.825 |
| Clopidogrel, n (%) | 17 (25.0) | 15 (27.3) | 2 (15.4) | 0.373 |
Values for continuous variables are shown as mean ± standard deviation after analysis by Student’s t-test; variables not normally distributed are shown as median and interquartile range after analysis by the Mann-Whitney U test; values are presented as number (%) and analysis after analysis by the chi-square test. ABI, ankle brachial index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; CRP, C-reactive protein; ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; CCB, calcium-channel blocker.
Values of P < 0.05 were considered statistically significant.
Multivariate logistic regression analysis of the factors significantly associated with PAD (statin use, iPTH, CRP, and sclerostin) revealing that sclerostin (odds ratio: 1.050, 95% confidence interval: 1.013-1.089, P = 0.008) was a significant independent predictor of PAD in elderly participants (Table 2). Two-dimensional scattered plots of left ABI values (r = -0.265, P = 0.029) or right ABI values (r = -0.420, P < 0.001) were negatively associated with serum sclerostin levels among the 68 elderly subjects as shown in Figure 1A and 1B.
Table 2.
Multivariate logistic regression analysis of the factors correlated to peripheral artery disease among the 68 elderly participants
| Variables | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Sclerostin (pmoL/L) | 1.050 | 1.013-1.089 | 0.008* |
| Statin used | 5.593 | 0.815-38.396 | 0.080 |
| Intact parathyroid hormone (pg/mL) | 1.022 | 0.997-1.047 | 0.082 |
| C-reactive protein (0.1 mg/dL) | 1.063 | 0.890-1.269 | 0.502 |
P < 0.05 was considered statistically significant in the multivariate logistic regression analysis (adopted factors: statin used, intact parathyroid hormone, C-reactive protein and sclerostin).
Figure 1.
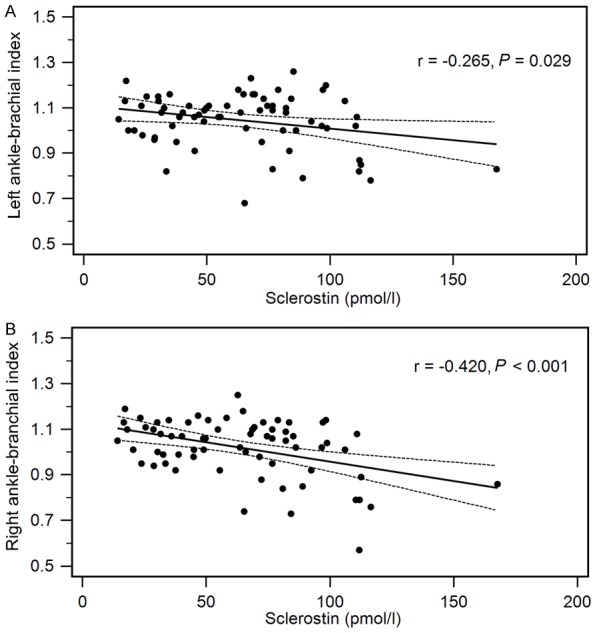
Two-dimensional scattered plots of serum sclerostin levels with (A) left ABI values (B) right ABI values among the 68 elderly subjects. Dashed lines represent 95% confidence interval.
Discussion
In the present study, we found that elderly adults with PAD had higher serum iPTH, CRP, and sclerostin levels and a high prevalence of statin use. Serum sclerostin values were found to be associated with PAD in this elderly population.
The prevalence of PAD is sharply age-related, rising > 10% among those aged 60-70 years [2]. One study in Taiwan reported that the overall prevalence of asymptomatic PAD was 8.4% in the elderly [17]. In the present study, the prevalence of PAD in patients over the age of 65 years was 19.1%. PAD is an expression of systemic atherosclerosis and well established as a risk factor for future cardiovascular events [18]. Statins effectively reduce TCH and LDL-C levels and are the most efficient drugs available today in the treatment of hypercholesterolemia, which takes a step in the growth of the atherosclerotic plaque [19]. Therefore, there would be more statin use in patients with atherosclerosis-related diseases, including PAD. Our results note a higher percentage of statin use in PAD elderly participants.
Vascular calcification and remodeling are involved in the development and progression of atherosclerosis, and PTH takes part via direct PTH receptor interaction on the vessel as well as indirectly via inflammation and vascular dysfunction [20]. Inflammatory activation participates in the development of atherosclerosis and may play a part in the generation of PAD [18]. CRP is an inflammatory marker, which is related to atherosclerosis and cardiovascular disease, including PAD [21]. Previous studies have noted that higher CRP levels are positively correlated with PAD [22,23]. Our results showed that serum iPTH and CRP levels were higher in the low ABI group than in the normal ABI group among the elderly participants.
The Wnt/β-catenin signaling pathway has been introduced as one of the inducers in this vas-cular osteochondrogenesis process and is also involved in the development of vascular calcification and atherosclerosis [5,6]. Sclerostin inhibits the Wnt/β-catenin signaling pathway, which can enhance the intracellular degradation of β-catenin and inhibit osteoblast differentiation and proliferation [24]. The transgenic introduction of human sclerostin in apolipoprotein E-deficient (ApoE-/-) mice (SOSTTg.ApoE-/-) and the administration of recombinant mouse sclerostin inhibited angiotensin II-induced aortic aneurysm and atherosclerosis [25]. Clinical studies have noted high serum sclerostin levels, which correlated positively with acute ischemic stroke, carotid intima-media thickness in humans, atherosclerotic disease in patients undergoing hemodialysis or those with diabetes, and peripheral arterial stiffness in renal transplantation recipients [6-8,13,26,27]. Our results also noted that serum sclerostin levels were higher in the low ABI group than in the normal ABI group in the elderly participants. After adjustment for a variety of confounders in multivariate logistic regression analysis, serum sclerostin levels remained independent predictors of PAD in elderly participants in our study.
There are several limitations of this study. First, this is an observational, single-center study with a limited number of elderly participants enrolled, and the possibility of bias cannot be excluded. Second, occlusive disease distal to the ankle is sensitive to the patient’s height and is not detected by the ABI test [2]. Moreover, the PAD subjects were small and they had only slightly decreased ABI levels. Further studies performed on advanced clinical PAD stages could confirm its association with higher serum sclerostin levels in elderly people.
In conclusion, high serum sclerostin levels are a risk factor for PAD in elderly people. Further long-term prospective studies are needed to elucidate the causal relationship between serum sclerostin and PAD in elderly people.
Acknowledgements
This study was supported by a grant from the Buddhist Tzu Chi General Hospital, Hualien, Taiwan (TCRD100-02).
Disclosure of conflict of interest
None.
References
- 1.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–40. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 2.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509–26. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 3.Dua A, Lee CJ. Epidemiology of peripheral arterial disease and critical limb ischemia. Tech Vasc Interv Radiol. 2016;19:91–5. doi: 10.1053/j.tvir.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Davies MG. Criticial limb ischemia: epidemiology. Methodist Debakey Cardiovasc J. 2012;8:10–14. doi: 10.14797/mdcj-8-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evrard S, Delanaye P, Kamel S, Cristol JP, Cavalier E SFBC/SN joined working group on vascular calcifications. Vascular calcification: from pathophysiology to biomarkers. Clin Chim Acta. 2015;438:401–14. doi: 10.1016/j.cca.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 6.Kirkpantur A, Balci M, Turkvatan A, Afsar B. Independent association between serum sclerostin levels and carotid artery atherosclerosis in prevalent haemodialysis patients. Clin Kidney J. 2015;8:737–43. doi: 10.1093/ckj/sfv077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morales-Santana S, García-Fontana B, García-Martín A, Rozas-Moreno P, García-Salcedo JA, Reyes-García R, Muñoz-Torres M. Atherosclerotic disease in type 2 diabetes is associated with an increase in sclerostin levels. Diabetes Care. 2013;36:1667–74. doi: 10.2337/dc12-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruzzese A, Lacquaniti A, Cernaro V, Ricciardi CA, Loddo S, Romeo A, Montalto G, Costantino G, Torre F, Pettinato G, Salamone I, Aloisi C, Santoro D, Buemi M. Sclerostin levels in uremic patients: a link between bone and vascular disease. Ren Fail. 2016;38:759–64. doi: 10.3109/0886022X.2016.1160207. [DOI] [PubMed] [Google Scholar]
- 9.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus: provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 10.Wang JH, Lee CJ, Hsieh JC, Chen YC, Hsu BG. Serum atrial natriuretic peptide level inversely associates with metabolic syndrome in older adults. Geriatr Gerontol Int. 2014;14:640–646. doi: 10.1111/ggi.12151. [DOI] [PubMed] [Google Scholar]
- 11.Lee CJ, Wang JH, Chen YC, Chen ML, Yang CF, Hsu BG. Serum osteopontin level correlates with carotid-femoral pulse wave velocity in geriatric persons. Biomed Res Int. 2014;2014:570698. doi: 10.1155/2014/570698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai JP, Wang JH, Lee CJ, Chen YC, Hsu BG. Positive correlation of serum adipocyte fatty acid binding protein levels with carotid-femoral pulse wave velocity in geriatric population. BMC Geriatr. 2015;15:88. doi: 10.1186/s12877-015-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu BG, Liou HH, Lee CJ, Chen YC, Ho GJ, Lee MC. Serum sclerostin as an independent marker of peripheral arterial stiffness in renal transplantation recipients-a cross-sectional study. Medicine. 2016;95:e3300. doi: 10.1097/MD.0000000000003300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho GJ, Chen YC, Yin WY, Chang YJ, Lee MC, Hsu BG. Fasting serum long-acting natriuretic peptide correlates with ankle brachial index in renal transplant recipients. Exp Clin Transplant. 2013;11:303–9. doi: 10.6002/ect.2012.0224. [DOI] [PubMed] [Google Scholar]
- 15.Chen YC, Hsu BG, Ho CC, Lee CJ, Lee MC. Elevated serum osteoprotegerin may predict peripheral arterial disease after kidney transplantation: a single-center prospective crosssectional study in Taiwan. PeerJ. 2017;5:e3847. doi: 10.7717/peerj.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreira AC, Macedo FY. A review of simple, non-invasive means of assessing peripheral arterial disease and implications for medical management. Ann Med. 2010;42:139–50. doi: 10.3109/07853890903521070. [DOI] [PubMed] [Google Scholar]
- 17.Chen YJ, Lin MS, Hsu KY, Chen CR, Chen CM, Chen W. Prevalence of asymptomatic peripheral arterial disease and related risk factors in younger and elderly patients in Taiwan. Angiology. 2014;65:396–401. doi: 10.1177/0003319713480842. [DOI] [PubMed] [Google Scholar]
- 18.Hamburg NM, Creager MA. Pathophysiology of intermittent claudication in peripheral artery disease. Circ J. 2017;81:281–9. doi: 10.1253/circj.CJ-16-1286. [DOI] [PubMed] [Google Scholar]
- 19.Markel A. Statins and peripheral arterial disease. Int Angiol. 2015;34:416–27. [PubMed] [Google Scholar]
- 20.Hagström E, Michaëlsson K, Melhus H, Hansen T, Ahlström H, Johansson L, Ingelsson E, Sundström J, Lind L, Arnlöv J. Plasma parathyroid hormone is associated with subclinical and clinical atherosclerotic disease in 2 community-based cohorts. Arterioscler Thromb Vasc Biol. 2014;34:1567–73. doi: 10.1161/ATVBAHA.113.303062. [DOI] [PubMed] [Google Scholar]
- 21.Albert MA, Ridker PM. C-reactive protein as a risk predictor: do race/ethnicity and gender make a difference? Circulation. 2006;114:e67–74. doi: 10.1161/CIRCULATIONAHA.106.613570. [DOI] [PubMed] [Google Scholar]
- 22.Hozawa A, Ohmori K, Kuriyama S, Shimazu T, Niu K, Watando A, Ebihara S, Matsui T, Ichiki M, Nagatomi R, Sasaki H, Tsuji I. C-reactive protein and peripheral artery disease among Japanese elderly: the tsurugaya project. Hypertens Res. 2004;27:955–61. doi: 10.1291/hypres.27.955. [DOI] [PubMed] [Google Scholar]
- 23.Sugiura T, Yoshikawa D, Ishii H, Suzuki S, Kumagai S, Inoue Y, Okumura S, Isobe S, Hayashi M, Ando H, Amano T, Murohara T. Relation of omega-3 fatty acid and C-reactive protein to peripheral artery disease in patients with coronary artery disease. Heart Vessels. 2014;29:449–55. doi: 10.1007/s00380-013-0384-4. [DOI] [PubMed] [Google Scholar]
- 24.Boudin E, Van Hul W. Mechanisms in endocrinology: genetics of human bone formation. Eur J Endocrinol. 2017;177:R69–R83. doi: 10.1530/EJE-16-0990. [DOI] [PubMed] [Google Scholar]
- 25.Krishna SM, Seto SW, Jose RJ, Li J, Morton SK, Biros E, Wang Y, Nsengiyumva V, Lindeman JH, Loots GG, Rush CM, Craig JM, Golledge J. Wnt signaling pathway inhibitor sclerostin inhibits angiotensin II-induced aortic aneurysm and atherosclerosis. Arterioscler Thromb Vasc Biol. 2017;37:553–66. doi: 10.1161/ATVBAHA.116.308723. [DOI] [PubMed] [Google Scholar]
- 26.He XW, Wang E, Bao YY, Wang F, Zhu M, Hu XF, Jin XP. High serum levels of sclerostin and Dickkopf-1 are associated with acute ischaemic stroke. Atherosclerosis. 2016;253:22–8. doi: 10.1016/j.atherosclerosis.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Gaudio A, Fiore V, Rapisarda R, Sidoti MH, Xourafa A, Catalano A, Tringali G, Zanoli L, Signorelli SS, Fiore CE. Sclerostin is a possible candidate marker of arterial stiffness: results from a cohort study in Catania. Mol Med Rep. 2017;15:3420–4. doi: 10.3892/mmr.2017.6390. [DOI] [PubMed] [Google Scholar]


